Abstract
Intracameral injection of bevacizumab (Avastin) helped in the successful regression of an anterior chamber neovascular membrane in a painful blind eye. The effect was persistent even after six months of follow-up. This is the first report on intracameral administration of bevacizumab with six months of follow-up.
Keywords: Avastin, bevacizumab, intracameral injection
Intravitreal administration of bevacizumab (Avastin, Genetech, Inc, San Francisco, CA), a humanized monoclonal antibody to vascular endothelial growth factor (VEGF) has recently been reported to be of benefit in choroidal neovascular membrane,1,2 retinal neovascularization in proliferative diabetic retinopathy3 and iris neovascularization.4,5 We observed rapid resolution of anterior chamber neovascularization following intracameral injection of bevacizumab in a patient with painful blind eye.
Case Report
A 31-year-old woman presented with pain and redness in her left eye. Visual acuity in the left eye was no perception of light and 20/20 in the right eye. The right eye was normal. The left eye was blind for the past 20 years following an injury. On examination the left eye showed circumcorneal congestion, anterior chamber cells and flare, peripheral anterior synechiae, ectropion uveae and an active fibrovascular membrane [Fig. 1A] on the iris and over the partially absorbed cataractous lens. The intraocular pressure was 6 mmHg. Contact B-scan ultrasonography revealed a total retinal detachment. Earlier the patient was treated with long-term topical steroids and cycloplegics with no significant relief of symptoms. So the patient was offered an off-label intracameral injection of 1.00 mg of bevacizumab (0.04 ml of Avastin, Genentech, INC, San Francisco, CA at a concentration of 25 mg /ml). The consent of the patient was obtained after explaining the risks and benefits of the treatment. One week following the intracameral injection the circumcorneal congestion disappeared and the anterior chamber inflammation decreased and there was dramatic regression of neovascularization [Fig. 1B]. The post injection intraocular pressure was 8 mmHg on day one and after one week. After six months this response to treatment sustained and the patient remained symptom-free [Fig. 1C].
Figure 1.
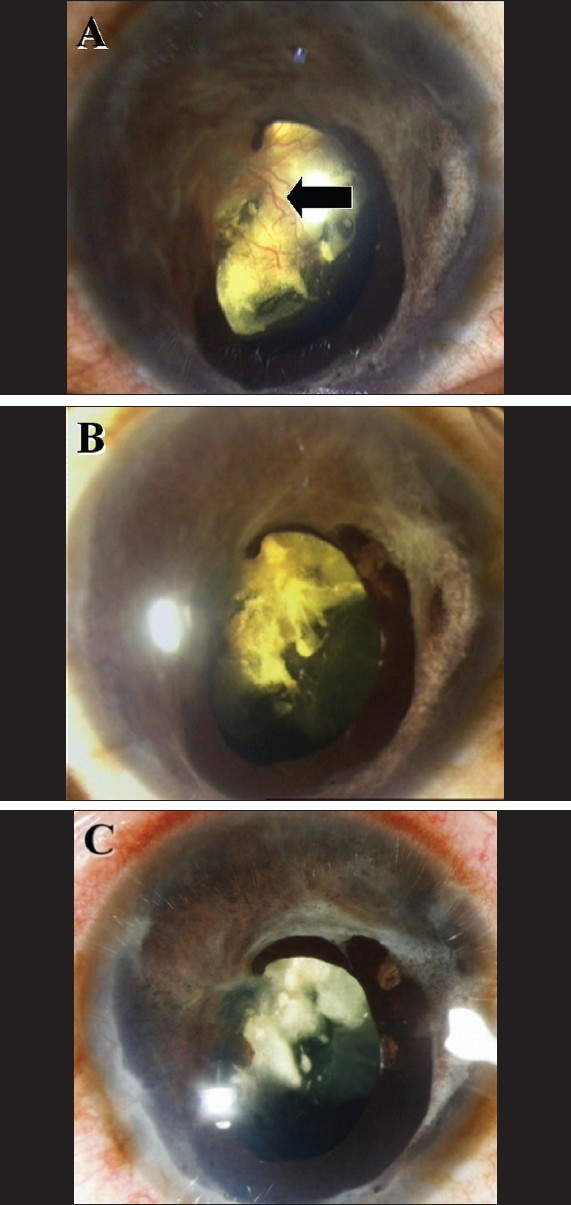
(A) Slit-lamp photograph showing circumcorneal congestion and neovascularization over the iris and lens capsule (Black arrow); (B and C) Photographs showing the total regression of neovascularization one week and six months respectively following intracameral injection of bevacizumab (Avastin)
Discussion
Genentech (San Francisco, CA) developed a monoclonal antibody against VEGF that was tested as a cancer therapy with the idea that reducing the vascular supply to a tumor may inhibit growth of the cancer. VEGF is a protein and is the most important growth factor for neovascularization in a variety of tissues including the eye.
Hypoxia stimulates the secretion of VEGF in retinal pigment epithelial cells6 and VEGF production increases with neovascularization of the iris in primates.7 In retinal detachment there is alteration in retinal perfusion arising from separation of the choroidal blood supply from the retinal pigment epithelium and can result in relative retinal ischemia. This ischemia stimulates the production of VEGF in retinal pericytes, endothelial cells, the retinal pigment epithelium and possibly other cell types.8 The VEGF is either bound to the cell-surface or basement-membrane proteoglycans containing heparin (VEGF189, 286) or freely diffusible within the vitreous cavity (VEGF121, 165).9 Diffusible VEGF follows its concentration gradient from the vitreous to the anterior segment and is cleared through the trabecular meshwork. Neovascularization can arise anywhere along this course. Inhibitions by means of antibody, antibody fragment or aptamer binding are strategies used in medicine to reduce the effects of VEGF in a variety of diseases. Our patient received 1 mg of bevacizumab, an antibody to VEGF, as an intracameral injection. The complete regression of neovascular membrane was noted after a week. We expected recurrence of neovascularization after some time, but there was no recurrence even after six months. Lloyd Paul Aiello and associates have mentioned in their article on VEGF in ocular fluid that "cell death without ischemia would have less vasoproliferative potential, since increased VEGF production would not be possible".8 In our patient the eye is going for phthisical state and maybe the cells responsible for the production of VEGF are dying without ischemia. The existing load of VEGF was taken care of by the therapy and there was no new VEGF production. Probably this is the reason why the patient did not have recurrence.
Regression of retinal and iris neovascularization after intravitreal injection of bevacizumab in human eyes has been reported.3,4,5 Although there is one report10 on intracameral administration of bevacizumab with one month follow-up, we believe that this is the first report on intracameral administration of bevacizumab with six months of follow-up. This case clearly demonstrates the dramatic effect of bevacizumab on ocular neovascularization, which might help in widening the spectrum of bevacizumab usage in ocular diseases.
Acknowledgments
We thank Dr. Richard F Spaide of Vitreous-Retina-Macula consultants of New York, NY, USA.
References
- 1.Rosenfeld PJ, Moshfegh AA, Puliafito CA. Optical Coherence Tomography findings after intravitreal injection of bevacizumab (Avastin) for neovascular age related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–5. [PubMed] [Google Scholar]
- 2.Avery RL, Pieramjci DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–72. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina. 2006;26:275–8. doi: 10.1097/00006982-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (Avastin) treatment. Retina. 2006;26:352–4. doi: 10.1097/00006982-200603000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Davidorf FH, Mouser JG, Derick RJ. Rapid improvement of rubeosis iridis from a single bevacizumab (Avastin) injection. Retina. 2006;26:354–6. doi: 10.1097/00006982-200603000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Shima D, Adamis AP, Yeo TK. Hypoxic regulation of vascular permeability factor (vascular endothelial growth factor) mRNA and protein secretion by human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1993;34:900. [Google Scholar]
- 7.Adamis AP, Miller JW, O′Reilly M. Vascular permeability factor (vascular endothelial growth factor) is produced in the retina and elevated levels are present in the aqueous humor of patients with iris neovascularization. Invest Ophthalmol Vis Sci. 1993;34:1440. [Google Scholar]
- 8.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–7. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 9.Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;267:26031–7. [PubMed] [Google Scholar]
- 10.Grisanti S, Biester S, Peters S, Tatar O, Ziemssen F, Bartz-Schmidt KU, et al. Intracameral bevacizumab for iris rubeosis. Am J Ophthalmol. 2006;142:158–60. doi: 10.1016/j.ajo.2006.02.045. [DOI] [PubMed] [Google Scholar]


