Abstract
Age-related macular degeneration (ARMD) is the most common cause for visual impairment in the elderly in western countries. Recently several anti-vascular endothelial growth factor (VEGF) drugs like pegaptanib sodium (Macugen), ranibizumab (Lucentis) and bevacizumab (Avastin) are available for use in the management of wet ARMD. A major limitation of these drugs is that they require multiple intravitreal injections, every 4 to 6 weeks interval for a period of 2 years. Moreover, most of these drugs are too expensive for the general masses to afford in developing nations. Avastin, though used "off-label", offers a comparable result at affordable cost, however, long term results are awaited. The drug industry should review the entire pricing policy of these drugs in developing countries like India, and develop affordable alternative compounds. The article reviews the economic burden and affordability issues of these Anti-VEGF drugs in ARMD.
Keywords: Age-related macular degeneration, avastin, lucentis, macugen
Age-related macular degeneration (ARMD) is the most common cause for visual impairment in the elderly in western countries. Three population-based studies, namely the Beaver Dam Eye Study,1 Blue Mountain Eye study2 and the Rotterdam Study3 report the prevalence rates to be 1.7% in the US, 1.4% in Australia and 1.2% in Netherlands respectively. The prevalence in India varies from 2.7% (early ARMD) to 0.6% (late ARMD) in South India4 to 4.7% in North India.5
The 60+ years age group is a fast-growing age group worldwide and by 2025, is estimated to constitute approximately one-third of the population of many developed countries.6 This shift of age group of the world population is expected to significantly increase the number of ARMD patients seeking treatment and burden the current eye care infrastructure.
Age-related macular degeneration has a significant impact in affected patients because it affects an older eye where vision is already deteriorating due to multiple coexisting ocular or systemic diseases and is often bilateral, thus, markedly lowering their ability to perform activities of daily living, deteriorating the quality of life and requirement for social care and support services. Besides direct costs like inpatient and outpatient expenses, health visits, nursing care and social services, ARMD also causes work absence and lost productivity.
For the UK, it is estimated that the average annual per patient cost is £4,240 for people with ARMD against £490 for the control group, which translates into annual costs of approximately £860 million.7 The ARMD Burden of Illness study showed that management of ARMD patients costs eight times more money (average annual per patient costs €6000 to €12000) than for control patients in general medical care (average annual per patient costs €700 to €1800) which translates into expenditure of billions of euros per year8 [Table 1]. Recent studies attempting to assess the economic burden of ARMD, indicate there are significant gaps in our understanding of the costs of ARMD (particularly in respect to indirect costs) and research should be augmented by more comprehensive studies to integrate the various components of ARMD-related costs.9,10
Table 1.
Yearly fi nancial burden of age-related macular degeneration patients worldwide 8

Anti vascular endothelial growth factor (VEGF) drugs and their economic burden in the Indian subcontinent
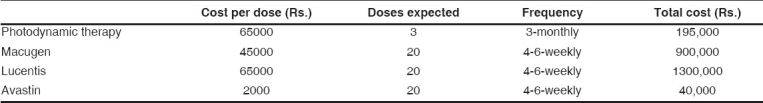
The management of subfoveal wet ARMD with current modalities of treatment is an expensive deal. A few years back, the advent of photodynamic therapy with verteporfin initiated a fresh approach to the management of ARMD by stabilizing vision in selected cases (mostly classic type, though indications were loosely expanded). A single treatment with verteporfin costs approximately Rs. 65000 and required on an average 2 to 3 treatments. The main advantage is that it is a noninvasive procedure (besides the dye injection) and though the cost seems exorbitant, several insurance companies and government agencies usually cover the costs required for treatment. Despite reimbursement from various agencies, a large number of patients of wet ARMD are undergoing transpupillary thermotherapy (TTT), often labeled as poor man′s PDT; its efficacy is questionable and it has lately been abandoned.
In view of better understood angiogenesis, several anti-VEGF drugs like pegaptanib sodium (Macugen), ranibizumab (Lucentis) and bevacizumab (Avastin) are available for use in the management of wet ARMD. Due to their recent launch, limited data are available regarding their long-term outcomes and comparative studies are underway to determine the best treatment modality as monotherapy or in combination therapy. However, recent studies have shown promising outcomes11,12,13,14 and anti-VEGF drugs may seem to be a popular treatment in the near future at least.
A few constraints limit the widespread usage of these drugs. Firstly, the treatment is invasive and involves intravitreal injection of these drugs. Secondly, multiple such treatments are required at four to six-week intervals for a period of two years. Thirdly and most importantly, most of these drugs are too expensive for the general masses and are unaffordable in developing nations. The economic burden is huge due to the cumulative multiple injection costs, treatment for iatrogenic complications caused by these injections, hospital costs, surgeon visits, social care and rehabilitative services.
Two years of treatment with pegaptanib with approximately 20 six-weekly injections will cost Rs. 9,00,000, while a similar regime with ranibizumab will cost about Rs. 13,00,000 [Table 2]. Though both these drugs have shown promise and have been approved for treatment of ARMD, the high costs of total treatment limits their usage in the population at large. Bevacizumab, an anti-VEGF drug used in the treatment of metastatic colorectal cancer, is gaining popularity primarily due its comparable results and a cheap total cost of treatment. Though it is still used "off-label", a single dose of Avastin would cost approximately Rs. 2,000 and two years of treatment with Avastin with approximately 20 six-weekly injections will cost only Rs. 40,000, which is much more affordable [Table 2].
Table 2.
Comparison of total estimated cost for different anti-vascular endothelial growth factor drugs
The 60+ years age group is at risk for ARMD and constitutes 7.5% of the Indian population (75 million).15 About one million of them will suffer from ARMD (considering a 1.5% prevalence). Wet ARMD will constitute about 10% of these cases (0.1 million) and will require treatment. Considering that about 18 to 22% of the Indian population is below the poverty line, they cannot afford these treatments. Of the rest, India′s per capita income is a mere $720 (compared to $43740 of the United States)16 and most Indians cannot afford these treatments, unless costs are covered by insurance companies or sponsored by government agencies.
Considering that each dose of ranibizumab costs approximately €1200, 10 doses in a year will cost €12000 and the total burden for an estimated 0.1 million patients of wet ARMD in India will be approximately €1.2 billion as drugs cost only. Direct and indirect costs will further add to this economic burden. This economic burden is comparable to other countries [Table 1]. Thus, such eyes often end up being treated with TTT, laser photocoagulation or no treatment at all leading to eventual blindness.
Conclusion
Research initiatives continue at a rapid pace by apex organizations and pharmaceutical companies worldwide to find a safe and effective treatment for ARMD. Anti-VEGF drugs have provided a ray of hope but involve the use of multiple intravitreal injections, which not only increase the risk of complications, but are expensive too. Undergoing these expensive treatments in developing countries like India is not economically viable for the majority of the population. Though bevacizumab is still an off-label drug, promising results at a very cheap cost has prompted its use in a wide spectrum of ocular diseases.17,18 As research continues, very soon we may see newer and more effective agents offering treatment options for ARMD. The drug industry should not only review the entire pricing policy of these drugs in developing countries like India, but also look for affordable alternative compounds.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1992;99:933–43. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-related maculopathy in Australia: The Blue Mountains Eye Study. Ophthalmology. 1995;102:1450–60. doi: 10.1016/s0161-6420(95)30846-9. [DOI] [PubMed] [Google Scholar]
- 3.Vingerling JR, Dielemans I, Hofman A, Grobbee DE, Hijmering M, Kramer CF, et al. The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology. 1995;102:205–10. doi: 10.1016/s0161-6420(95)31034-2. [DOI] [PubMed] [Google Scholar]
- 4.Nirmalan PK, Katz J, Robin AL, Tielsch JM, Namperumalsamy P, Kim R, et al. Prevalence of vitreoretinal disorders in a rural population of southern India: The Aravind Comprehensive Eye Study. Arch Ophthalmol. 2004;122:581–6. doi: 10.1001/archopht.122.4.581. [DOI] [PubMed] [Google Scholar]
- 5.Jain IS, Prasad P, Gupta A, Ram J, Dhir SP. Senile macular degeneration in northern India. Indian J Ophthalmol. 1984;32:343–6. [PubMed] [Google Scholar]
- 6.Who.int [homepage on the Internet] United States: Active ageing: A Policy Framework (PDF); 2002. [Last accessed on 2007 Mar 3]. Available from: http://www.who.int/ageing/publications/active/en/index.html. [Google Scholar]
- 7.Rnib.org.uk [homepage on the Internet]. United Kingdom: Royal National Institute of the Blind. The cost of sight loss in the UK. RNIB Campaign Report 23; 2004. [Last accessed on 2007 Mar 3]. Available from: http://onlineshop.rnib.org.uk/display_item.asp?n=11andc=467andsc=0andid=864andit=2andl=3. [Google Scholar]
- 8.Amdalliance.org [homepage on the Internet]. United States: AMD Alliance International. AMD action summit media briefing (PPT); 2006. [Last accessed on 2007 Mar 3]. Available from: http://www.amdalliance.org/resources/newsarticles.php. [Google Scholar]
- 9.Schmier JK, Jones ML, Halpern MT. The burden of age-related macular degeneration. Pharmacoeconomics. 2006;24:319–34. doi: 10.2165/00019053-200624040-00003. [DOI] [PubMed] [Google Scholar]
- 10.Ke KM, Chakravarthy U, O′Neill C. Economic cost of age-related macular degeneration: A review of recent research. Drugs Aging. 2006;23:217–25. doi: 10.2165/00002512-200623030-00004. [DOI] [PubMed] [Google Scholar]
- 11.Gonzales CR VEGF Inhibition Study in Ocular neovascularization (V.I.S.I.O.N.) Clinical Trial Group. Enhanced efficacy associated with early treatment of neovascular age-related macular degeneration with pegaptanib sodium: An exploratory analysis. Retina. 2005;85:815–27. doi: 10.1097/00006982-200510000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration: 2-year results of the MARINA study. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 13.Costa RA, Jorge R, Calucci D, Cardillo JA, Melo LA, Jr, Scott IU. Intravitreal bevacizumab for choroidal neovascularization caused by AMD (IBeNA Study): Results of a phase 1 dose-escalation study. Invest Ophthalmol Vis Sci. 2006;47:4569–78. doi: 10.1167/iovs.06-0433. [DOI] [PubMed] [Google Scholar]
- 14.Chen CY, Wong TY, Heriot WJ. Intravitreal Bevacizumab (Avastin) for Neovascular Age-related Macular Degeneration: A Short-term Study. Am J Ophthalmol. 2007;143:510–2. doi: 10.1016/j.ajo.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Worldbank.org [homepage on the Internet] United States: World Development Indicators Database; [Last updated on 2006 Apr]; [Last accessed on 2007 Mar 3]. Available from: http://www.worldbank.org/data. [Google Scholar]
- 16.Censusindia.gov.in [homepage on the Internet] India: Registrar General and Census Commissioner; [Last accessed on 2007 Mar 3]. Available from: http://www.censusindia.gov.in. [Google Scholar]
- 17.Shah PK, Narendran V, Tawansy KA, Raghuram A, Narendran K. Intravitreal bevacizumab (Avastin) for post laser anterior segment ischemia in aggressive posterior retinopathy of prematurity. Indian J Ophthalmol. 2007;55:75–6. doi: 10.4103/0301-4738.29505. [DOI] [PubMed] [Google Scholar]
- 18.Iturralde D, Spaide RF, Meyerle CB, Klancnik JM, Yannuzzi LA, Fisher YL, et al. Intravitreal bevacizumab (Avastin) treatment of macular edema in central retinal vein occlusion: A short-term study. Retina. 2006;26:279–84. doi: 10.1097/00006982-200603000-00005. [DOI] [PubMed] [Google Scholar]


