Abstract
In the last few years anti-vascular endothelial growth factor (VEGF) therapy has changed the paradigm in the treatment of neovascular age-related macular degeneration (ARMD). Besides, its potential use in the treatment of diabetic retinopathy and other possible proliferative vascular disorders has also shown promise. Clinical trial results have shown tremendous beneficial effect of ranibizumab in ARMD. Off-label use of bevacizumab has also shown similar benefit but long-term and clinical trial results do not exist. Some of the potential questions in the use of anti-VEGF are recurring cost, possible long-term effect on physiological function of VEGF and determination of endpoint of treatment. Overall, the use of anti-VEGF therapy in ocular angiogenesis has proven to be beneficial at least now.
Keywords: Age-related macular degeneration, angiogenesis, anti-vascular endothelial growth factor, bevacizumab, complement pathway, pegaptanib, pigment epithelium derived factor, ranibizumab
Recently, pharmacologic inhibition of abnormal angiogenesis has been a novel approach in the treatment of neovascular age-related macular degeneration (ARMD)1 and in various retinal vascular disorders like retinopathy of prematurity, diabetes and retinal vascular occlusion.2,3,4,5,6 It is known that angiogenesis is the formation of new blood vessels associated with sprouting or splitting from existing vessels and is the process through which a vascular network refines. In adults, new blood vessels are formed exclusively through angiogenesis, which is essential for normal biologic functions.7,8,9,10 It is a complex process involving multiple growth factors and cell adhesion molecules which is induced by any hypoxic or ischemic stimuli in ocular diseases like ARMD, diabetic retinopathy or proliferative retinopathy.
The purpose of this article is to provide the readers information about the molecular basis of angiogenesis and clinical implications of available anti-VEGF molecules in the treatment of aberrant angiogenesis.
Molecular Biology of Ocular Angiogenesis
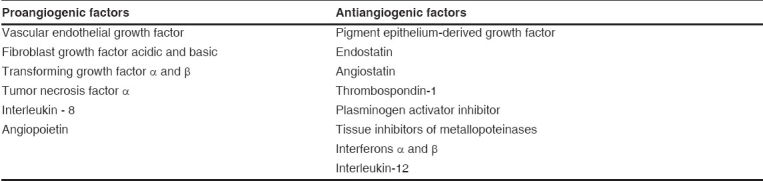
Physiologically, this angiogenic cascade occurs due to the carefully balanced interplay of growth promoting and growth inhibiting factors in the internal milieu of the eye. Increasing evidence suggests that vascular endothelial growth factor (VEGF) is the primary promoting factor besides fibroblast growth factor (FGF), transforming growth factor (TGF α and β ), angiopoietin (1 and 2) and many more contributing proangiogenic factors [Table 1]. Presently, intraocular anti-VEGF therapy has shown impressive effectiveness in the treatment of intraocular neovascularization, namely ARMD.11,12,13 The VEGF levels were shown to correlate both spatially and temporally with iris neovasculariazation in a monkey model.14 In the human eye, elevated levels of VEGF in vitreous and aqueous strongly correlate with retinal ischemia-associated neovascularization in diabetic retinopathy, retinal vein occlusion and retinopathy of prematurity.15,16,17 Increased VEGF expression was also demonstrated in the retinal and choroidal vessels of subjects with diabetes suggesting a close correlation of intraocular VEGF and intraocular neovascularization.18 Evidence also suggests VEGF is over-expressed in the retinal pigment epithelium (RPE) of ARMD and in transdifferentiated RPE cells of surgically excised choroidal neovascular membrane.19 Increased levels of VEGF have also been observed in vitreous and aqueous humor in the eyes of patients with polypoidal choroidal vasculopathy (PCV) and choroidal neovascularization (CNV) secondary to ARMD and pathological myopia compared to normal individuals.20,21,22,23 Intraocular level of VEGF and pigment epithelium derived factor (PEDF) in ARMD as compared to age-matched normal control eyes suggested that a critical balance between PEDF and VEGF is important and that PEDF may counteract the angiogenic potential of VEGF,24 as also seen in diabetic patients.25
Table 1.
Important proangiogenic and antiangiogenic factors
The VEGF-A gene has been localized to chromosome 6p12.3. Alternate splicing of this gene results in the production of five biologically active isoforms (VEGF 121 , VEGF 145 , VEGF 165 , VEGF 189 and VEGF 206 ). 26,27,28,29,30,31,32,33 Several studies have demonstrated the presence of complement components in the drusen and RPE of ARMD patients and the role of aberrant complement activation in ARMD. The complement component, particularly C3 and C5a can up-regulate the secretion of VEGF from RPE cells. It was shown recently in an animal model of ARMD that genetic ablation of the receptor for C3a and C5a reduces VEGF expression and that antibody-mediated neutralization of C3 and C5a or pharmacological blockade of their receptor also reduces CNV. Antibody-mediated neutralization or pharmacological blockade of their receptor can be a major therapeutic target for ARMD.34 Besides VEGF independent pathways like carboxyethylpyrrole (CEP), protein modifications (Bruch′s membrane) have also shown to stimulate angiogenesis. This also suggests that besides VEGF, other potential therapeutic targets can be of value in limiting CNV in ARMD in future.35
Anti-VEGF Therapy
Presently, available anti-VEGF drugs are approved by the food and drug administration (FDA) only for use in ARMD. Clinical trials are underway for their use in other retinal vascular diseases.
Ranibizumab (Lucentis) and pegaptanib sodium (Macugen) are the only two FDA-approved intravitreal anti-VEGF drugs for the treatment of neovascular ARMD. In December 2004, the US FDA approved pegaptanib sodium (Macugen) as an anti-VEGF RNA aptamer for the treatment of all types of neovascular ARMD. It was the first aptamer to be successfully developed as a therapeutic agent in humans.36
Pegaptanib is an aptamer i.e., ribonucleic acid (RNA) oligonucleotide that has high affinity and specificity for binding proteins. It is a 28- base RNA aptamer covalently linked to two branched 20kD polyethylene glycol moieties which bind and block VEGF, specifically the 165-amino acid residue (VEGF 165 ) [Fig. 1]. They bind with high specificity and affinity to target molecules.36,37 To prolong activity at the site of action, the sugar backbone of pegaptanib was modified to prevent degradation by endogenous endonucleases and exonucleases and the polyethylene glycol moieties, to increase the half-life of the drug in the vitreous cavity. Pegaptanib differs from other anti-VEGF therapies in that it binds near the heparin-binding domain of VEGF-A, thus preventing VEGF 165 and larger isoforms from attaching to the VEGF receptors, instead of targeting all active VEGF-A isoforms.36
Figure 1.
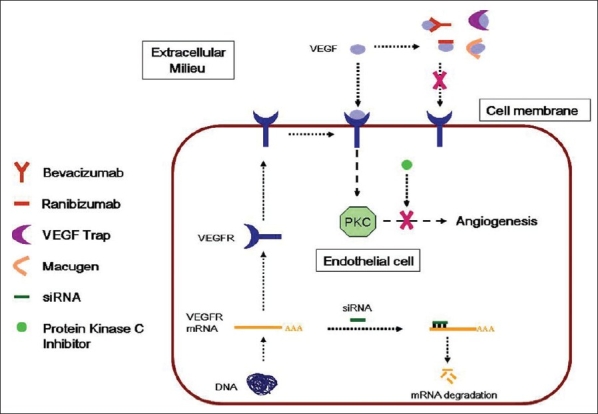
Schematic diagram showing the site of action of different anti-VEGF
The VEGF inhibition studies in the ocular neovascularization (VISION) trial was a large multicenter prospective, randomized double-masked, dose-ranging trial of pegaptanib sodium in patients with a wide range of vision and all subfoveal types of CNV secondary to ARMD.37 It was found that 70% of the patients met the primary end point (< 15 letters loss) in the 0.3 mg dose versus 55% of the controls ( P < 0.001). The secondary endpoint analysis showed 9.5% of patients lost > 30 letters versus 22% in the control group. Thirty one per cent patients in the 0.3 mg of pegaptanib arm with baseline visual acuity (VA) ≥ 20/200 ended up with worse than 20/200 vision compared to 50% in the control group at Week 54. The long-term safety of every six weeks injection of Macugen is not known. However, endophthalmitis, a potentially serious adverse event was seen in 1.3% of 890 patients with a per injection rate of 0.16%. This was similar to the rates identified in a comprehensive review of more than 15,000 intravitreal injections.38 Hence the risk associated with intraocular injection of Macugen was no different from intraocular injection of other drugs. Authors also mentioned that careful attention to proper injection technique can minimize the risk of endophthalmitis.37
Ranibizumab is a chimeric molecule that includes a nonbinding human sequence which makes it less antigenic in primates and a high affinity epitope that binds to VEGF-A. It was designed specifically to treat neovascular ARMD by manipulating the structure of a murine full-length monoclonal antibody (A.4.6.1) directed against the human VEGF-A. The humanized form is called bevacizumab. The Fab form of A.4.6.1 was humanized and referred to as rhuFab VI (Fab12). It was then affinity matured using phase display technology to generate the Y0317 variant, also known as ranibizumab [rhuFab V2; Fig. 2].39 Ranibizumab binds to and inhibits the biological activity of all the active forms of VEGF-A [Fig. 1].
Figure 2.
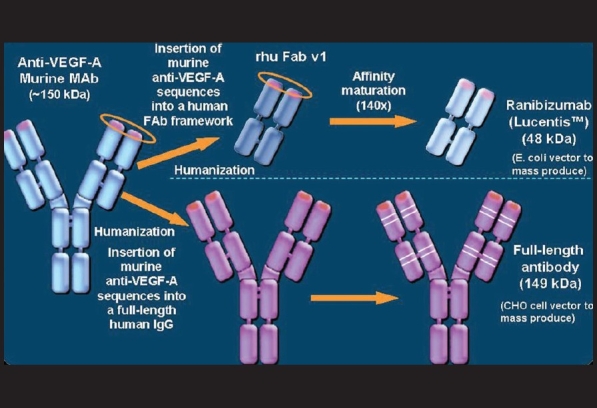
Humanization of ranibizumab and bevacizumab (Courtesy: Novartis Ophthalmics, India)
Ranibizumab (Lucentis) has shown to be associated with clinically and statistically significant benefits with respect to VA and angiographic lesions in neovascular ARMD.11,12,13 At 12 months, 94.5% of 0.3 mg of ranibizumab and 94.6% of 0.5 mg of ranibizumab lost fewer than 15 letters compared to 62.2% in controls for minimally classic or occult lesions;11 33.8% of 0.5 mg Lucentis gained ≥ 15 letters and 24.8% in the 0.3 mg group which was maintained till 24 months. When compared with verteporfin, 94.3% of patients receiving 0.3 mg of Lucentis and 96.4% patients in the 0.5 mg group lost fewer than 15 letters compared to 64.3% in the verteporfin-treated group at 24 months for classic lesions.12 In the 0.3 mg group 35.7% patients and 40.3% in the 0.5 mg group improved by ≥ 15 letters compared with 5.6% in the verteporfin group. Hence, ranibizumab was found to be superior to verteporfin. Even the combination of ranibizumab with verteporfin has shown 90.5% patients losing < 15 letters compared to verteporfin alone (67.9%, P < 0.001).13 Hence irrespective of lesion type ranibizumab has been found to improve vision. Results of the ANCHOR, MARINA and FOCUS11,12,13 studies have clearly shown that there is always a stage of significant initial gain in vision in the first three months. Following this, a gradual stability is maintained. This may also suggest that initial three injections every month may be necessary to achieve the initial gain in vision. This possibly can be followed on need basis. Clinical trials have been designed to answer even these questions.
Bevacizumab (Avastin) was approved by the FDA exclusively for the treatment of certain types of cancer including metastatic colorectal cancer. It has also been extensively used as an off-label drug for various ophthalmic diseases. 40,41,42,43,44,45,46,47 Besides, bevacizumab is not only used as an intravitreal application but was first introduced as a systemically delivered drug. It has also shown benefit of improved vision in neovascular ARMD and reduction of macular edema in diabetic retinopathy. Most of the published reports are either small case series or anecdotal reports. However, extensive publications on intravitreal bevacizumab suggest that the drug has shown its beneficial effect at least in short-term follow-up and appears to be a part of preferred practice in the treatment of CNV or retinal vascular disease. The long-term safety is yet to be determined demanding a randomized clinical trial for intraocular use. A prospective, non-randomized, open-label trial was performed to investigate the safety and tolerability of three escalating doses of bevacizumab (1.0, 1.5 and 2.0 mg) administered as a single intravitreal injection in wet ARMD.48 No systemic or serious drug-related adverse events were observed. Subconjuctival hemorrhage and conjunctival hyperemia were observed frequently at the injection site. Even though the mean best corrected visual acuity (BCVA) significantly improved from baseline ( P < 0.001) at one and six weeks, the change was significantly different at 12 weeks suggesting a dose-related response. The most favorable macular remodeling (OCT/ angiography) was observed in patients in the 2.0 mg dose group at weeks 6 and 12 and at week 6 in patients in the 1.5 mg dose group. Combination therapy of PDT-verteporfin and intravitreal bevacizumab has also shown short-term benefit.49 The mean BCVA showed improvement of 1.49 ETDRS lines (+0.6 to +2.4) and 0.98 lines (- 0.4 to +2.8) at 12 and 24 weeks respectively.
Possibly, the complications of ARMD treatments trial (CATT) (sponsored by the National Eye Institute of National Institute of Health Bethesda, USA) will prove the effectiveness of bevacizumab (http://irvaronsjournal.blogspot.com/2007/09/catt-study-update-2-avastin-vs-lucentis.html). The study will compare monthly injections using bevacizumab to monthly injections of ranibizumab as well as compare three-monthly injections of bevacizumab followed by "as needed" injections to the same regimen using ranibizumab. The trial should determine whether bevacizumab or ranibizumab is better and whether the "as needed" injection regimen is as good as monthly injections. "As needed" means a patient will receive another injection only if there is fluid on the OCT, new vision loss, new hemorrhage or new growth of the neovascularization. The "as needed" regimen is used to reduce the total number of injections that must be given to control the neovascularization and its leakage. Similarly, in the UK, the HTA (NHS Health Technology Assessment) clinical trials (http://www.oxfordshire.nhs.uk/documents/2007February28minutes.pdf) programme is considering to fund a trial of bevacizumab versus ranibizumab with further randomization to photodynamic therapy.
Recently, systematic review has synthesized the randomized clinical trial (RCT) evidence for effectiveness of ranibizumab and pegaptanib for subfoveal CNV associated with ARMD.50 The benefits of ranibizumab and pegaptanib were shown to be statistically significant in any lesion type. Patients receiving pegaptanib or ranibizumab were less likely to lose 15 letters, which means that a patient could live independently, or to deteriorate to the level of legal blindness (≤ 20/200) than those receiving sham injection and/or photodynamic therapy. The benefits of continued treatment appeared to be maintained after two years of follow-up with either drug. It was also found in the review that the outcome measures of loss of fewer than 15 letters and gain of ≥ 15 letters, the 95% confidence intervals did not overlap and patients receiving ranibizumab appeared to experience greater benefit compared to patients receiving pegaptanib. The adverse effects were mild to moderate transient events. Endophthalmitis was seen in 1.3% patients receiving pegaptanib in the first year and none in the second year whereas it ranged from 1.4 to 1.9% in patients receiving 0.5 mg ranibizumab. The review concluded that both pegaptanib and ranibizumab are clinically effective in the treatment of subfoveal CNV secondary to ARMD and fewer patients showed improvement of vision with pegaptanib than with ranibizumab. It also suggested a clinical trial comparing pegaptanib with ranibizumab and bevacizumab including a wide range of lesion types of ARMD and perform economic evaluation with prospective collection of data on quality of life, utilities, resources and costs.
Brown et al.51 have shown the total value of each treatment modality for ARMD. Value-based medicine is the practice of medicine based upon the patient value (improvement in the quality of life and length of life) conferred by an intervention. It has been found that conversion of data of intravitreal pegaptanib every six weeks for two years for wet ARMD to value-based medicine format, including the disutility occurring secondary to adverse events confer an improvement in the quality of life of 5.9%. In contrast, the value gain for intravitreal therapy with ranibizumab was found to be >15%. This shows the cost-effectiveness of the treatment for the patient. Since bevacizumab is similar to ranibizumab and since case series data suggest good outcome, one can speculate that the value gain may be similar or greater than ranibizumab. This estimation will be possible only when prospective data are available from a trial.
In clinical practice, the decision to use a particular intravitreal drug depends on its evidence-based visual outcome as well as the safety profile. Presently, the three FDA-approved drugs for the treatment of ARMD are verteporfin therapy, pegaptanib and ranibizumab. Due to varied patients′ recruitment in the VISION study, it is difficult to comment on the outcome of pegaptanib for ARMD. About 20% of patients in the pegaptanib-treated group may experience significant vision gain in the early treatment of ARMD.37 It is important to note that it is extremely difficult to conclude on the comparative efficacy of pegaptanib or other anti-VEGF drugs unless there is head to head comparison. It is clear from the evidence that at least ranibizumab can improve vision significantly in any subtype of ARMD.11,12,13
Anti-VEGF usefulness in the treatment of diabetic macular edema is still under clinical trial. Anecdotal report does suggest beneficial effect and clinical trial results are awaited.43 Intravitreal bevacizumab has also been used in the treatment of aggressive posterior retinopathy of prematurity.52 Anecdotal reports also show the effect of intravitreal bevacizumab in the treatment of central retinal vein occlusion, branch retinal vein occlusion53,54 and neovascular glaucoma.55 This appears to be a potential treatment but at present results of clinical trial are not available about the safety, efficacy and long-term outcome of anti-VEGF in various retinal vascular diseases. Use of anti-VEGF drugs (other than FDA-approved) in indications other than ARMD needs caution and ethical issues need to be addressed.
It is evident now that anti-VEGF therapy has shown tremendous promise as a treatment for ocular disease, primarily ARMD and potential for retinal vascular disease. There is variability in the efficacy of different agents which may relate to the development of the drug or type of clinical trial. Other promising anti-VEGF therapies for future use are VEGF Trap and Tyrosine kinase inhibitors, which are undergoing clinical trial.
The major concerns in the long-term management of such cases with anti-VEGF are:
Repeated intravitreal injections
Systemic risk for cerebrovascular accidents
Determination of end point
Possible retinal neural toxicity due to cumulative dosing
Speculation of altering the VEGF physiological function in the eye
Recurring economic burden to the patient
It may be possible that the future may include a combination approach that has the potential to decrease the frequency of dosing, improve efficacy and provide additional blockade of the angiogenic cascade.39 In a nutshell, with increasing life expectancy, we will face more patients with ARMD and diabetics. Searching for a cost-effective approach which is functionally beneficial is imperative. One must also understand that the present scientific evidence should allow us to select therapies that can restore quality of central vision and allow patients to experience the benefit of treatment.56
Conclusion
Use of anti-VEGF drugs (intraocular) has shown tremendous potential in the treatment of neovascular ARMD. Even though there is enough potential to show its benefit in retinal vascular diseases (vein occlusion and diabetic retinopathy), randomized clinical trials are necessary to prove its long-term efficacy. Off-label uses of intravitreal anti-VEGF drugs need to be addressed with caution. It is also important to critically evaluate the available evidence in the literature while we wait for the clinical trial results.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Csaky K. Anti-Vascular Endothelial Factor for neovascular age related macular degeneration. Ophthalmology. 2003;110:879–81. doi: 10.1016/s0161-6420(03)00095-2. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham ET, Jr, Adamis AP, Altaweel M, Aiello LP, Bressler NM, D′Amico DJ, et al. A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005;112:1747–57. doi: 10.1016/j.ophtha.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Jorge R, Costa RA, Calucci D, Cintra LP, Scott IU. Intravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE study) Retina. 2006;26:1006–13. doi: 10.1097/01.iae.0000246884.76018.63. [DOI] [PubMed] [Google Scholar]
- 4.Haritoglou C, Kook D, Neubauer A, Wolf A, Priglinger S, Strauss R, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006;26:999–1005. doi: 10.1097/01.iae.0000247165.38655.bf. [DOI] [PubMed] [Google Scholar]
- 5.Isaacs TW, Barry C. Rapid resolution of severe disc new vessels in proliferative diabetic retinopathy following a single intravitreal injection of bevacizumab (Avastin) Clin Experiment Ophthalmol. 2006;34:802–3. doi: 10.1111/j.1442-9071.2006.01378.x. [DOI] [PubMed] [Google Scholar]
- 6.Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695. doi: 10.1016/j.ophtha.2006.05.064. e1-15. [DOI] [PubMed] [Google Scholar]
- 7.Ruiter DJ, Schlingemann RO, Westphal JR, Denijn M, Rietveld FJ, De Waal RM. Angiogenesis in wound healing and tumour metastasis. Behring Inst Mitt. 1993;92:258–72. [PubMed] [Google Scholar]
- 8.Ferrara N, Chen H, Davis-Smyth T, Gerber HP, Nguyen TN, Peers D, et al. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat Med. 1998;4:336–40. doi: 10.1038/nm0398-336. [DOI] [PubMed] [Google Scholar]
- 9.Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001;107:409–17. doi: 10.1172/JCI11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiefer FN, Neysari S, Humar R, Li W, Munk VC, Battegay EJ. Hypertension and angiogenesis. Curr Pharm Des. 2003;9:1733–44. doi: 10.2174/1381612033454540. [DOI] [PubMed] [Google Scholar]
- 11.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 12.Brown DM, Kaiser PK, Michaels M, Soubrane G, Heier JS, Kim RY, et al. Ranibizumab versus Verteporfin for neovascular age related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 13.Heier JS, Boyer DS, Ciulla TA, Ferrone TJ, Jumper JM, Gentile RC, et al. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age related macular degeneration. Arch Ophthalmol. 2006;124:1532–42. doi: 10.1001/archopht.124.11.1532. [DOI] [PubMed] [Google Scholar]
- 14.Miller J, Adamis AP, Shima DT, D′Amore PA, Moulton RS, O′Reilly MS, et al. Vascular endothelial growth factor/ vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol. 1994;145:574–84. [PMC free article] [PubMed] [Google Scholar]
- 15.Adamis AP, Miller JW, Bernal MT, D′Amico DJ, Folkman J, Yeo TK, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994;118:445–50. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- 16.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–7. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 17.Malecazi F, Clamens S, Simorre-Pinatel V, Mathis A, Chollet P, Favard C, et al. Detection of vascular endothelial growth factor-like activity in proliferative diabetic retinopthy. Arch Ophthalmol. 1994;112:1476–82. doi: 10.1001/archopht.1994.01090230090028. [DOI] [PubMed] [Google Scholar]
- 18.Lutty GA, McLeod S, Merges C, Diggs A, Plouét J. Localization of VEGF in human retina and choroid. Arch Ophthalmol. 1996;114:971–7. doi: 10.1001/archopht.1996.01100140179011. [DOI] [PubMed] [Google Scholar]
- 19.Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR. Trans-differentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age related macular degeneration related choroidal neovascular membranes. Invest Ophthalmol Vis Sci. 1996;37:855–68. [PubMed] [Google Scholar]
- 20.Neufeld G, Tzafra C, Gengrnovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 21.Watanabe O, Suzuma K, Suzuma I, Ohashi H, Ojima T, Kurimoto M, et al. Vitreous levels of angiopoitein 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 1998;139:476–81. doi: 10.1016/j.ajo.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002;133:70–7. doi: 10.1016/s0002-9394(01)01269-7. [DOI] [PubMed] [Google Scholar]
- 23.Tong JP, Chan WM, Liu DT, Lai TY, Choy KW, Pang CP, et al. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol. 2006;141:456–62. doi: 10.1016/j.ajo.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Tsai DC, Charng MJ, Lee FL, Hsu WM, Chen SJ. Different plasma levels of vascular endothelial growth factor and nitric oxide between patients with choroidal and retinal neovascularization. Ophthalmoligca. 2006;220:246–51. doi: 10.1159/000093079. [DOI] [PubMed] [Google Scholar]
- 25.Bhutto IA, McLeod DS, Hasegawa T, Kim SY, Merges C, Tong P, et al. Pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged human choroids and eye with age-related macular degeneration. Exp Eye Res. 2006;82:99–110. doi: 10.1016/j.exer.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Churchill AJ, Carter JG, Lovell HC, Ramsden C, Turner SJ, Yeung A, et al. VEGF polymorphisms are associated with neovascular age-related macular degeneration. Hum Mol Genet. 2006;15:2955–61. doi: 10.1093/hmg/ddl238. [DOI] [PubMed] [Google Scholar]
- 27.Banyasz I, Bokodi G, Vannay A, Szebeni B, Treszl A, Vasarhelyi B, et al. Genetic polymorphisms of vascular endothelial growth factor and angiopoietin 2 in retinopathy of prematurity. Curr Eye Res. 2006;31:685–90. doi: 10.1080/02713680600801123. [DOI] [PubMed] [Google Scholar]
- 28.Suganthalakshmi B, Anand R, Kim R, Mahalakshmi R, Karthikprakash S, Namperumalsamy P, et al. Association of VEGF and eNOS gene polymorphisms in type 2 diabetic retinopathy. Mol Vis. 2006;12:336–41. [PubMed] [Google Scholar]
- 29.Nam EJ, Han SW, Kim SU, Cho JH, Sa KH, Lee WK, et al. Association of vascular endothelial growth factor gene polymorphisms with Behcet disease in a Korean population. Hum Immunol. 2005;66:1068–73. doi: 10.1016/j.humimm.2005.08.238. [DOI] [PubMed] [Google Scholar]
- 30.Awata T, Kurihara S, Takata N, Neda T, Iizuka H, Ohkubo T, et al. Functional VEGF C-634G polymorphism is associated with development of diabetic macular edema and correlated with macular retinal thickness in type 2 diabetes. Biochem Biophys Res Commun. 2005;333:679–85. doi: 10.1016/j.bbrc.2005.05.167. [DOI] [PubMed] [Google Scholar]
- 31.Vannay A, Dunai G, Banyasz I, Szabo M, Vamos R, Treszl A, et al. Association of genetic polymorphisms of vascular endothelial growth factor and risk for proliferative retinopathy of prematurity. Pediatr Res. 2005;57:396–8. doi: 10.1203/01.PDR.0000153867.80238.E0. [DOI] [PubMed] [Google Scholar]
- 32.Cooke RW, Drury JA, Mountford R, Clark D. Genetic polymorphisms and retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2004;45:1712–5. doi: 10.1167/iovs.03-1303. [DOI] [PubMed] [Google Scholar]
- 33.Awata T, Inoue K, Kurihara S, Ohkubo T, Watanabe M, Inukai K, et al. A common polymorphism in the 5′-untranslated region of the VEGF gene is associated with diabetic retinopathy in type 2 diabetes. Diabetes. 2002;51:1635–9. doi: 10.2337/diabetes.51.5.1635. [DOI] [PubMed] [Google Scholar]
- 34.Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci. 2006;103:2328–33. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ebrahem Q, Renganathan K, Sears J, Vasanji A, Gu X, Lu L, et al. Carboxyethylpyrrole oxidative protein modifications stimulate neovascularization: Implications for age-related macular degeneration. Proc Natl Acad Sci. 2006;103:13480–4. doi: 10.1073/pnas.0601552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eugene WM, Shima DT, Calias P, Cunningham ET, Jr, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev. 2006;5:123–32. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- 37.Gragoudas ES, Adamis AP, Cunningham ET, Feinsod M, Guyer DR VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for age related macular degeneration. N Engl J Med. 2004;351:2805–16. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 38.Jager RD, Aiello P, Patel SC, Cunningham ET., Jr Risk of intravitreous injection: A comprehensive review. Retina. 2004;24:676–98. doi: 10.1097/00006982-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Kaiser PK. Antivascular Endothelial Growth Factor Agents and their development: Therapeutic implications in ocular disease. Am J Ophthalmol. 2006;142:660–8. doi: 10.1016/j.ajo.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 40.Moschos MM, Brouzas D, Apostolopoulos M, Koutsandrea C, Loukianou E, Moschos M. Intravitreal use of bevacizumab (Avastin) for choroidal neovascularization due to ARMD: A preliminary multifocal-ERG and OCT study: Ultifocal-ERG after use of bevacizumab in ARMD. Doc Ophthalmol. 2007;114:37–44. doi: 10.1007/s10633-006-9036-7. [DOI] [PubMed] [Google Scholar]
- 41.Aisenbrey S, Ziemssen F, Volker M, Gelisken F, Szurman P, Jaissle G, et al. Intravitreal bevacizumab (Avastin) for occult choroidal neovascularization in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2007;245:941–8. doi: 10.1007/s00417-006-0471-7. [DOI] [PubMed] [Google Scholar]
- 42.Tewari A, Dhalla MS, Apte RS. Intravitreal bevacizumab for treatment of choroidal neovascularization in pathologic myopia. Retina. 2006;26:1093–4. doi: 10.1097/01.iae.0000254896.78766.74. [DOI] [PubMed] [Google Scholar]
- 43.Haritoglou C, Kook D, Neubauer A, Wolf A, Priglinger S, Strauss R, et al. Intravitreal bevacizumab (Avastin) therapy for persistent diffuse diabetic macular edema. Retina. 2006;26:999–1005. doi: 10.1097/01.iae.0000247165.38655.bf. [DOI] [PubMed] [Google Scholar]
- 44.Yoganathan P, Deramo VA, Lai JC, Tibrewala RK, Fastenberg DM. Visual improvement following intravitreal bevacizumab (Avastin) in exudative age-related macular degeneration. Retina. 2006;26:994–8. doi: 10.1097/01.iae.0000244380.34082.67. [DOI] [PubMed] [Google Scholar]
- 45.Dhalla MS, Shah GK, Blinder KJ, Ryan EH, Jr, Mittra RA, Tewari A. Combined photodynamic therapy with verteporfin and intravitreal bevacizumab for choroidal neovascularization in age-related macular degeneration. Retina. 2006;26:988–93. doi: 10.1097/01.iae.0000247164.70376.91. [DOI] [PubMed] [Google Scholar]
- 46.Aggio FB, Farah ME, Silva WC, Melo GB. Intravitreal bevacizumab for exudative age-related macular degeneration after multiple treatments. Graefes Arch Clin Exp Ophthalmol. 2006 doi: 10.1007/s00417-006-0412-5. Dec 1; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Jonas JB, Harder B, Spandau UH, Kamppeter BA, Libondi T, Sauder G. Bevacizumab for occult subfoveal neovascularization in age-related macular degeneration. Eur J Ophthalmol. 2006;16:774–5. doi: 10.1177/112067210601600522. [DOI] [PubMed] [Google Scholar]
- 48.Costa RA, Jorge R, Calucci D, Cardillo JA, Melo LA., Jr Intravitreal Bevacizumab for choroidal neovascularisation caused by AMD (IbeNA Study): Results of a phase 1 dose escalation study. Invest Ophthalmol Vis Sci. 2006;47:4569–78. doi: 10.1167/iovs.06-0433. [DOI] [PubMed] [Google Scholar]
- 49.Costa RA, Jorge R, Calucci D, Melo LA, Jr, Cardillo JA, Scott IU. Intravitreal Bevacizumab (Avastin) in combination with verteporfin photodynamic therapy for choroidal neovascularization associated with age related macular degeneration (IbeVe Study) Graefe′s Arch Clin Exp Ophthalmol. doi: 10.1007/s00417-007-0557-x. 2007 Feb 28; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Takeda AL, Colquitt JL, Clegg AJ, Jones J. Pegaptanib and ranibizumab for neovascular age related macular degeneration: A systematic review. Br J Ophthalmol. doi: 10.1136/bjo.2007.118562. 2007 May 2; [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown MM, Brown GC, Brown H. Value based medicine and interventions for macular degeneration. Curr Opin Ophthalmol. 2007;18:194–200. doi: 10.1097/ICU.0b013e3281377209. [DOI] [PubMed] [Google Scholar]
- 52.Shah PK, Narendran V, Tawansy KA, Raghuram A, Narendran K. Intravitreal bevacizumab (Avastin) for post laser anterior segment ischemia in aggressive posterior retinopathy of prematurity. Indian J Ophthalmol. 2007;55:75–6. doi: 10.4103/0301-4738.29505. [DOI] [PubMed] [Google Scholar]
- 53.Schaal KB, Hoh AE, Scheuerle A, Schutt F, Dithmar S. Bevacizumab for the treatment of macular edema secondary to retinal vein occlusion. Ophthalmologe. 2007;104:285–9. doi: 10.1007/s00347-007-1509-x. [DOI] [PubMed] [Google Scholar]
- 54.Stahl A, Agostini H, Hansen LL, Feltgen N. Bevacizumab in retinal vein occlusion-results of a prospective case series. Graefes Arch Clin Exp Ophthalmol. doi: 10.1007/s00417-007-0569-6. 2007 Mar 14; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 55.Kahook MY, Schuman JS, Noecker RJ. Intravitreal bevacizumab in a patient with neovascular glaucoma. Ophthalmic Surg Lasers Imaging. 2006;37:144–6. [PubMed] [Google Scholar]
- 56.Kroll P, Meyer CH. Which treatment is best for which AMD patient? Br J Ophthalmol. 2006;90:128–30. doi: 10.1136/bjo.2005.083337. [DOI] [PMC free article] [PubMed] [Google Scholar]


