Abstract
Recent developments may provide an opportunity to improve outcome in individuals who develop neovascular age-related macular degeneration (ARMD). Several therapies have been introduced that show promise for halting the progression of this disorder. However, data from controlled clinical trials to test the relative efficacy of different management strategies across the subtypes of disease remain limited. New treatment modalities that target the neovascularization process, including leakage from choroidal neovascularization (CNV), are currently being developed. Vascular endothelial growth factor (VEGF) has been implicated as a key mediator in the pathogenesis of ARMD-related CNV. Anti-VEGF strategies show promise as potential therapeutic agents for the treatment of CNV and are currently undergoing active clinical investigation. Such strategies include anti-VEGF antibodies, anti-VEGF aptamer, gene therapy and protein kinase C inhibition. This article reviews the mechanism of action and rationale for anti-VEGF drugs in ARMD.
Keywords: Age-related macular degeneration, anti-vascular endothelial growth factor
Choroidal neovascularization (CNV) in patients with age-related macular degeneration (ARMD) is the leading cause of irreversible vision loss in the elderly. Neovascularization derived from choroidal blood vessels usually breaks through Bruch′s membrane and grows under the retinal pigmented epithelium (RPE). Currently available therapeutic modalities are not highly efficacious in the treatment of CNV from ARMD. To date, the two treatments shown by large clinical trials to have some efficacy are thermal laser treatment (photocoagulation) and photodynamic therapy (PDT). Laser photocoagulation is only useful in a small percentage of patients that have well-defined areas of classic CNV.1,2,3 In addition, central vision is preserved only if these lesions are in either an extrafoveal or juxtafoveal location. Moreover, recurrences after laser photocoagulation are common and occur in approximately 50% of patients.3 Photodynamic therapy with verteporfin (Visudyne ® , Novartis AG, Basel, Switzerland) has been shown to reduce vision loss for subfoveal CNV secondary to ARMD, particularly if angiography demonstrates the lesion to be predominantly classic CNV.4 However, many patients have lesions that are not amenable to treatment and vision improvement is unusual.
New treatment modalities that target the neovascularization process including leakage from CNV are currently being developed. Vascular endothelial growth factor has been demonstrated in human specimens of CNV and animal models have confirmed that this protein is capable of inducing CNV.5 Therefore, targeted inhibition of VEGF seems to be a reasonable approach for the treatment of CNV.
VEGF appears to be an important growth factor for angiogenesis and has been shown to be necessary in normal vascular development. VEGF is highly selective for vascular endothelial cells and induces angiogenesis by serving as a potent endothelial cell mitogen. It has been shown to be secreted by hypoxic RPE cells and induces endothelial cell proliferation and retinal vascular permeability. VEGF has also been shown to be necessary and sufficient for the development of retinal and iris neovascularization in experimental models.6 It has been identified as a major mediator of retinal ischemia-associated neovascularization. VEGF is up-regulated by hypoxia and its levels are increased in the vitreous and retina of patients and laboratory animals with active neovascularization from ischemic retinopathies such as proliferative diabetic retinopathy, central retinal vein occlusion and retinopathy of prematurity. Polarized secretion of VEGF by RPE cells is thought to direct VEGF toward the choroidal vasculature, where it may regulate choroidal integrity by binding to its receptors on the adjacent choriocapillaris. The importance of VEGF for the development of ARMD-related CNV has led to the development of strategies to block its effects. Such strategies include anti-VEGF antibodies, anti-VEGF aptamer, gene therapy and protein kinase C inhibition. The hope is that blocking the actions of VEGF will prove to be an effective strategy for the treatment of CNV.6 Angiogenesis is the development of new capillaries from preexisting vascular network. It may occur in a variety of ocular disorders, such as retinopathy of prematurity, retinal artery or vein occlusion, diabetic retinopathy and ARMD. The discovery of such factors as VEGF and their mechanisms of action has led to the development of drugs specifically targeting the molecules or their signal transduction pathways.7
Rationale
VEGF appears to be one of the major regulators in CNV among the angiogenic factors studied so far; VEGF plays a central role in the development of CNV. The VEGF family includes placenta growth factor, VEGF-A, VEGF-B, VEGF-C, VEGF-D and VEGF-E. Briefly, VEGF-A plays a pivotal role in the development of pathologic angiogenesis in ischemic and inflammatory diseases. VEGF is a 35- to 45-kd homodimeric protein originally isolated as a vasopermeability factor and later cloned and identified as an angiogenesis factor. Up to six different VEGF isoforms are derived through alternative splicing of messenger RNA (mRNA). VEGF165 appears to be the isoform most responsible for pathologic ocular neovascularization. Hypoxia is a major regulator of VEGF expression which distinguishes VEGF from other growth factors that have been postulated to have a role in ocular neovascular diseases, including insulin-like growth factor-1, fibroblast growth factors (FGF), epidermal growth factor and placenta growth factor. Many cells in the eye produce VEGF and within the retina, these include RPE, pericytes, endothelial cells, glial cells, muller cells and ganglion cells. In the human eye, elevated vitreous and aqueous VEGF levels strongly correlate with retinal ischemia-associated neovascularization in conditions like diabetic retinopathy, retinal vein occlusion and retinopathy of prematurity.7
The importance of VEGF as a therapeutic target derives from its roles in two of the most basic processes within a typical lesion of advanced ARMD: neovascularization and vascular leakage. The neovascular form is responsible for 80 to 90% of cases of severe vision loss due to ARMD. Given the increasing prevalence of neovascular ARMD and the burden of associated vision loss, it is important to define treatment benefits that are meaningful to the patient. Neovascular ARMD often has a poor prognosis, resulting in a rapid and progressive loss of visual acuity and contrast sensitivity. Such losses have a profound effect on patients′ quality of life and their ability to perform everyday tasks. Photodynamic therapy, currently the most thoroughly investigated definitive therapy, is useful mainly for the classic types of neovascular ARMD. However, most angiographic lesions of patients who undergo fluorescein angiography for neovascular ARMD are subfoveal and occult; only 20% of subfoveal lesions are predominantly classic. Other treatment options such as submacular surgery and steroid-based therapies appear less favorable on current evidence. The strong supportive evidence from animal studies defined VEGF as an optimal therapeutic target. It is hoped that by using a more selective and less destructive approach, vision loss induced by the treatment itself might be reduced. VEGF over expression induces endothelial cell proliferation and increases vascular permeability, properties that can be detected clinically as the presence of subretinal fluid or enlarging CNV. Anti-VEGF therapy might reduce subretinal fluid, a theoretic possibility with VEGF inhibition,8 resulting in short-term vision improvement.
Mechanism of Action
The role of VEGF as a critical factor in the control of the growth of abnormal blood vessels from the choroid directly attacks a central problem in this disease. The profound vascular permeability induced by VEGF is potentially of even greater importance in the treatment of established neovascular ARMD lesions, in which leakage of fluid from new vessels causes visual loss through retinal edema and exudation, subretinal fluid and hemorrhage.7
Anti-VEGF aptamers are stable small RNA-like molecules that bind exclusively and with high affinity to the 165-kDa isoform of human VEGF [Fig. 1]. Pegaptanib sodium, an oligonucleotide known as an aptamer, binds and inhibits only the extracellular isoforms of VEGF that are at least 165 amino acids in length.9 Multiple biologically active forms of VEGF-A are generated by both alternative mRNA splicing and posttranslational modification (proteolytic cleavage),10 and two of these forms (VEGF165 and VEGF121) have been detected in choroidal neovascular lesions. Pegaptanib (Macugen, Pfizer) can only bind and inhibit the larger VEGF165 isoform.
Figure 1.
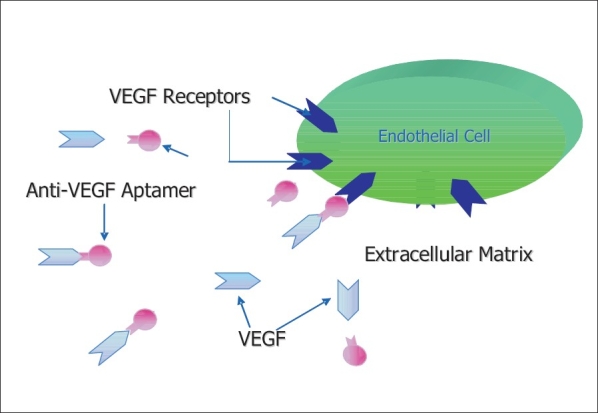
Mechanism of Anti-VEGF inhibition - The aptamer prevents binding of VEGF to normal receptor
In contrast to pegaptanib, bevacizumab (Avastin; Genentech, South San Francisco) a full-length, humanized monoclonal antibody against VEGF and ranibizumab (Lucentis; Genentech, South San Francisco, California), a recombinant, humanized, monoclonal antibody antigen-binding fragment (Fab), bind and neutralize all the biologically active forms of VEGF.11,12,13 The similar VEGF binding properties of bevacizumab and ranibizumab can be explained by their common molecular lineage. Both drugs are proteins that were genetically modified from the same murine monoclonal antibody against VEGF. The two proteins differ in their size and affinity for VEGF. Whereas bevacizumab is a humanized, murine full-length antibody with two binding sites for VEGF, ranibizumab is a humanized, murine antigen-binding fragment (Fab) with only a single affinity-matured binding site for VEGF.13 Bevacizumab is currently approved as an intravenous treatment for metastatic colorectal cancer. There is anecdotal evidence that off-label use intravitreal bevacizumab improves short-term visual outcomes in patients with neovascular ARMD.14
VEGF-Trap(R1R2) is a fusion protein that combines ligand-binding elements taken from the extracellular domains of VEGFR-1 and VEGFR-2 fused to the Fc portion of IgG. This potent high-affinity VEGF blocker effectively suppresses tumor growth and vascularization in vivo , resulting in almost completely avascular tumors. Subcutaneous injections or a single intravitreous injection of VEGF-Trap(R1R2) strongly suppressed CNV in mice with laser-induced rupture of Bruch′s membrane, and subretinal neovascularization in transgenic mice expressing VEGF in photoreceptor cells.15
RNAi is a double-stranded piece of interference RNA that is taken up by chorioretinal cells, activating a protein that breaks down the antisense mRNA. Destruction of VEGF mRNA prevents the production of VEGF protein. The whole process is catalytic, so the RNAi may be a very potent and efficient blockade of VEGF. RNAi may have a long biologic half-life, indicating a much longer interval between intravitreal injections. Anti-VEGF RNAi for the treatment of CNV is currently being tested in clinical trials.15
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Rasmussen H, Chu KW, Campochiaro P, Gehlbach PL, Haller JA, Handa JT, et al. Clinical protocol. An open-label, phase I, single administration, dose-escalation study of ADGVPEDF.11D (ADPEDF) in neovascular age-related macular degeneration (AMD). Hum Gene Ther. 2001;12:2029–2032. [PubMed] [Google Scholar]
- 2.Macular Photocoagulation Study Group. Krypton laser photocoagulation for neovascular lesions of age-related macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1990;108:816–24. doi: 10.1001/archopht.1990.01070080058036. [DOI] [PubMed] [Google Scholar]
- 3.Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration. Results of a randomized clinical trial. Arch Ophthalmol. 1991;109:1220, 31. doi: 10.1001/archopht.1991.01080090044025. [DOI] [PubMed] [Google Scholar]
- 4.Blumenkranz MS, Bressler NM, Bressler SB, Donati G, Fish GE, Haynes LA, et al. Verteporfin therapy for subfoveal choroidal neovascularization in age-related macular degeneration: Three-year results of an open-label extension of 2 randomized clinical trails-TAP report no. 5. Arch Ophthalmol. 2002;120:1307, 14. doi: 10.1001/archopht.120.10.1307. [DOI] [PubMed] [Google Scholar]
- 5.Kvanta A, Algvere PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996;37:1929–34. [PubMed] [Google Scholar]
- 6.Barouch FC, Miller JW. Anti-vascular endothelial growth factor strategies for the treatment of choroidal neovascularization from age-related macular degeneration. Int Ophthalmol Clin. 2004;44:23–32. doi: 10.1097/00004397-200404430-00005. [DOI] [PubMed] [Google Scholar]
- 7.Lu M, Adamis AP. Molecular biology of choroidal neovascularization. Ophthalmol Clin North Am. 2006;19:323–34. doi: 10.1016/j.ohc.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Aiello LP, Bursell SE, Clermont A, Duh E, Ishii H, Takagi C, et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997;46:1473–80. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- 9.Adamis AP, Shima DT. The role of vascular endothelial growth factor in ocular health and disease. Retina. 2005;25:111–8. doi: 10.1097/00006982-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab: An anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 12.Presta LG, Chen H, O′Connor SJ, Chisholm V, Meng YG, Krummen L, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–9. [PubMed] [Google Scholar]
- 13.Chen Y, Wiesmann C, Fuh G, Li B, Christinger HW, McKay P, et al. Selection and analysis of an optimized anti-VEGF antibody: Crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol. 1999;293:865–81. doi: 10.1006/jmbi.1999.3192. [DOI] [PubMed] [Google Scholar]
- 14.Shams N, Ianchulev T. Role of vascular endothelial growth factor in ocular angiogenesis. Ophthalmol Clin North Am. 2006;19:335–44. doi: 10.1016/j.ohc.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Kaiser PK. Antivascular endothelial growth factor agents and their development: Therapeutic implications in ocular diseases. Am J Ophthalmol. 2006;142:660–8. doi: 10.1016/j.ajo.2006.05.061. [DOI] [PubMed] [Google Scholar]


