Abstract
Age-related macular degeneration (AMD) is now considered an important and leading cause of blindness among elderly patients in developed and developing countries. AMD has two forms, dry and wet; both can lead to visual loss. However, occurrence of subfoveal choroidal neovascular (CNV) membrane in the wet form results in severe visual impairment.
Treatment options for choroidal neovascularization are available in order to maintain and in some cases improve vision. Photodynamic therapy (PDT) has been used to treat both classic and occult membranes. It has known to cause choroidal hypoperfusion and production of vascular endothelial growth factor.
Intravitreal steroid can possibly reduce the damage caused due to these undesirable effects. In the recent past, intravitreal injection of triamcinolone acetonide (IVTA) has been used extensively as an adjunct to PDT in AMD in order to reduce the number of PDT sessions and evaluate possible beneficial effects on vision.
This article reviews the pharmacological attributes of triamcinolone, available evidence of its use as monotherapy or combination therapy to treat AMD, ocular side-effects thereof and ongoing clinical trials on IVTA.
Keywords: Age-related macular degeneration, choroidal neovascularization, intravitreal steroid
Age-related macular degeneration (AMD) is now considered an important and leading cause of blindness among elderly patients in developed and developing countries. Age-related macular degeneration has two forms, dry and wet; both can lead to visual loss. However, occurrence of subfoveal choroidal neovascular membrane (CNVM) in the wet form results in severe visual impairment. Treatment options used to treat the wet form of AMD were laser photocoagulation, low-dose radiation, interferon, transpupillary thermotherapy with or without ICG-enhancement and more recently, photodynamic therapy (PDT).
Photodynamic therapy with verteporfin has been shown by the TAP study1 to be effective in the treatment of predominantly classic subfoveal CNVM. Verteporfin in PDT (VIP study)2 showed it to be so in occult CNVM. In general, patients do require multiple sessions (3.5 to 5 sessions on an average)1,2 of PDT, thereby hiking the cost. Studies have also revealed that PDT could cause choroidal hypoperfusion and upregulate the production of vascular endothelial growth factor (VEGF). Yet, what detrimental effects these changes have on vision remains speculative.
In the recent past, intravitreal injection of triamcinolone acetonide (IVTA) has been used extensively as an adjunct to PDT in AMD in order to reduce the number of PDT sessions and evaluate possible beneficial effects on vision.
This article reviews the pharmacological attributes of triamcinolone, available evidence of its use as monotherapy or combination therapy to treat AMD, ocular side-effects thereof and ongoing clinical trials on IVTA.
Pharmacological Attributes
Chemical composition
Triamcinolone is a synthetic steroid of the glucocorticoid family having a fluorine instead of hydrogen atom in the ninth position.
It is used clinically as an anti-inflammatory drug and an immunomodulator in a variety of diseases.
It is commercially available as esters; a white powder practically insoluble in water but soluble in alcohol, chloroform.
Vitreous concentration of triamcinolone after intravitreal injection is 1.22 ± 0.24 µg/ml.3
Mechanism of action
Triamcinolone acetonide appears to modulate the permeability and adhesion of endothelial cells in culture.
It down-regulates cytokine-induced expression of the intercellular adhesion molecule - 1(ICAM-1).4
Interferon gamma-induction of vascular permeability is brought down by triamcinolone.4
Wang et al.5 have demonstrated that matrix metalloproteinase was down-regulated following incubation with triamcinolone.
Ciulla et al.6 have shown that triamcinolone inhibited laser-induced choroidal neovascularization in rats.
Steroids help re-absorption of fluid and down-regulate inflammatory stimuli.
Technique of intravitreal injection
It is preferable to give intravitreal triamcinolone under an aseptic condition to minimize the potential risk of endophthalmitis. Preoperative topical antibiotics can be used as per the physician′s discretion. Topical povidone iodine application enhances safety.7,8
Commercially available triamcinolone can be used for injection. To prevent inflammation due to the vehicle benzyl alcohol, the drug can be passed through a Millipore filter or can be centrifuged; however, in such methods, the concentration of the drug and its dosage may not be accurate. High performance liquid chromatography can precisely determine the concentration.9
The injection is loaded into a tuberculin syringe and a 30-G needle is used for the injection. The procedure is done under topical anesthesia. It is injected 3.5-4 mm from the limbus, usually in the inferotemporal quadrant. Fundus evaluation after the procedure is performed to examine the arterial perfusion at the optic nerve head and to determine the need for anterior chamber paracentesis. Topical use of antibiotics is continued for a week after the injection. In the postoperative period, measurement of intraocular pressure (IOP) is recommended for two to three months.10
Clinical Evidence
Use of IVTA as monotherapy
There have been studies with small sample sizes on the treatment of subfoveal CNVM due to AMD, with IVTA. It was first described by Penfold et al. ,11 in a pilot study using IVTA in 30 eyes, not amenable to laser photocoagulation. The overall visual outcome was better in the treatment group than in the untreated group.
Table 1 summarizes the results of published reports. In all these studies, there was no masking of investigators and patients. The duration of follow-up was only up to one year. The only study with a large number (n= 151) showed that though the size of membrane decreased with IVTA, there was no significant reduction in the risk of developing severe visual loss. Two were randomized control trials and the one with a larger sample size did not show any significant difference between cases and controls.
Table 1.
Studies of monotherapy with intravitreal injection of triamcinolone acetonide in age-related macular degeneration
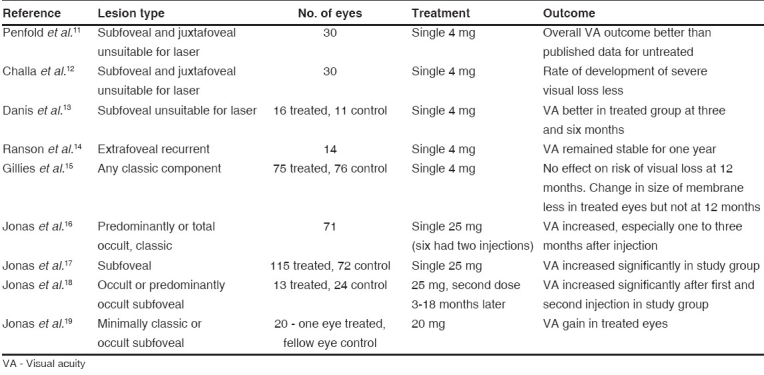
The efficacy of IVTA monotherapy remains controversial.
Use of IVTA as a combined therapy (PDT and IVTA)
Photodynamic therapy with verteporfin has been shown to be effective in the treatment of choroidal neovascularization secondary to AMD [Fig. 1]. Though the leakage from the neovascular complex, on fluorescein angiogram, declines with treatment, moderate to severe vision loss is noted after PDT. This can be due to collateral hypoxic damage to the adjacent choriocapillaris and retinal pigment epithelial atrophy following PDT. There is also release of VEGF following PDT which can cause transient visual disturbance secondary to hyperpermeability induced by it.20 This was demonstrated by multifocal electroretinography.21
Figure 1.
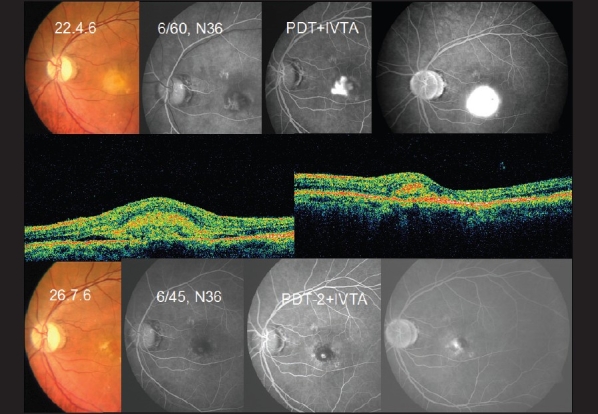
A 57-year old male with reduced vision following cataract surgery. On examination, he was diagnosed to have classic subfoveal choroidal neovascular membrane. FFA showed classic subfoveal membrane (top panel). He underwent PDT with IVTA following which his vision had improved by 1 line and the membrane has reduced in size. FFA showed reduction in size (bottom panel). OCT (middle panel) showed marked reduction in thickness of CNVM following treatment. He underwent a second sitting of PDT with IVTA
Triamcinolone acetonide due to its anti-angiogenic and anti-inflammatory effects can reduce the re-perfusion of CNVM and also reduce the edema secondary to inflammation. Spaide et al. ,22 in their pilot study of PDT with IVTA showed improvement in visual acuity. Subsequently, there have been a number of reports of IVTA combined with PDT for all types of membranes due to AMD [Table 2].
Table 2.
Studies of combination therapy with intravitreal injection of triamcinolone acetonide and photodynamic therapy in age-related macular degeneration
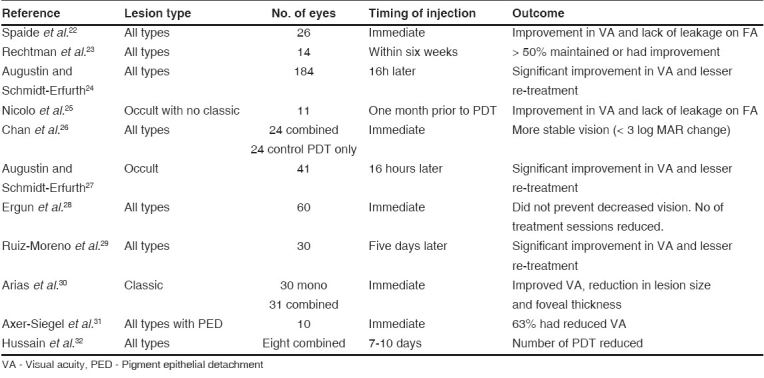
These studies indicate that combined treatment as a first line of management reduces the need for multiple sessions of PDT, avoids further vision loss and improves visual acuity. However, reduction in visual acuity of three or more Snellen lines was noted in two studies.28,31 Except one,30 all of the published studies were nonrandomized. So, the results need to be interpreted cautiously before making a clinical decision of using IVTA combined with PDT.
Ocular Complications
(a) Due to injection procedure
Endophthalmitis
Both infectious and sterile endophthalmitis were reported.
In one study33 the incidence of culture-positive endophthalmitis was 0.87%, while in other reports no case was observed; this difference could be due to following strict asepsis during the administration of the drug and partly due to prophylactic use of antibiotics. Sterile endophthalmitis could occur due to solvent agent. Pseudo hypopyon occurs due to triamcinolone crystals entering the anterior chamber.
(b) Due to the triamcinolone
Glaucoma
IOP elevation is reported in 19 to 43% of eyes after IVTA. It occurs most often between one and three months of injection. Jonas et al. ,10 reported IOP greater than 21 mm of Hg, 30 mm of Hg, 35 mm of Hg and 40 mm of Hg in 41.2%, 11.4%, 5.5% and 1.8% patients respectively. Rise in IOP is well controlled with one to two topical anti-glaucoma medications; around 3% need filtering surgery.
Cataract
Following IVTA, progression of cataract has been reported in 0 to 57%. One study34 has shown that progression of cataract could be associated with increased IOP. Cataract surgery after IVTA does not harbor a markedly elevated frequency or a markedly changed profile of surgical complications.35
Constraints of using IVTA
A cause of concern with the use of IVTA is the vehicle benzyl alcohol which can cause inflammatory responses. There have been methods described to remove it; however, the concentration of the drug obtained after removal is not uniform. We usually do not undertake measures to purify the drug, however, some others use only the purified form.15,23,24,29 These need to be standardized
Various investigators have given the injection at various intervals following PDT. More research with respect to its ideal timing, frequency and dosage will help standardize the procedure.
The drug has a propensity to cause rise in IOP and cataract. Hence careful evaluation and follow-up is needed.
There is as yet no satisfactory answer to the question: how frequently can IVTA be repeated?
Non-availability of randomized clinical trial, a gold standard in evaluating the efficacy of IVTA.
Ongoing Clinical Trials
-
VERTACL (Verteporfin Triamcinolone for AMD, sponsored by the National Eye Institute, Bethesda, MD)
Study design: This is a masked, randomized, multi-center clinical study of participants randomized to one of the following treatment arms:
1.25 mg intravitreal bevacizumab at baseline (visit 0) and then at each of weeks 6, 12 (month 3), 18, 26 (month 6), 32, 38 (month 9), 45 and 52 (month 12).
1.25 mg intravitreal bevacizumab + half-fluence verteporfin-PDT + 1 mg preservative-free triamcinolone acetonide (TAC-PF) at baseline (visit 0), then as needed at months 3, 6, 9, 12 with 1.25 mg intravitreal bevacizumab alone as needed at weeks 6, 18, 32 and 45.
Primary objective: To investigate whether, compared to bevacizumab alone, combination bevacizumab, PDT, TAC-PF therapy results in a similar functional outcome and reduces the number of intravitreal injections in participants with neovascular AMD. The results of this investigation will contribute substantially to planning future definitive studies on anti-VEGF/PDT/steroid combination therapies.
VisTA (Visudyne with intravitreal triamcinolone acetonide under Richard Spaide).
To determine whether PDT in combination with 4 mg intravitreal triamcinolone will reduce the average loss from baseline of best corrected visual acuity (BCVA) as compared with PDT without intravitreal triamcinolone at 12 months in subjects with occult subfoveal and minimally classic subfoveal CNVM secondary to AMD. The IVTA will be given as either a 1mg or 4 mg dose. This study will also evaluate the safety of PDT in combination with IVTA. An interim statistical readout will be performed when the first 60 patients have completed six months of follow-up evaluation. Recruitment is going on.
Comparing PDT combined with triamcinolone vs. PDT combined with Macugen (sponsored by Novartis/ QLT under Peter Kaiser).
NAPP study (Neovascular AMD, peri-ocular corticosteroids and PDT trial)
Pilot randomized controlled clinical study to test the efficacy of peri-ocular steroid in reducing the risk of persistent or progressive leakage from CNVM at three to six months after initial standard PDT with verteporfin treatment in patients with CNVM.
With the recent invasion of the VEGF inhibitors, triamcinolone has taken a back seat in the treatment armamentarium. These anti-VEGF drugs have undergone randomized control trials and in the current clinical scenario stand a better chance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: One-year results of 2 randomized clinical trials-TAP report. Arch Ophthalmol. 1999;117:1329–45. [PubMed] [Google Scholar]
- 2.Verteporfin in Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: Two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization--verteporfin in photodynamic therapy report 2. Am J Ophthalomol. 2001;1331:541–60. doi: 10.1016/s0002-9394(01)00967-9. [DOI] [PubMed] [Google Scholar]
- 3.Inoue M, Takeda K, Morita K, Yamada M, Tanigawara Y, Oguchi Y. Vitreous concentrations of triamcinolone acetonide in human eyes after intravitreal or subtenon injection. Am J Ophthalmol. 2004;138:1046–8. doi: 10.1016/j.ajo.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Penfold PL, Wen Li, Madigen MC, King NJ, Provis JM. Modulation of permeability and adhesion molocule epxression by human choroidal endothelail cells. Invest Ophthalmol Vis Sci. 2002;43:3125–30. [PubMed] [Google Scholar]
- 5.Wang YS, Friedrichs U, Eicler W, Hoffmann S, Wiedemann P. Inhibitory effects of triamcinolone acetonide on bFGF- induced migration and tube formation in choroidal microvascular endothelial cells. Graefes Arch Clin Exp Ophthalmol. 2002;240:42–8. doi: 10.1007/s00417-001-0398-y. [DOI] [PubMed] [Google Scholar]
- 6.Ciulla TA, Criswell MH, Danis RP, Hill TE. Intravitreal triamcinolone acetonide inhibits choroidal neovascularization in a laser-treated rat model. Arch Opthalmol. 2001;119:399–404. doi: 10.1001/archopht.119.3.399. [DOI] [PubMed] [Google Scholar]
- 7.Aiello LP, Brucker AJ, Chang S, Cunningham ET, Jr, D′Amico DJ, Flynn HW, Jr, et al. Evolving guidelines for intravitreousl injections. Retina. 2004;24:S3–19. doi: 10.1097/00006982-200410001-00002. [DOI] [PubMed] [Google Scholar]
- 8.Vedantham V, Kim R. Intravitreal injection of triamcinolone acetonide for diabetic macular edema: Principles and practice. Indian J Ophthalmol. 2006;54:133–7. doi: 10.4103/0301-4738.25840. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura A, Kokayashi A, Segawa Y, Sakurai M, Shirao E, Shirao Y, et al. Isolating triamcinolone acetonide particles for intravitreal use with a porous membrane filter. Retina. 2003;23:777–9. doi: 10.1097/00006982-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Jonas JB, Degenring RF, Kreissig I, Akkoyun I, Kamppeter BA. Intraocular pressure elevation after inrtavitreal triamcinolone acetonide injection. Ophthalmology. 2005;112:593–8. doi: 10.1016/j.ophtha.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 11.Penfold PL, Gyory JF, Hunyor AB, Billson FA. Exudative macular degeneration and intravitreal triamcinolone. A pilot study. Aust N Z J Ophthalmol. 1995;23:293–8. doi: 10.1111/j.1442-9071.1995.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 12.Challa JK, Gillies MC, Penfold PL, Gyory JF, Hunyor AB, Billson FA. Exudative macular degeneration and intravitreal traimcinolone: 18 month follow up. Aust N Z J Ophthalmol. 1998;26:277–81. doi: 10.1111/j.1442-9071.1998.tb01330.x. [DOI] [PubMed] [Google Scholar]
- 13.Danis RP, Ciulla TA, Pratt LM, Anliker W. Intravitreal triamcinolone acetonide in exudative age-related macular degeneration. Retina. 2000;20:244–50. [PubMed] [Google Scholar]
- 14.Ranson NT, Danis RP, Ciulla TA, Pratt L. Intravitreal triamcinolone in subfoveal recurrence of choroidal neovascularisation after laser treatment in macular degeneration. Br J Ophthalmol. 2002;86:527–9. doi: 10.1136/bjo.86.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillies MC, Simpson JM, Luo W, Penfold P, Hunyor AB, Chua W, et al. A randomized clinical trial of a single dose of intravitreal triamcinolone acetonide for neovascular age-related macular degeneration: One-year results. Arch Ophthalmol. 2003;121:667–73. doi: 10.1001/archopht.121.5.667. [DOI] [PubMed] [Google Scholar]
- 16.Jonas JB, Kreissig I, Hugger P, Sauder G, Panda-Jonas S, Degenring R. Intravitreal triamcinolone acetonide for exudative age related macular degeneration. Br J Ophthalmol. 2003;87:462–8. doi: 10.1136/bjo.87.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonas JB, Kreissig I, Degenring R, Friedemann T, Akkoyun I. Exudative age-related macular degeneration treated by intravitreal triamcinolone acetonide. A prospective comparative nonrandomized study. Eye. 2005;19:153, 70. doi: 10.1038/sj.eye.6701438. [DOI] [PubMed] [Google Scholar]
- 18.Jonas JB, Akkoyun I, Budde WM, Kreissig I, Degenring RF. Intravitreal reinjection of triamcinolone for exudative age-related macular degeneration. Arch Ophthalmol. 2004;122:218–22. doi: 10.1001/archopht.122.2.218. [DOI] [PubMed] [Google Scholar]
- 19.Jonas JB, Spandau UH, Harder B, Vossmerbaeumer U, Kamppeter BA. Intereye difference in exudative age-related macular degeneration with minimally classic or occult subfoveal neovascularization after unilateral intravitreal injection of triamcinolone acetonide. Am J Ophthalmol. 2006;141:228. doi: 10.1016/j.ajo.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt-Erfurth U, Schotzen-Schrehardu, Cursiefen C, Michels S, Beckendorf A, Naumann GO. Influence of Photodynamic therapy on expression of Vascular endothelial growth factor, Vascular endothelial growth factor receptor 3 and Pigment epithelial- growth factor. Invest Opthalmol Vis Sci. 2003;44:4473–80. doi: 10.1167/iovs.02-1115. [DOI] [PubMed] [Google Scholar]
- 21.Lai TY, Chan WM, Lam DS. Transient reduction in retinal function revealed by multifocal electroretinogram following photodynamic therapy. Am J Ophthalmol. 2004;137:826–33. doi: 10.1016/j.ajo.2003.11.079. [DOI] [PubMed] [Google Scholar]
- 22.Spaide RF, Sorenson J, Maranan L. Combined photodynamic therapy with verteporfin and intravitreal triamcinolone acetonide for choroidal neovascularization. Ophthalmology. 2003;110:1517–25. doi: 10.1016/S0161-6420(03)00544-X. [DOI] [PubMed] [Google Scholar]
- 23.Rechtman E, Danis RP, Pratt LM, Harris A. Intravitreal triamcinolone with photodynamic therapy for subfoveal choroidal neovascularisation in age related macular degeneration. Br J Opthalmol. 2004;88:344–7. doi: 10.1136/bjo.2003.027177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Augustin AJ, Schmidt-Erfurth U. Verteporfin therapy combined with intravitreal triamcinolone in all types of choroidal neovascularization due to age-related macular degeneration. Ophthalmology. 2006;113:14–22. doi: 10.1016/j.ophtha.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Nicolo M, Ghiglione D, Lai S, Nasciuti F, Cicinelli S, Calabria G. Occult with no classic choroidal neovascularization secondary to age-related macular degeneration treated by intravitreal triamcinolone and photodynamic therapy with verteporfin. Retina. 2006;26:58–64. doi: 10.1097/00006982-200601000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Chan WM, Lai TY, Wong AL, Tong JP, Liu DT, Lam DS. Combined photodynamic therapy and intravitreal triamcinolone injection for the treatment of subfoveal choroidal neovascularisation in age related macular degeneration: A comparative study. Br J Ophthalmol. 2006;90:337–41. doi: 10.1136/bjo.2005.081299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Augustin AJ, Schmidt-Erfurth U. Verteporfin and intravitreal triamcinolone acetonide combination therapy for occult choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol. 2006;141:638–45. doi: 10.1016/j.ajo.2005.11.058. [DOI] [PubMed] [Google Scholar]
- 28.Ergun E, Maar N, Ansari-Shahrezaei S, Wimpissinger B, Krepler K, Wedrich A, et al. Photodynamic therapy with verteporfin and intravitreal triamcinolone acetonide in the treatment of neovascular age-related macular degeneration. Am J Ophthalmol. 2006;142:10–6. doi: 10.1016/j.ajo.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz-Moreno JM, Montero JA, Barile S, Zarbin MA. Photodynamic therapy and high-dose intravitreal triamcinolone to treat exudative age-related macular degeneration: 1-year outcome. Retina. 2006;26:602–12. doi: 10.1097/01.iae.0000224942.59481.c9. [DOI] [PubMed] [Google Scholar]
- 30.Arias L, Garcia-Arumi J, Ramon JM, Badia M, Rubio M, Pujol O. Photodynamic therapy with intravitreal triamcinolone in predominantly classic choroidal neovascularization: one-year results of a randomized study. Ophthalmology. 2006;113:2243–50. doi: 10.1016/j.ophtha.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 31.Axer-Siegel R, Ehrlich R, Avisar I, Kramerr M, Rosenblatt I, Priel E, et al. Combined photodynamic therapy and intravitreal triamcinolone acetonide injection for neovascular age-related macular degeneration with pigment epithelium detachment. Ophthalmic Surg Lasers Imaging. 2006;37:455–61. doi: 10.3928/15428877-20061101-02. [DOI] [PubMed] [Google Scholar]
- 32.Hussain N, Das T, Rawal H, Kallukuri SB, Mohan Ram LS, Khanna R. Combination therapy of intravitreal triamcinolone and photodynamic therapy with verteporfin for subfoveal choroidal neovascularization. Indian J Ophthalmol. 2006;54:247–50. doi: 10.4103/0301-4738.27949. [DOI] [PubMed] [Google Scholar]
- 33.Moshfeghi DM, Kaiser PK, Scott IU, Sears JE, Benz M, Sinesterra JP, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Opthalmol. 2003;136:791–6. doi: 10.1016/s0002-9394(03)00483-5. [DOI] [PubMed] [Google Scholar]
- 34.Jonas JB, Kreissig I, Degenring RF. Cataract surgery after intravitreal injection of triamcinolone acetonide. Eye. 2004;18:361–4. doi: 10.1038/sj.eye.6700654. [DOI] [PubMed] [Google Scholar]
- 35.Gillies MC, Kuzniarz M, Craig J, Ball M, Luo W, Simpson JM. Intravitreal triamcinolone-induced elevated intraocular pressure is associated with the development of posterior subcapsular cataract. Ophthalmology. 2005;112:139–43. doi: 10.1016/j.ophtha.2004.07.017. [DOI] [PubMed] [Google Scholar]


