Abstract
Background:
The successful lowering of the intraocular pressure after glaucoma filtration surgery depends mostly on the nature of the healing response, which is also the single most important modifiable factor.
Aims:
To evaluate and compare the effectiveness of two oxidated regenerated cellulose material, Interceed and Surgicel on wound healing reaction after glaucoma filtration surgery.
Setting and Design:
University hospital, prospective study.
Materials and Methods:
Full thickness filtration surgery was carried out on three groups of rabbits. Interceed and Surgicel was applied in Groups 1 and 2 respectively. The third group was the controls. Intraocular pressure, anterior chamber depth and bleb appearance were checked on the first, third, seventh and 14th days. The rabbits were sacrificed on the14th day and the trabeculectomy area with overlying conjunctiva was excised, fixed, stained and evaluated histopathologically.
Statistics:
The values obtained from the clinical and histopathologic evaluation were statistically analyzed using non-parametric tests (Mann Whitney-U and Kruskall Wallis tests) in SPSS for Windows v-10. P values under 0.05 for statistical significance in comparisons were considered significant.
Results
The groups were similar with respect to intraocular pressure, anterior chamber depth, bleb appearance and number of the fibroblasts and neutrophils on the seventh and 14th days. Mean number of the eosinophils and vessels was significantly less in Groups 1 and 2 (P = 0.014, P = 0.20 respectively). Macrophages in Group 2 were significantly less than Group 1 (P = 0.047).
Conclusion
Both these agents seem to suppress vascularization. Since they have no significant effect on fibroblast proliferation, it is controversial to talk about wound healing modulation.
Keywords: Glaucoma filtration surgery, interceed, surgicel, wound healing
Introduction
Glaucoma filtration surgery (GFS) involves the creation of a new drainage channel for the aqueous humor to flow out of the eye and thus lower intraocular pressure (IOP).1 The successful lowering of IOP depends mostly on the nature of the healing response, which is also the single most important modifiable factor.2
Subconjunctival adhesions are the leading causes of the failure after GFS. Increased permeability of the blood vessels in the traumatized tissue produces inflammatory exudates rich in plasma proteins, such as fibrinogen. Under optimal conditions, the majority of fibrinous attachments so formed are absorbed within a few days by fibrinolytic mechanisms. If they persist, fibroblastic proliferation may occur, causing adhesion formation.3
Capability for adhesion formation is not identical for different tissues of the human body. The GFS site differs from any ocular area in one fundamental aspect: it is bathed by aqueous humor and its contents can significantly affect the healing response level.1,4 Normal rabbit aqueous humor has been shown to be powerfully chemo attractant to rabbit Tenon′s capsule fibroblasts5 and similar effects of human aqueous were also confirmed.6 The factors reported to stimulate fibroblasts in the aqueous humor are fibroblast growth factor (FGF), epidermal growth factor (EGF), transforming growth factor (TGF) beta-1, insulin-like growth factor (IGF), fibronectin, transferrin and interleukin.6 All of these substances stimulate fibroblast proliferation, migration and collagen production to some degree.1
Wound healing modulation after glaucoma filtration surgery already has been achieved by a growing number of approaches.6-9 Research in this area has strongly focused on inflammatory response following surgical trauma. Anti- inflammatory and antimetabolite derivatives are commonly used to inhibit the synthesis of inflammatory mediators leading to decreased granulocyte and mast cell degranulation, fibrin formation and fibroblast proliferation.7,8 Subconjunctival adhesion prevention with adhesion barriers may also control wound healing response and reduce failure.
Surgicel absorbable hemostat (AH), one of the earlier membrane hemostat materials, is designed for use adjunctively in surgical procedures to assist in the control of capillary, venous and small arterial hemorrhage when ligation or other conventional methods of control are impractical or ineffective.10 It is made of oxidated regenerated cellulose (ORC). Hemostasis alone reduces adhesion formation by preventing the flow of the active mediators for wound healing to the surgical site. Beside this indirect effect, although it is not designed primarily as an adhesion barrier, it also precedes peritoneal membrane adhesion barriers. Larsson et al., showed that adhesions following cecal trauma were significantly less common in rats treated with Surgicel.11 In spite of most studies12,13 that found Surgicel to be effective in preventing adhesion formation, some14,15 did not confirm this observation.
Interceed is made of the same material (ORC) as Surgicel, but it lasts longer in the peritoneal cavity. It is the first product which was designed as an adhesion preventing agent and it was found to be effective in many clinical studies in gynecologic and abdominal surgery.9,16
The aim of the study was to evaluate and compare the effectiveness of two membrane adhesion barriers made of ORC material, Surgicel and Interceed, on wound healing reaction after glaucoma filtration surgery.
Materials and Methods
This study was performed by the Ophthalmology and Pathology departments of Firat University School of Medicine. The experiments adhered to the ARVO statement for the use of animals in ophthalmic and vision research. Twelve six-month-old male albino rabbits were used for this study, with mean body weight of 2.87 ± 0.36 kg. Three study groups were formed, each consisted of four rabbits.
Experimental filtration surgery
Full thickness filtration surgery was performed in one eye of all rabbits in the following manner: After the rabbits were anesthetized with intramuscular injections of xyalazine hydrochloride 5 mg/kg (Rompun, Bayer, Istanbul-Turkey) and ketamin hydrochloride 25 mg/kg (Ketalar, Eczacibasi, Istanbul-Turkey). The right eyes of the rabbits were cleaned and draped for surgery. A drop of oxybuprocain hydrochloride (Benoxinate, ThiloandCo Gmbh, Puurs-Belgium) was instilled and the traction suture was placed to the upper eyelid. A limbal- based conjunctival flap was fashioned and a full thickness scleral block excision was carried out next to the limbus in the superior quadrant of all the eyes. A peripheral iridectomy was then performed and hemostasis was established. At this point, Interceed (TC7, Johnson and Johnson Medical Inc. Arlington, Tx, USA) and Surgicel (Surgicel Absorbable Hemostat, Johnson and Johnson Medical Inc. Arlington, Tx, USA), prepared in 3 x 4 mm dimensions, were placed to the opened sclera of the eyes which formed Groups 1 and 2 respectively. The membranes was draped with the conjunctival flap and left there. No material was applied in Group 3 (control group). At the end of the procedure, the conjunctiva was closed with a running 8-0 polyglactin suture and the eyes were patched after instillation of ophthalmic ointment consisting of oxytetracyclin and polymyxin B sulphate. All the eyes were operated by the same surgeon.
Postoperative follow-up
All groups received tobramycin and dexamethasone drops thrice daily for 14 days. IOP, anterior chamber depth and bleb appearance were checked and recorded on the first, third, seventh and 14th postoperative days. Schiotz indentation tonometer was used for IOP measurements. Anterior chamber depth was evaluated with the help of a penlight and estimated as Grade 0 (flat chamber), Grade I (narrow chamber) and Grade II (chamber with a normal depth). Bleb appearance was also evaluated with inspection and graded as Grade 0 (no bleb or hardly visible non-functioning bleb), Grade I (slightly elevated and functioning bleb) and Grade II (grossly elevated and functioning bleb).
Histological preparation
Animals were sacrificed by overdose of intravenous pentobarbital anesthesia at the end of the 14th day. After the placement of a blepharostat, a fixation suture was placed at 3 mm behind the operation site, helping conjunctiva remain attached to the sclera. Square-shaped corneoscleral blocks (10 × 10 mm) with overlying conjunctiva were then dissected from all operated eyes having the trabeculectomy site on the center of the samples. The blocks were immediately fixed with 10% formalin and then buried in paraffin. Five-micrometer thick sections crossing the visible or estimated fistula site were cut using a microtome (Leica). The samples were stained with hematoxylin and eosin. Three sections were cut at a minimum of 20 micrometers apart to provide different population of cells in each section.
Light microscopic analysis of the specimens was performed with the ×40 objective of a standard light microscope (BH2 Olympus Photomicroscope) and ×10 eyepieces. Histopathologic analysis of the surgical site and surrounding subconjunctival area consisted of: (1) Cell counts per area (fibroblast, lymphocyte, eosinophil, neutrophil and macrophage); (2) Vessel count per area; (3) Presence of edema and fibrosis; (4) Presence of foreign body reaction; (5) Patency of the fistula tract. All counts were made in two microscope areas and means ± standard deviations were used. Only clearly identified cells with their peculiar nuclear and cytoplasmic features were counted. Foreign body reaction was evaluated only in trabeculectomy site which was draped with the membrane barriers. The foreign body reaction around the conjunctival closure area which was due to suture was omitted.
Statistical analysis
The values obtained from the clinical and histopathologic evaluation were statistically analyzed using non-parametric tests (Mann Whitney-U and Kruskall Wallis tests) in SPSS for Windows. P values were used to show statistical significance in comparisons and a P value under 0.05 was considered significant.
Results
The standardized surgical procedures and the administration of the protocols were well tolerated by the animals. None of the eyes showed signs of infection. Anterior chamber depth was assessed as normal in all examinations of the groups. Fig. 1 gives data about the appearance of filtration blebs by means of the number of the elevated and functioning blebs. Mean and SD of the grades in the study groups were as follows: 0.75 ± 0.96, 0.5 ± 1.0, 0.5 ± 1.0 (Group 1); 0.5 ± 0.58, 0.5 ± 0.58, 1.25 ± 0.96 (Group 2) and 1.25 ± 0.96, 1.25 ± 0.96, 1.0 ± 1.0 (Group 3) on the first, third and seventh postoperative days respectively. All the filtering blebs failed on the 14th day and all groups were found to be statistically similar in terms of the bleb appearance (Kruskall Wallis test).
Figure 1.
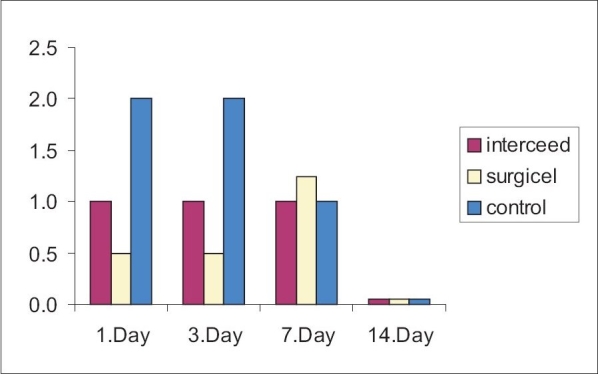
Number of elevated and functioning blebs in various study groups
Fig. 2 shows the mean IOP in the postoperative examinations. Mean IOP on first day examination was found to be significantly low in Group 2 as compared to the other groups. Also, mean IOP on the third day was found to be significantly low in Group 3 as compared to the other groups. On the other hand, no overall difference was found between groups on the seventh and 14th day examinations.
Figure 2.

Mean intraocular pressures on 1st - 14th days in various study groups
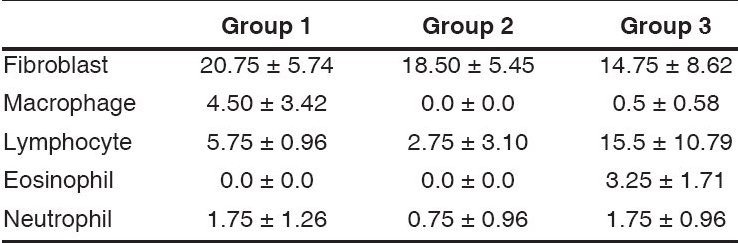
Histopathologic analysis of the sections from the operation site of the control group displayed one patent and three closed fistula tract. The number of the patent fistula tracts was two in Group 1 and three in Group 2. Groups were found statistically similar with respect to the appearance of the fistula tract. Table 1 shows the mean cell counts in the light microscopic examinations. Mean number of eosinophils in Groups 1 and 2 was significantly less than Group 3 (P = 0.014 for both groups; Mann Whitney U test). Mean number of lymphocytes in Group 2 was significantly less than Group 3 (P = 0.043, Mann Whitney U test). Group 1 and 2 were found similar with respect to the number of lymphocytes. Subconjunctival space of Group 2 contained significantly small number of macrophages than Group 1 (P = 0.047, Mann Whitney U test). Histopathologic examination of the sections showed no foreign body reaction in any group.
Table 1.
Cell counts as mean ± standard deviations in various study groups
A thick, dense layer of connective tissue lying under the epithelium was detected in all groups. The active fibroblasts of Tenon′s layer were increased in number, elongated and oriented parallel to each other. The elongated fibroblasts also invaded old scleral collagen. None of the specimens showed the histopathologic signs of suspended fibroblast proliferation. Active macrophages, which were widespread in Group 1, appeared mostly around the fistula tract on which barrier membranes had been implanted. Groups 2 and 3 showed no significant increase of macrophages around the fistula tract. Newly formed subepithelial connective tissue was rich in blood vessels. Mean number of vessels were 3.5 ± 0.58, 3.5 ± 1.29 and 9.75 ± 2.36 in Groups 1 to 3 respectively. Mean number of vessels in Group 1 and Group 2 was significantly less than Group 3 (P = 0.019 and P = 0.020 respectively; Mann Whitney U test). No statistical significance was found between the numbers of vessels of Group 1 and Group 2. Figs. 3 to 5 show the photographs of the light microscopic sections of Groups 1 to 3 respectively.
Figure 3.
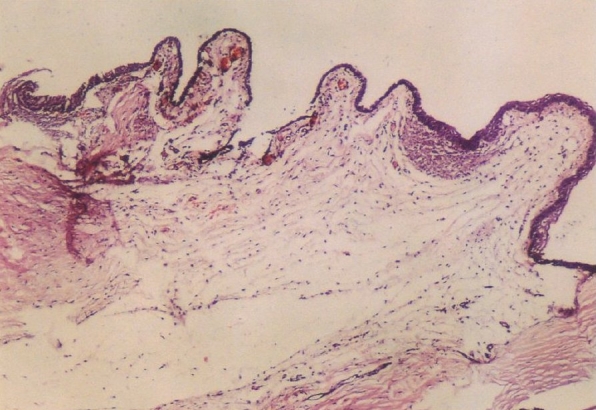
Photomicrograph of the operation site from an interceedtreated (Group-1) animal. Under the thickened conjunctival epithelium, newly formed connective tissue contains activated fibroblasts. Inflammatory infiltration is mild to moderate. Moderately increased vascular patterns are seen (hematoxylin and eosin stain, original magnification ×40)
Figure 5.
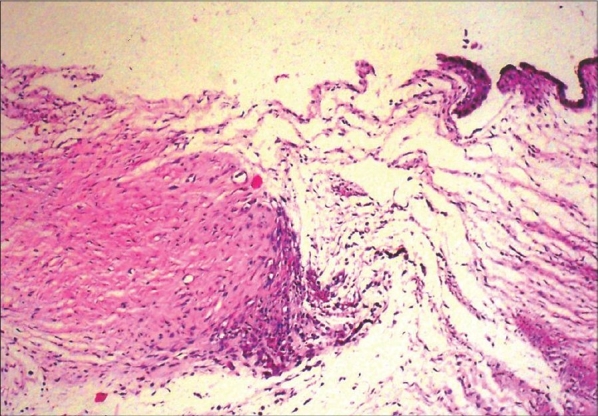
Photomicrograph of operation site of a control (Group-3) animal shows numerous activated fibroblasts and new collagen under the thickened conjunctival epithelium. Subconjunctival area displays moderate lymphocyte infi ltration and much more frequent vascular patterns (hematoxylin and eosin stain, original magnification ×40)
Discussion
The conjunctival wound healing initiates with the inflammatory phase, which is characterized by the movement of intravascular components to the extravascular area. By the end of this stage, a clot is formed and facilitates the movement of other cellular components into the wound. Among them, fibroblasts originating mostly from adjacent tissues (Tenon′s capsule and episclera) appear on the third day and become the dominant cellular component of the wound in the second (fibroblastic) phase. Angiogenesis immediately follows the fibroblast migration and these two form a granulation tissue. Wound closure is achieved by the epithelialization with the migration and proliferation of the epithelial cells and by contraction of the myofibroblasts, which originated from fibroblasts. Contraction starts at five to seven days and is maximally observed at the fourth to fifth weeks. Remodeling, the last phase of healing begins during the fibroblastic phase and may last for more than a year.7,8
Rabbits and monkeys are the commonly used animal models for wound healing studies because of their suitably sized globes.6 We used rabbits because they are less expensive, docile and easy to care. Filtration surgery tends to fail in animal experiments, which indicates that the behavior of animal eyes is different from that of humans. It may not be possible to achieve a functioning bleb in an animal even with adjunctive therapy for wound healing modulation.6 So the failure of the fistula does not mean the failure of the method. Histopathological evaluation, mainly fibroblastic activity and vascular proliferation, gives us a better idea about the success. The wound healing process generates in a relatively short time in animals, compared to the human being. In the rabbit model, young fibrovascular tissue was seen in the fistula by the third day and it peaks within the first two weeks after experimental glaucoma filtration surgery.1,17 Using this data, we selected to evaluate wound healing reaction at 14th day.
The studies about wound healing modulation after GFS are far from accessing the end point.6-9 One of the important reasons of the failure after GFS is subconjunctival adhesion. Adhesion formation in general may be reduced by several routes: reduction of the initial inflammatory response and subsequent exudation (topical corticosteroids), inhibition of fibroblastic proliferation (such as mitomycine-C), promotion of fibrinolysis and mechanical separation of surfaces.18 The last one is achieved by barrier agents which include mechanical barriers and viscous solutions.3 The ideal barrier should be non-reactive, bioabsorbable and easy to use; also it should persist during the critical stages of wound healing.
There is no study about the use of barrier agents to modulate the wound healing reaction after GFS in the ophthalmic literature. The use of barrier agents in strabismus surgery was found controversial.19,20 Yacobi et] al., reported that the use of ORC sleeves significantly increases the formation of postoperative adhesions.19 However, Hwang and Chang reported that the combined use of ORC, 5-fluorouracil and viscoat could delay the adjustment time after adjustable strabismus surgery in rabbits.20
The exact mechanism of action of ORC in wound healing modulation is unknown. In spite of the fact that it is a physical barrier in the beginning, there is convincing data about its breakdown products being biologically active. Interaction of ?nterceed with macrophages may result in a decreased secretion of matrix components, inflammatory mediators like interleukin- 1 beta and cellular growth factors.21
Barrier agents are widely used in gynecologic surgery. In spite of being produced from the same material, clinical experience as a barrier agent is quite different with Interceed and Surgicel. As far as we know from their intraperitoneal applications, Interceed fits the requirements and it has been approved for clinical use.22 On the other hand, Surgicel AH has not been even proposed as an adhesion-preventing product by its producer. There is a small number of studies which support the retarding effects of Surgicel on wound healing reaction.23-25 It is also advised to remove the material after the achievement of hemostasis.23 The main reason for this precaution is the unpredictable volume of the product when it is used in large amounts. The small pieces used and the easily reachable position of Surgicel after GFS could make this precaution unjustified. The manufacturer of surgicel notice that, during its placement, surgicel block must not overlap the wound edges. In that case, wound healig may be postphoned. We were also very careful during the placement since capture of the membrane between the conjunctival edges might interfere with conjunctival closure. This feature makes Surgicel useful for our purpose when it postpones the healing in the subconjunctival area and keeps compartments apart.
The most confusing point about the effects and biodegradation behavior of these products in the GFS is the presence of aqueous humor in the environment. Body fluids other than whole blood showed no reaction with Surgicel AH. It could be disadvantageous if we desired only its hemostatic effect but in our situation we hardly need any hemostasis. In the absence of hemorrhage, only the barrier effect of Surgicel comes into play and helps modulation of wound healing preventing adhesions.
In vivo and in vitro biodegradation and solubilization of ORC was well studied for the conditions in which it is used as intraperitoneal adhesion barrier. Dimitrijevich et al., collected peritoneal fluid, serum and urine from the rabbits on whom ORC were surgically implanted on their uterine horns and analyzed for carbohydrate components utilizing high-performance liquid chromatography with pulsed amperometric detection.26 They reported that oligomeric products were evident in peritoneal fluid from the implantation site, with no apparent accumulation in either the serum or the urine. The size and amount of products rapidly decreased and by Day 4, peritoneal lavages were free of oligomers.26 In vitro solubilization was studied by the same authors and method, with the presence of serum/plasma and hydrolytic enzymes. They reported that ORC first undergoes chain shortening to give oligomers which, in the presence of plasma or serum, are further hydrolyzed to smaller fragments, including glucuronic acid and glucose.27
To conclude, it is not easy to talk about any significant interaction of ORC materials with wound healing reaction with our small study size. In spite of statistically insignificant differences of overall IOP and filtrating bleb, the results of the histopathologic examination are convincing for the future use of these membrane barriers in GFS. In that manner, we thought the decreased number of vessels in study groups comparing controls may show less aggravated wound healing reaction. More studies on glaucomatous human eyes should be performed to explore the effects of these agents.
Figure 4.
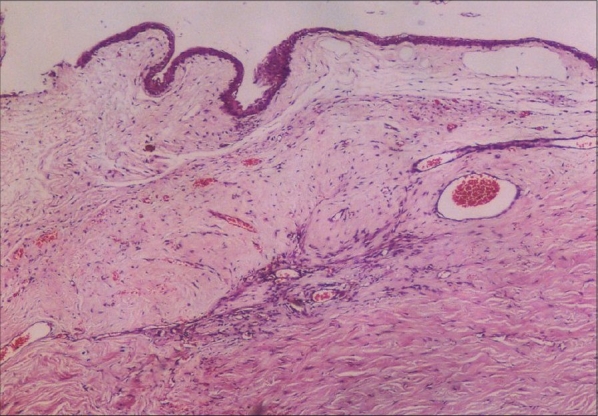
Photomicrograph of operation site of a Surgicel-treated (Group-2) animal shows the disorganized and comparatively hypocellular connective tissue. Vascular patterns are slightly increased (hematoxylin and eosin stain, original magnification ×40)
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Khaw PT, Occleston NL, Schultz GS, Grierson I, Sherwood MB, Larkin G. Activation and supression of fibroblast function. Eye. 1994;8:188–95. doi: 10.1038/eye.1994.44. [DOI] [PubMed] [Google Scholar]
- 2.Akyol N, Demir T, Kükner A, Çolakoǧlu N. Effects of systemic octreotide, local mytomycine-C and local corticosteroids on wound-healing reaction after glaucoma surgery. Int Ophthalmol. 2001;24:235–41. doi: 10.1023/a:1025401430745. [DOI] [PubMed] [Google Scholar]
- 3.Yoldemir T, Saǧol S, Adakan S, Öztekin K, Özşener S, Karadadaş N. Comparison of the reduction of postoperative adhesions by two barriers, one solution and two pharmacologic agents in the rat uterine model. Fertil Steril. 2002;78:335–9. doi: 10.1016/s0015-0282(02)03224-7. [DOI] [PubMed] [Google Scholar]
- 4.Cunliffe IA, Rees RC, Rennie IG. The effect of TGF-beta1 and TGF-beta2 on the proliferation of human Tenon′s capsule fibroblasts in tissue culture. Acta Ophthalmol Scand. 1996;74:31–5. doi: 10.1111/j.1600-0420.1996.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 5.Joseph JP, Grierson I, Hitchings RA. Normal rabbit aquous humour, fibronectin and fibroblast conditioned medium are chemoattractant to Tenon′s capsule fibroblasts. Eye. 1987;1:585–92. doi: 10.1038/eye.1987.90. [DOI] [PubMed] [Google Scholar]
- 6.Joseph JP, Miller MH, Hitchings RA. Wound healing as a barrier to successful filtration surgery. Eye. 1988;2:S113–23. doi: 10.1038/eye.1988.138. [DOI] [PubMed] [Google Scholar]
- 7.Costa VP, Spaeth GL, Eiferman RA, Orengo-Nania S. Wound healing modulation in glaucoma filtraton surgery. Ophthalmic Surg. 1993;24:152–70. [PubMed] [Google Scholar]
- 8.Kook MS, Lee DA. Improving the success of glaucoma surgery by controlling wound healing. Ophthalmol Clin North Am. 1995;8:393–9. [Google Scholar]
- 9.Khaw PT, Wilkins M. Antifibrotic agents in glaucoma surgery. In: Yanoff M, Duker JS, editors. Ophthalmology. 1999. p. 31.1. Ch 12. [Google Scholar]
- 10.Matthew IR, Browne RM, Frame JW, Millar BG. Subperiosteal behaviour of alginate and cellulose wound dressing materials. Biomaterials. 1995;16:275–8. doi: 10.1016/0142-9612(95)93254-b. [DOI] [PubMed] [Google Scholar]
- 11.Larsson B, Nisell H, Branberg I. Surgicel: An absorbable hemostatic material-in prevention of peritoneal adhesions in rats. Acta Chir Scand. 1978;144:375–8. [PubMed] [Google Scholar]
- 12.Rafferty AT. Absorbable haemostatic materials and intraperitoneal adhesion formation. Br J Surg. 1980;67:57. doi: 10.1002/bjs.1800670118. [DOI] [PubMed] [Google Scholar]
- 13.Galan N, Leader A, Malkinson T, Taylor PJ. Adhesion prophylaxis in rabbits with surgical and two absorbable microsurgical sutures. J Reprod Med. 1983;28:662–4. [PubMed] [Google Scholar]
- 14.Schroder M, Willumsen H, Hansen JP, Hansen OH. Peritoneal adhesion formation after the use of oxidized cellulase (Surgicel) and gelatin sponge (Spongostan) in rats. Acta Chir Scand. 1982;148:595–6. [PubMed] [Google Scholar]
- 15.Vemini M, Meshorer A, Katz Z, Rozenman D, Lancet M. Prevention of reformation of pelvic adhesions by ″barrier″ methods. Int J Fertil. 1984;29:194–6. [PubMed] [Google Scholar]
- 16.Steinleitner A, Lopez G, Suarez M, Lambert H. An evaluation of flowgel as an intraperitoneal barrier for prevention of postsurgical adhesion reformation. Fertil Steril. 1992;57:305–8. [PubMed] [Google Scholar]
- 17.Miller MH, Grierson I, Unger WI, Hitchings RA. Wound healing in animal model of glaucoma fistulizing surgery in the rabbit. Ophthalmic Surg. 1989;20:350–7. [PubMed] [Google Scholar]
- 18.Bakkum EA, van Blitterswijk CA, Emeis JJ, Trimbos JB, Dalmeijer RA, Trimbos-Kemper TC. Long-term analysis of peritoneal plasminogen activator activity and adhesion formation after surgical trauma in the rat model. Fertil Steril. 1996;66:1018–22. doi: 10.1016/s0015-0282(16)58700-7. [DOI] [PubMed] [Google Scholar]
- 19.Yacobi Y, Hamed LM, Kaul KS, Fanous MM. Reduction of postoperative adhesions secondary to strabismus surgery in rabbits. Ophthalmic Surg. 1992;23:123–8. [PubMed] [Google Scholar]
- 20.Hwang JM, Chang BL. Combined effect of Interceed and 5-fluorouracil on dalayed adjustable strabismus surgery. Br J Ophthalmol. 1999;83:788–91. doi: 10.1136/bjo.83.7.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy S, Santanam N, Reddy PP, Rock JA, Murphy AA, Parthasarathy S. Interaction of interceed oxidized regenerated cellulose with macrophages: A potential mechanism by which Interceed may prevent adhesions. Am J Obstet Gynecol. 1997;177:1315–21. doi: 10.1016/s0002-9378(97)70070-x. [DOI] [PubMed] [Google Scholar]
- 22.Al-Jaroudi D, Tulandi T. Adhesion prevention in gynecologic surgery. Obstet Gynecol Surv. 2004;59:360–7. doi: 10.1097/00006254-200405000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Sabel M, Stummer W. The use of local agents: Surgicel and Surgifoam. Eur Spine J. 2004;13:97–101. doi: 10.1007/s00586-004-0735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen JK, Krogsgaard J, Nielsen KM, Norgaard EB. A comparison between 2 absorbable hemostatic agents: Gelatin sponge (Spongostan)and oxidized regenerated cellulose (Surgicel) Int J Oral Surg. 1984;13:406–10. doi: 10.1016/s0300-9785(84)80066-6. [DOI] [PubMed] [Google Scholar]
- 25.Mattsson T, Anneroth G, Kondell PA, Nordenram A. ACP and Surgicel in bone hemostasis: A comparative experimental and histologic study. Swed Dent J. 1990;14:57–62. [PubMed] [Google Scholar]
- 26.Dimitrijevich SD, Tatarko M, Gracy RW, Wise GE, Oakford LX. In vivo degradation of oxidized, regenerated cellulose. Carbonhydrate Res. 1990;198:331–41. doi: 10.1016/0008-6215(90)84303-c. [DOI] [PubMed] [Google Scholar]
- 27.Dimitrijevich SD, Tatarko M, Gracy RW, Linsky CB, Olsen C. Biodegradation of oxidized regenerated cellulose. Carbonhydrate Res. 1990;195:247–56. doi: 10.1016/0008-6215(90)84169-u. [DOI] [PubMed] [Google Scholar]


