Dear Editor,
Bevacizumab is monoclonal antibody approved by the US FDA for metastatic colorectal cancer.1 Off-label intravitreal bevacizumab is gaining popularity among ophthalmologists worldwide, including India,2 due to its reported efficacy. Herein, we report the safety profile of bevacizumab through our recent experience over the last 14 months (1 st January ′06 to 28 th February ′07) of using 1000 intravitreal injections of bevacizumab prepared in single dosage form (while maintaining sterility) for the treatment of choroidal neovascularization, proliferative diabetic retinopathy, and macular edema due to diabetic retinopathy or vascular occlusion.
Intravitreal bevacizumab injections were prepared by a qualified pharmacist in the pharmacy laboratory. From the commercially available 4 ml vial containing 100 mg bevacizumab (Avastin, Genentech, Inc. South San Francisco, CA), 0.2 ml fractions were transferred under strict aseptic conditions (class 10 environment) into 2 ml glass ampoules having long necks, using a 2 ml syringe fitted with a 20 G needle. The filled ampoules consisting of bevacizumab fractions were flame-sealed manually with a brief exposure of the neck for 5 to 7 seconds [Fig. 1]. During the entire formulation process cold chain (2 to 8°C) was maintained and elevation of temperature was observed upto 15°C. The cold chain was continued immediately after sealing till the samples were stored and administered. Twenty fractions were dispensed from each 4 ml vial of bevacizumab. For quality checking, one random sample was selected for sterility testing.
Figure 1.
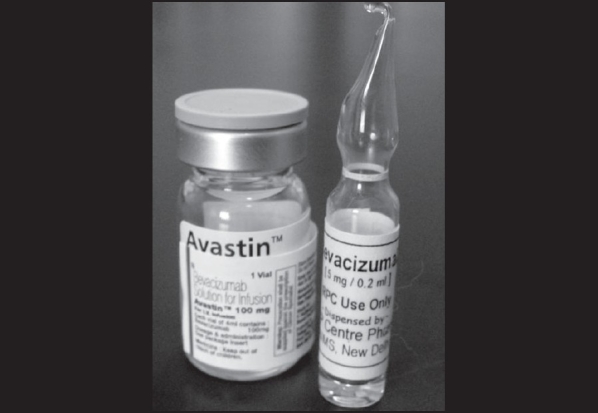
Aliquoted 2 ml glass ampoules containing 5 mg/0.2 ml, dispensed under sterile conditions from 4 ml vials containing 100 mg of bevacizumab—for Dr. R. P. Centre use only
Over the period of 14 months, 50 vials were dispensed into 1000 ampoules, which were used in 480 patients. In all cases, 1.25 mg/0.05 ml of bevacizumab from one ampoule was used for intravitreal injection in one eye only. All the patients were examined with slit lamp biomicroscopy at day 1 and day 7 post-injection for signs of intraocular inflammation/infection. Among the 480 patients, only one eye (0.001% of injections) developed endophthalmitis. The patient was a 36-year-old lady having peripapillary choroidal neovascularization [Fig. 2] in her left eye with a best-corrected vision (BCVA) of 20/200. Two days after intravitreal injection, this patient developed hypopyon and vitreous exudates, which were more at the injection site, i.e., the inferotemporal retina [Fig. 3]. She recovered BCVA of 10/200 at 3 months after intravitreal vancomycin and ceftazidime followed by parsplana vitrectomy. Further, two patients (0.004% of eyes) had experienced mild iridocyclitis in the first week post-injection and were successfully treated with topical prednisolone acetate. In all the other patients, intravitreal bevacizumab was well tolerated.
Figure 2.
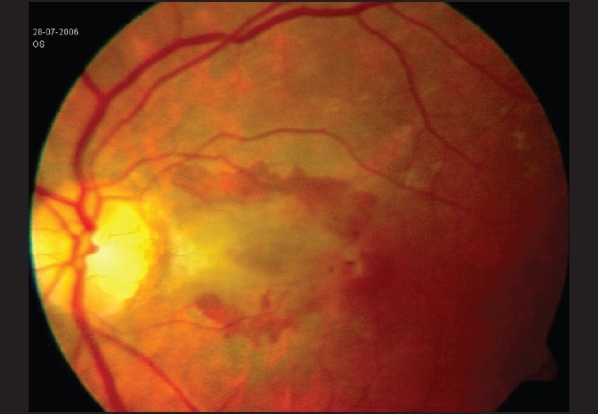
Patient with peripapillary choroidal neovascular membrane involving the papillo-macular region in the left eye
Figure 3.
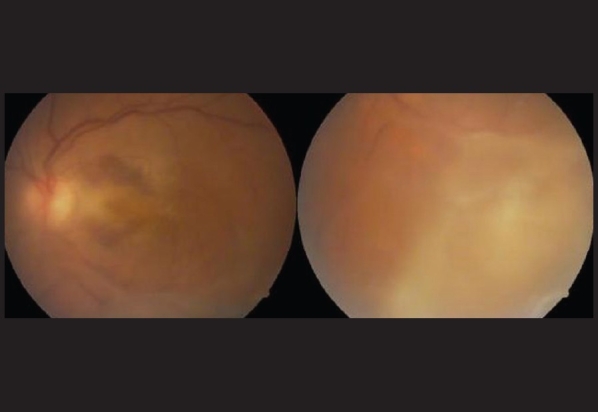
Two days after intravitreal bevacizumab injection
The available therapeutic modalities like pegaptanib, ranibizumab, and photodynamic therapy for treatment of ocular pathologies are quite expensive and unaffordable for patients from the low-income strata. Bevacizumab has been found to be well tolerated and is devoid of any significant retinal toxicity in various preclinical and clinical studies.3,4 Therefore, cost-effective formulations of bevacizumab can be explored as an alterative and economical approach to treat a variety of retinal pathologies in developing and underdeveloped countries, where the per capita income is low. In developing countries like India, where the per capita GDP is USD 543 (March 2006), the majority of patients cannot afford to pay USD 730 for a single vial of bevacizumab.5 Therefore, efforts were made to make 20 fractions of 0.2 ml from a single vial, thus decreasing the cost to USD 38 per injection. This type of hospital pharmacy support not only reduces the cost-per-injection but also allows ready availability of bevacizumab in single dosage while maintaining the sterility.
References
- 1.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 2.Shah PK, Narendran V, Tawansy KA, Raghuram A, Narendran K. Intravitreal bevacizumab (Avastin) for post laser anterior segment ischemia in aggressive posterior retinopathy of prematurity. Indian J Ophthalmol. 2007;55:75–6. doi: 10.4103/0301-4738.29505. [DOI] [PubMed] [Google Scholar]
- 3.Bakri SJ, Cameron JD, McCannel CA, Pulido JS, Marler RJ. Absence of histologic retinal toxicity of intravitreal bevacizumab in a rabbit model. Am J Ophthalmol. 2006;142:162–4. doi: 10.1016/j.ajo.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 4.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–72. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Fung AE, Rosenfeld PJ, Reichel E. The international intravitreal bevacizumab safety survey: Using the internet to assess drug safety worldwide. Br J Ophthalmol. 2006;90:1344–9. doi: 10.1136/bjo.2006.099598. [DOI] [PMC free article] [PubMed] [Google Scholar]


