Dear Editor,
We read with great interest the article titled ″Intra-cameral injection of bevacizumab (Avastin) to treat anterior chamber neovascular membrane in a painful blind eye″ by Raghuram et al.1
We would like to share our experience of an interventional case series in which 14 patients of diabetic retinopathy (DR) and vascular occlusions complicated with neovascular glaucoma and raised intraocular pressure (IOP) causing pain and circum-corneal congestion were included. All these patients were treated with intra-vitreal (1.25 mg/0.05 ml) and intra-cameral (0.25 mg/0.02 ml) bevacizumab (Avastin) injection after an informed consent from the patients. There was regression of iris neovascularization (NVI) in 8 days (1 to 14 days) on an average [Fig. 1]. Nine patients underwent phaco-trabeculectomy with intraocular lens implantation (those with significant cataract) or trabeculectomy followed by pan-retinal photocoagulation (PRP) to ablate the ischemic retina. Four patients underwent PRP and medical management of glaucoma and one was lost to follow-up. On an average follow-up of 6 months (range 3 to 12 months), two patients showed more than two-line improvement in vision and the rest all had stabilization of vision. All patients showed stabilization of IOP with well-formed filtering blebs.
Figure 1.
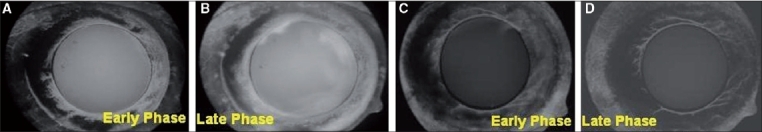
(A, B) Early and late phases of anterior segment fluorescein angiography showing dye leakage from iris neovascularization prior to intra-cameral Avastin injection. (C, D) Early and late phases of anterior segment fluorescein angiography showing reduced dye leakage from iris neovascularization after intra-cameral Avastin injection
Two patients had a recurrence of iris NVI after 14 and 16 weeks of Avastin injection, respectively, presenting with hyphema [Fig. 2]. Intra-cameral injection of Avastin had to be repeated in both these cases. Both cases at their last follow-up at 8 months were stable with no evidence of recurrence of NVI or neovascularization of the angle (NVA).
Figure 2.
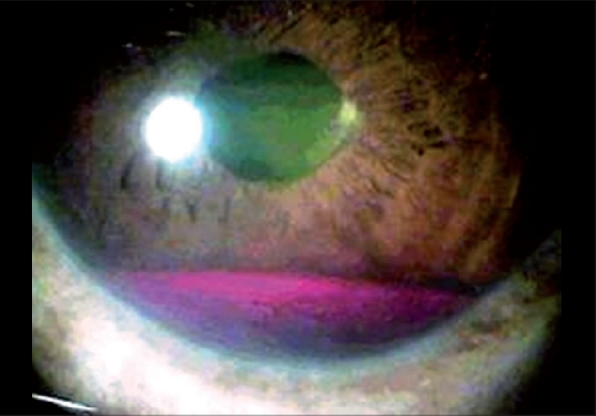
Recurrence of iris neovascularisation presenting as hyphema
We have used intra-cameral Avastin in a dose of 0.25 mg/ 0.02 ml in comparison to 1 mg/0.04 ml used in the article by Raghuram et al.1 and found this low dose to be as effective in regressing NVI and NVA. In our cases of DR and vascular occlusions, the NVI and NVA were caused secondary to the production of VEGF from the ischemic retina;2 and we targeted the source of VEGF by intra-vitreal Avastin and ablating the ischemic retina with laser photocoagulation thus trying to avoid a recurrence of NVI/NVA. However, in comparison to the case reported by Raghuram et al.,1 we had a recurrence in two cases probably because of active production of vascular endothelial growth factor (VEGF) from the ischemic cells in comparison to cells dying without ischemia in a phthisical eye. The advantages of intra-cameral Avastin were - rapid regression of NVI avoiding a delay in surgery to normalize IOP in these cases with already compromised perfusion to optic nerve head secondary to diabetic retinopathy and vascular occlusions, and increasing the success rate of glaucoma surgery by avoiding intra-operative bleed.
To conclude, intra-cameral Avastin in lower doses was effective in causing regression of NVI/NVA which may be temporary in cases of DR and vascular occlusions with an active production of VEGF; and a more long-term regression in these cases may require targeting the production of VEGF from the posterior segment by additional intra-vitreal Avastin,3 laser photocoagulation or cryopexy. Potential systemic side-effects have been reported with intra-vitreal Avastin injection and we need to weigh the risk-benefit ratio before using this drug.
References
- 1.Raghuram A, Saravanan VR, Narendran V. Intra-cameral injection of Bevacizumab (Avastin) to treat anterior chamber neovascular membrane in a painful blind eye. Indian J Ophthalmol. 2007;55:460–2. doi: 10.4103/0301-4738.36484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–7. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 3.Avery R, Pearlman J, Pieramici D, Rabena M, Castellarin A, Nasir M, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695–705. doi: 10.1016/j.ophtha.2006.05.064. [DOI] [PubMed] [Google Scholar]


