Abstract
Standard recommended guidelines for diagnosis of infectious keratitis do exist. Based on an extensive Medline literature search, the various investigative modalities available for aiding the diagnosis of microbial keratitis have been reviewed and described briefly. Preferred practice patterns have been outlined and the importance of routine pre-treatment cultures in the primary management of infectious keratitis has been highlighted. Corneal scraping, tear samples and corneal biopsy are few of the specimens needed to carry out the investigative procedures for diagnosis and for initiating therapy in cases of microbial keratitis. In bacterial, fungal and amoebic keratitis, microscopic examination of smears is essential for rapid diagnosis. Potassium hydroxide (KOH) wet mount, Gram′s stain and Giemsa stain are widely used and are important for clinicians to start empirical therapy before microbial culture results are available. The usefulness of performing corneal cultures in all cases of suspected infectious keratitis has been well established. In cases of suspected viral keratitis, therapy can be initiated on clinical judgment alone. If a viral culture is needed, scrapings should directly be inoculated into the viral transport media. In vivo confocal microscopy is a useful adjunct to slit lamp bio-microscopy for supplementing diagnosis in most cases and establishing early diagnosis in many cases of non-responding fungal and amoebic keratitis. This is a non-invasive, high resolution technique which allows rapid detection of Acanthamoeba cysts and trophozoites and fungal hyphae in the cornea long before laboratory cultures give conclusive results. Other new modalities for detection of microbial keratitis include molecular diagnostic techniques like polymerase chain reaction, and genetic finger printing by pulsed field gel electrophoresis.
Keywords: Corneal biopsy, corneal scraping, culture, infectious keratitis, investigations, microbiology
Microbial keratitis is a sight-threatening condition with significant ocular morbidity that requires prompt and appropriate management. To minimize complications and permanent sequelae, timely antimicrobial treatment must be initiated on the basis of clinical and microbiological evaluation.1
Various investigative modalities [Table 1] supplement the clinical diagnosis and provide supportive evidence for planning therapy. Apart from its diagnostic value, corneal scraping allows improved antibiotic penetration and therapeutic debridement of necrotic tissue. Though recognized to be useful in proper management, there is no worldwide consensus on which investigative modality to apply in various cases of infectious keratitis.
Table 1.
List of investigative modalities useful for diagnosis and planning management of patients with infectious keratitis
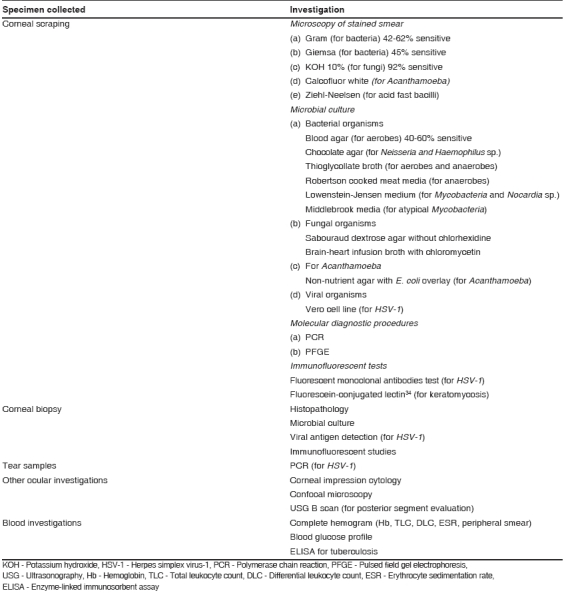
Microbial culture, considered to be the gold standard,2-4 and direct microscopic detection of causative organisms are the two important microbiological investigations that are widely used. Standard practice patterns vary from country to country and hospital to hospital depending on facilities and expertise that are locally available. Nevertheless, this section reviews the principles of diagnosis and management that should be considered as a baseline internationally accepted standard of care.
Sample Collection: Practical Information
To determine the causative organism, meticulous collection of microbiological specimens is of critical importance. The ulcer is scraped for microscopy, culture and drug sensitivity and any further investigations, if indicated.
Steps in scraping a corneal ulcer
Explain the procedure to the patient and obtain informed consent.
Scraping should be performed under magnification of a slit lamp or operating microscope. Pre-prepared scraping and treatment kits should ideally be available in the emergency room. Routine scraping kits should include a minimum of one slide for Gram staining and one agar plate for aerobic incubation. Anesthetize the affected eye with a topical anesthetic (4% lignocaine hydrochloride or 0.5% proparacaine hydrochloride).
Sterile gloves and an aseptic technique for handling of specimens should be used in all cases. Get an assistant to gently retract the lids.
With the help of a specimen collection aid like a Bard Parker no. 15 blade, 21-gauge needle, calcium alginate swab or a Kimura platinum spatula, gently but firmly scrape the surface of the ulcer. The base and leading edges of the ulcer are scraped from the periphery to the center. A prospective comparative evaluation of Bard Parker blade no. 15 and calcium alginate swab for collecting the corneal material showed that there was no significant difference in microbial yield between the two methods as reported previously.6
The material is smeared on two glass slides, one for potassium hydroxide (KOH) wet mount preparation and the other for Gram′s stain. It is smeared thinly within a marked area on the slide and heat-fixed for Gram′s and Giemsa stains. For KOH + calcofluor white (CFW) stain, the scraping is placed within a marked area on the glass slide and then covered with one drop each of 10% KOH and 1% CFW with Evan′s blue, followed by placement of a cover-slip.
The scraping is repeated several times using a fresh blade for each scrape or re-flaming and cooling the spatula, to obtain sufficient samples which are immediately transferred directly onto the culture plate and glass slide. Additional scrapings are inoculated onto blood agar, chocolate agar, non-nutrient agar, Sabouraud′s dextrose agar or potato dextrose agar in rows of C-shaped streaks and also inoculated into the depth of liquid media such as thioglycollate broth and brain-heart infusion broth as and when indicated.
All the culture media are kept at room temperature, preferably in an incubator after sample collection. All the culture media are then incubated according to the standard procedure.
Brief Description and Review of Literature of Investigative Modalities
Microscopic evaluation of smears
Smears are useful for providing information about the presence of inflammatory cells, for rapid diagnosis of bacterial and fungal infections and to corroborate culture results.
The material obtained from the smear is examined microscopically using Gram′s and Giemsa stains, KOH 10%, CFW and lactophenol cotton blue stain. CFW is a fluorescent dye that requires a fluorescent microscope for detection of Acanthamoeba cysts. Lactophenol cotton blue stain may also be used in cases of suspected Acanthamoeba keratitis and it has the advantage that it does not require a fluorescent microscope. For typical and atypical Mycobacteria andNocardia species, Kinyoun acid-fast (Ziehl-Neelsen) stain is a very reliable stain. The auramine-rhodamine stain is a histological technique used to visualize acid-fast bacilli using fluorescent microscopy.
KOH wet mount, Gram′s stain and Giemsa stain are widely used for the rapid detection of microbes;7 however, owing to misinterpretation, presence of artifacts and lack of detection of Candida and other yeasts, the sensitivity of these methods is highly variable. A recent study concluded that decisions can reliably be based on KOH + CFW stain of corneal scrapings for instituting early, specific therapy in mycotic keratitis. Bharathi et al.8 concluded that a KOH smear is of greater diagnostic value in the diagnosis of fungal keratitis, Nocardia keratitis and Acanthamoeba keratitis. The simple, sensitive technique of KOH wet mounting is recommended in all clinics without exception for establishing timely treatment. Gram′s stain was also found to be very dependable for making decisions in the treatment of bacterial keratitis, thereby enabling the clinician to start empirical treatment.
Microbial culture
Routine laboratory investigation should always include both bacterial and fungal media by the standard C-streak method.9 These media are incubated under appropriate atmospheric conditions, examined daily and require a specific period of time for positive growth depending on the organisms (24 h to 3 weeks). A study by O′Day et al.10 suggested that one-fourth of the fungal cultures did not become positive until 14-19 days after inoculation. In the evaluation of infectious keratitis, plating onto chocolate agar or blood agar alone is a reasonable alternative to sending multiple cultures.11
Microbial cultures are considered relevant if growth of the same organism is observed on more than one solid-phase medium, if there is confluent growth at the site of inoculation on one solid medium, if growth of one medium is consistent with direct microscopy findings (i.e., appropriate staining and morphology with Gram′s stain) and if the same organism is grown from repeated scraping.
In patients who have received empirical therapy without undergoing routine microbiological analysis, a delay in starting culture-guided antibiotic treatment has been noted in a study by Marangon et al.12 They reported that 56% of patients referred to their center were already on topical antibiotic therapy before culture specimen was obtained. Once treatment has been initiated, it may be more difficult to recover the organisms in culture for identification and sensitivity studies. Culture- positive rate may not be significantly decreased, but a delay in pathogen recovery may occur, as a result of pre-treatment. Similarly, McDonnel et al.13 and Kowal et al.14 reported that patients who were already on treatment prior to culture showed delayed healing of the ulcer, probably due to the toxic effect of ineffective and prolonged antibiotic therapy. Sub-optimal concentration of antibiotics does allow bacterial growth to occur in culture media. Hence, results of bacterial culture may be positive even after therapy and this should encourage all ophthalmologists to definitely scrape previously treated ulcers, though they may expect a delay in growth of the organism.
Corneal ulcers already treated with antibiotics show growth of Gram-negative bacteria, fungi and Acanthamoeba more commonly than fresh, non-treated cases as reported by Rodman et al.15 They also reported an incidence of about 9% of uncommon organisms recovered from corneal ulcers that were treated with antibiotics before sending culture specimens. If the cultures are negative, the ophthalmologist may consider stopping antibiotic treatment for 12-24 h and then re-culturing. Adequate microbiology studies, even in patients who have been treated empirically without microbiological evaluation, are important.
In a study by Kaye et al.16 there was no significant difference in the number of positive cultures from solid media (direct inoculation) used conventionally or liquid (indirect) culture media, both in patients and experimental pig corneas. The broth inoculation technique or the use of transport media17 is a promising alternative to the recommended direct plate inoculation, especially in private eye clinics and community settings.18
A survey of 30 years of laboratory experience concluded that the use of Gram′s stain and culture in combination seems to yield the highest percentage of bacterial recovery.19
The role of routine pre-treatment cultures in the primary management of ulcerative keratitis is well-established to be the accepted standard of care. Various studies by Kowal et al.14 and Morlet et al.20 highlight the importance of collecting data on both the isolates and antibiotic sensitivity of organisms grown in cultures from cases of microbial keratitis. McLeod et al.4 also reported the usefulness of performing corneal cultures in all cases of suspected microbial keratitis. In certain situations, purely empirical therapy without sending culture specimens has been advocated to be an acceptable practice.7 These exceptions include small (< 2 mm size), superficial and peripheral (not involving the visual axis) ulcers which are not associated with trauma with vegetable matter or other risk factors for unusual pathogens where empirical therapy with close daily follow-up may be reasonable and cost-effective.21
Although the ophthalmic literature uniformly recommends that microbiological investigations must be performed in all cases of infectious keratitis, these procedures require investment of a certain amount of time and expense by the ophthalmologist, the patient and ultimately the medical system in general. Hence, notwithstanding standard published and recommended guidelines, in actual practice it has been found that community ophthalmologists′ compliance with the recommended procedures in the management of corneal ulcers is inadequate.13 McDonnel et al. reported that only half of all corneal ulcers seen by community ophthalmologists in Southern California were sent for microbiological analysis.13
Alternatives to universal culture and sensitivity testing that might be considered include selectively performing cultures for more severe(i.e., ulcers that are large >2 mm size, central and deep) or suspected non-bacterial ulcers or routinely obtaining cultures in all cases, but pursuing identification and sensitivity studies only when it is required for therapy modification.4 Rather than an ′all or none′ approach to microbiological analysis, patients can best be treated on the clinical skills and judgment of the ophthalmologist at the first contact and then the therapy can be modified in accordance with the clinical response and investigative outcome.21 In general, the standard practice of obtaining cultures is followed more often by cornea specialists than general ophthalmologists.3
Finally, in conclusion, after reviewing all available literature and preferred practice patterns, we recommend the practice of routine microbiological analysis for all corneal ulcers. However, those clearly suspected to have purely viral etiology may be treated differently. Generally, initiating therapy for viral keratitis based on clinical evaluation is well accepted. However, if viral culture is required, isolation of herpes simplex virus (HSV-1) in culture provides the most reliable and specific method, and is considered as the ″Gold Standard″ in the laboratory diagnosis of herpes simplex keratitis (HSK). Using ″cell lines of corneal origin″, e.g., human corneal epithelial (HCE) cell line,22 for virus isolation may be beneficial under such circumstances, since these cells have been shown to be excellent substrates for the growth of HSV-1 isolated from the cornea. If viral culture is requested, scrapings should be placed directly into viral transport media and delivered promptly to the laboratory or refrigerated for a short time and transported on wet ice. Corneal impression cytology on a sterile glass slide has been described as a simple and inexpensive technique for diagnosis of HSV epithelial keratitis.23
As resistance patterns to commonly used ophthalmic antibiotics continue to shift, an important role for laboratory investigation of corneal ulcers, both for community surveillance of pathologic species and susceptibility characteristics, as well as for appropriate management of individual cases persists.
Confocal microscopy
In vivo confocal microscopy provides a real-time and non- invasive method for identifying corneal pathogens in early stages of infection. Compared to the slit-lamp, confocal microscopy offers better resolution and contrast. Thus, confocal microscopy can be used as a complement to slit- lamp biomicroscopy in diagnosis of the cause of infectious keratitis.24
Like the first-generation confocal microscopes, Heidelberg retinal tomograph-corneal module (HRTII) provides non- invasive, high-contrast, in vivo images of the cornea at different depths from epithelium to endothelium. Images of fungal structures are obtained immediately and allow early treatment to be started, before laboratory investigations conclude on the definitive diagnosis. They also play a useful role in rapid, non-invasive, in vivo detection of Acanthamoeba cysts and trophozoites in the cornea and in monitoring the efficacy of amebicide treatment.25
Thus, the new-generation confocal microscopes, now available, might be extremely useful in the management and prognosis of many corneal ulcers, helping in the differential diagnosis between keratomycosis and Acanthamoeba keratitis in the early phase of these diseases.26
Molecular diagnostic techniques
There has been a rapidly growing trend toward microbial characterization by applying molecular methods, as it prevents some of the limiting factors of culture-based bacterial detection and has significantly improved the diagnostic approach to infectious keratitis in the past decade. Polymerase chain reaction (PCR) has been shown to detect small amounts of microbial DNA and hence improve therapeutic efficacy. Direct PCR amplification and sequencing of bacterial genes encoding the small subunit of ribosomal RNA (16S rDNA) without prior cultivation allow the identification of fastidious or unculturable bacteria. PCR is a promising technique as a means to diagnose fungal keratitis27 and offers some advantages over culture methods, including rapid analysis and the ability to analyze specimens far from where they are collected.28
This technique showed promise as an alternative to standard microbiologic testing for rapid detection of bacteria, particularly in cases in which standard microbiologic test results were negative.29 Analysis of corneal scrapings by 16S rDNA PCR should be considered as a supplement to standard microbiological procedures.30
A reduced-sensitivity PCR can detect HSV DNA in tears from patients with clinically diagnosed HSV epithelial keratitis. The sensitivity of this system is higher than that of viral culture, while being low enough not to give a positive PCR result in normal tears. This PCR system is more sensitive than a viral culture system.31 PCR for HSV has been shown to be most useful to the clinician in atypical presentations of herpetic ocular disease.
Genetic fingerprinting of the total bacterial community is performed by denaturing gradient gel electrophoresis (DGGE). In pulsed field gel electrophoresis (PFGE), the orientation of the electric field across the gel is periodically changed (pulsed), allowing DNA fragments to be separated according to size. Results demonstrated that 16S rDNA genotyping in combination with DGGE fingerprinting are appropriate molecular methods for the investigation of severe bacterial infections which might not be detected by conventional cultivation.32
Molecular diagnosis of pathogenic agents is a newer technology for accurate identification of the causative agents, but is inapplicable to all patients with corneal ulcer, as it is more expensive.
Corneal biopsy
When the culture of scrapings of a progressive, non-responding corneal ulcer is negative, histological examination of biopsy specimen is indicated. Superficial keratectomy or corneal biopsy specimen may be obtained by a trephine or a sharp blade by free lamellar dissection for immunohistochemical and light microscopic examination. This approach is especially useful for the detection of fungi and Acanthamoeba in deep ulcers33 and could be excisional or incisional. Excisional biopsy is performed for peripheral lesions while incisional biopsy is done in cases where the visual axis is spared. A 1-mm margin of macroscopically uninvolved tissue should be included, where possible, to ensure that the active edge is sampled.
Conclusion
The documented emergence of resistant patterns to fluoroquinolones in ophthalmic practice is therefore accompanied by decreasing confidence in empirical therapy and by greater need for strategies for the microbiological evaluation of infectious keratitis, which are simple, reliable and cost-effective. Further research that simplifies the process of laboratory investigation for all practitioners and patients is encouraged.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Allan BD, Dart JK. Strategies for the management of microbial keratitis. Br J Ophthalmol. 1995;79:777–86. doi: 10.1136/bjo.79.8.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLeod SD. The role of cultures in the management of ulcerative keratitis. Cornea. 1997;16:381–2. [PubMed] [Google Scholar]
- 3.Levey SB, Katz HR, Abrams DA, Hirschbein MJ, Marsh MJ. The role of cultures in the management of ulcerative keratitis. Cornea. 1997;16:383–6. [PubMed] [Google Scholar]
- 4.Mcleod SD, Kolahdouz-Isfahani A, Rostamian K, Flowers CW, Lee PP, McDonnel PJ. The role of smears, cultures and antibiotic sensitivity testing in the management of suspected infectious keratitis. Ophthalmology. 1996;103:23–8. doi: 10.1016/s0161-6420(96)30738-0. [DOI] [PubMed] [Google Scholar]
- 5.Jacob P, Gopinathan U, Sharma S, Rao GN. Calcium alginate swab versus Bard Parker blade in the diagnosis of microbial keratitis. Cornea. 1995;14:360–4. doi: 10.1097/00003226-199507000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Benson WH, Lanier JD. Comparison of techniques for culturing corneal ulcers. Ophthalmology. 1992;99:800–4. doi: 10.1016/s0161-6420(92)31897-4. [DOI] [PubMed] [Google Scholar]
- 7.Sharma S, Kunimoto DY, Gopinathan U, Athmanathan S, Garg P, Rao GN. Evaluation of corneal scraping smear examination methods in the diagnosis of bacterial and fungal keratitis: A survey of eight years of laboratory experience. Cornea. 2002;21:643–7. doi: 10.1097/00003226-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Bharathi MJ, Ramakrishnan R, Meenakshi R, Mittal S, Shivakumar C, Srinivasan M. Microbiological diagnosis of infective keratitis: Comparative evaluation of direct microscopy and culture results. Br J Ophthalmol. 2006;90:1271–6. doi: 10.1136/bjo.2006.096230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones DB. Early diagnosis and therapy of bacterial corneal ulcers. Int Ophthalmol Clin. 1973;13:1–29. [PubMed] [Google Scholar]
- 10.O′Day DM, Akrabawi PL, Head WS, Ratner HB. Laboratory isolation techniques in human and experimental fungal infections. Am J Ophthalmol. 1979;87:688–93. doi: 10.1016/0002-9394(79)90305-2. [DOI] [PubMed] [Google Scholar]
- 11.Waxman E, Chechelnitsky M, Mannis MJ, Schwab IR. Single culture media in infectious keratitis. Cornea. 1999;18:257–61. doi: 10.1097/00003226-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Marangon FB, Miller D, Alfonso EC. Impact of prior therapy on the recovery and frequency of corneal pathogens. Cornea. 2004;23:158–64. doi: 10.1097/00003226-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 13.McDonnell PJ, Nobe J, Gauderman WJ, Lee P, Aiello A, Trousdale M. Community care of corneal ulcers. Am J Ophthalmol. 1992;114:531–8. doi: 10.1016/s0002-9394(14)74479-4. [DOI] [PubMed] [Google Scholar]
- 14.Kowal VO, Levey SB, Laibson PR, Rapuano CJ, Cohen EJ. Use of routine antibotic sensitivity testing for the management of corneal ulcers. Arch Ophthalmol. 1997;115:462–5. doi: 10.1001/archopht.1997.01100150464002. [DOI] [PubMed] [Google Scholar]
- 15.Rodman RC, Spisak S, Sugar A, Meyer RF, Soong HK, Musch DC. The utility of culturing corneal ulcers in a tertiary referral center versus a general ophthalmology clinic. Ophthalmology. 1997;104:1897–901. doi: 10.1016/s0161-6420(97)30010-4. [DOI] [PubMed] [Google Scholar]
- 16.Kaye SB, Rao PG, Smith G, Scott JA, Hoyles S, Morton CE, et al. Simplifying collection of corneal specimens in cases of suspected bacterial keratitis. J Clin Microbiol. 2003;41:3192–7. doi: 10.1128/JCM.41.7.3192-3197.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLeod SD, Kumar A, Cevallos V, Srinivasan M, Whitcher JP. Reliability of transport medium in the laboratory evaluation of corneal ulcers. Am J Ophthalmol. 2005;140:1027–31. doi: 10.1016/j.ajo.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 18.Schonheyder HC, Pedersen JK, Naeser K. Experience with a broth culture technique for diagnosis of bacterial keratitis. Acta Ophthalmol Scand. 1997;75:592–4. doi: 10.1111/j.1600-0420.1997.tb00156.x. [DOI] [PubMed] [Google Scholar]
- 19.Asbell P, Stenson S. Ulcerative keratitis: Survey of 30 years′ laboratory experience. Arch Ophthalmol. 1982;100:77–80. doi: 10.1001/archopht.1982.01030030079005. [DOI] [PubMed] [Google Scholar]
- 20.Morlet N, Dart J. Routine antibiotic sensitivity testing for corneal ulcers. Arch Ophthalmol. 1998;116:1262–3. doi: 10.1001/archopht.116.9.1262. [DOI] [PubMed] [Google Scholar]
- 21.McDonnell PJ. Empirical or culture-guided therapy for microbial keratitis? A plea for data. Arch Ophthalmol. 1996;114:84–7. doi: 10.1001/archopht.1996.01100130080013. [DOI] [PubMed] [Google Scholar]
- 22.Athmanathan S, Reddy SB, Nutheti R, Rao GN. Comparison of an immortalized human corneal epithelial cell line with Vero cells in the isolation of Herpes simplex virus-1 for the laboratory diagnosis of Herpes simplex keratitis. BMC Ophthalmol. 2002;2:3. doi: 10.1186/1471-2415-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Athmanathan S, Bandlapally SR, Rao GN. Collection of corneal impression cytology directly on a sterile glass slide for the detection of viral antigen: An inexpensive and simple technique for the diagnosis of HSV epithelial keratitis: A pilot study. BMC Ophthalmol. 2001;1:3. doi: 10.1186/1471-2415-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chew SJ, Beuerman RW, Assouline M, Kaufman HE, Barron BA, Hill JM. Early diagnosis of infectious keratitis with in vivo real time confocal microscopy. CLAO J. 1992;18:197–201. [PubMed] [Google Scholar]
- 25.Bourcier T, Dupas B, Borderie V, Chaumeil C, Larricart P, Baudouin C, et al. Heidelberg retina tomograph II findings of Acanthamoeba keratitis. Ocul Immunol Inflamm. 2005;13:487–92. doi: 10.1080/09273940590951098. [DOI] [PubMed] [Google Scholar]
- 26.Brasnu E, Bourcier T, Dupas B, Degorge S, Rodallec T, Laroche L, et al. In vivo confocal microscopy in fungal keratitis. Br J Ophthalmol. 2007;91:588–91. doi: 10.1136/bjo.2006.107243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagyalakshmi R, Therese KL, Madhavan HN. Application of semi-nested polymerase chain reaction targeting internal transcribed spacer region for rapid detection of panfungal genome directly from ocular specimens. Indian J Ophthalmol. 2007;55:261–5. doi: 10.4103/0301-4738.33037. [DOI] [PubMed] [Google Scholar]
- 28.Gaudio PA, Gopinathan U, Sangwan V, Hughes TE. Polymerase chain reaction based detection of fungi in infected corneas. Br J Ophthalmol. 2002;86:755–60. doi: 10.1136/bjo.86.7.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto S, Shimomura Y, Kinoshita S, Nishida K, Yamamoto R, Tano Y. Detection of herpes simplex virus DNA in human tear film by the polymerase chain reaction. Am J Ophthalmol. 1994;117:160–3. doi: 10.1016/s0002-9394(14)73071-5. [DOI] [PubMed] [Google Scholar]
- 30.Rudolph T, Welinder-Olsson C, Lind-Brandberg L, Stenevi U. 16S rDNA PCR analysis of infectious keratitis: A case series. Acta Ophthalmol Scand. 2004;82:463–7. doi: 10.1111/j.1395-3907.2004.00303.x. [DOI] [PubMed] [Google Scholar]
- 31.Dota A, Chie S, Norihiko Y, Yamamoto S, Koizumi S, Nishida K, et al. Detection of herpes simplex virus DNA in atypical epithelial keratitis using polymerase chain reaction. Br J Ophthalmol. 1999;83:957–60. doi: 10.1136/bjo.83.8.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schabereiter-Gurtner C, Maca S, Kaminsky S, Rölleke S, Lubitz W, Barisani-Asenbauer T. Investigation of an anaerobic microbial community associated with a corneal ulcer by denaturing gradient gel electrophoresis and 16S rDNA sequence analysis. Diagn Microbiol Infect Dis. 2002;43:193–9. doi: 10.1016/s0732-8893(02)00401-7. [DOI] [PubMed] [Google Scholar]
- 33.Lee P, Green WR. Corneal biopsy: Indications, techniques and a report of a series of 87 cases. Ophthalmology. 1990;97:718–21. doi: 10.1016/s0161-6420(90)32517-4. [DOI] [PubMed] [Google Scholar]
- 34.Robin JB, Nielson S, Trousdale MD. Fluorescein-conjugated lectin identification of a case of human keratomycosis. Am J Ophthalmol. 1986;102:797–8. doi: 10.1016/0002-9394(86)90413-7. [DOI] [PubMed] [Google Scholar]


