Abstract
Primary open angle glaucoma (POAG) is usually a chronic, slowly progressive disease. At present, all resources are directed towards reduction of intraocular pressure (IOP), the only known causal and treatable risk factor for glaucoma, and medical management is frequently the first choice in most cases. With the introduction of innovative tools for early diagnosis and newer medications for treatment, decision-making in diagnosis and treatment of glaucoma has become more complex. The philosophy of glaucoma management is to preserve the visual function and quality of life (QOL) of the individual with minimum effects on QOL in terms of cost, side effects, treatment regime, follow-up schedules as well as socioeconomic burden. Our aim should be not to treat just the IOP, optic disc or visual field, but to treat the patient as a whole so as to provide maximum benefit with minimal side effects. In this article, we describe the scientific approach to medical management, mainly of POAG.
Keywords: Medical management, primary open angle glaucoma, target intraocular pressure
Glaucoma is one of the major causes of visual loss worldwide.1- 5 With the introduction of innovative tools for early diagnosis and newer medications for treatment, decision-making in diagnosis and treatment of glaucoma has become more complex; in this article, we describe our approach to medical management. The approach we describe has evolved over the two decades of caring for glaucoma patients and with available evidence in the literature. We will focus mainly on the management of primary open angle glaucoma (POGG). As far as primary angle closure disease is concerned, once the iridotomy is performed (and the angle opened), medical management is similar to that of POAG. Differences will be discussed where relevant.
At the very outset, we will state our management philosophy. The philosophy of glaucoma management is to preserve the visual function and quality of life (QOL) of the individual. Essentially, (functional) vision should outlast the patient. Our aim is not to treat just the intraocular pressure (IOP), optic disc or visual field, but to treat the patient as a whole so as to provide maximum benefit with minimal side effects.
Some of the terms used in this article are defined below:
POAG: It is a chronic optic neuropathy with characteristic changes in the optic disc and corresponding typical defects in the visual field for which IOP is the only treatable risk factor.6 POAG is a diagnosis of exclusion.
Normal tension glaucoma (NTG): The definition is same as POAG except that the central corneal thickness (CCT) corrected IOP is less than 22 mmHg (mean + 2SD) on diurnal variation.7 Like POAG, it is a diagnosis of exclusion; most cases are managed like POAG.
Pre-perimetric glaucoma: It is the presence of characteristic optic disc and nerve fiber layer changes strongly suggestive of glaucoma but without field defects on conventional automated perimetry (white on white).8
Ocular hypertension (OHT): It is defined as the CCT corrected IOP above the 97.5 percentile in that population, with open angles on gonioscopy and no disc or field changes.9
Target IOP: Target IOP is the IOP at which the sum of the health-related quality of life (HRQOL) from preserved vision and the HRQOL from not having side effects from treatment is maximized. It can also be defined in other ways including the highest IOP in a given eye at which no clinically apparent nerve damage occurs.10
The basic principles that we follow in the management of a glaucoma patient are discussed below.
Establish a diagnosis
Establish a baseline IOP
Set a target IOP
Initiate therapy and to lower IOP to target
Follow-up
Establish A Diagnosis
Glaucoma is suspected (or diagnosed) after a comprehensive eye examination (CEE). There is no substitute or surrogate for a CEE. It includes slit lamp biomicroscopy, Goldmann applanation tonometry, gonioscopy (preferably dynamic, using an indentation lens), indirect ophthalmoscopy and stereoscopic examination of the optic disc and retinal nerve fiber layer (RNFL). Those suspected to have POAG need further investigations, the minimum being automated perimetry (24-2 or 30-2 program) for the detection of functional defects. The diagnosis of POAG is made using a combination of IOP, disc and field changes in the presence of an open angle.
Fig. 1 shows the flowchart for a work-up of a suspected glaucoma patient in our clinic.
Figure 1.
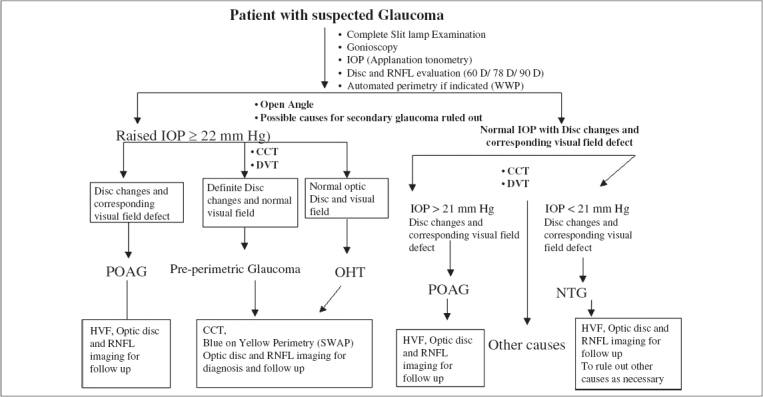
Flowchart showing the work-up of glaucoma suspect, IOP - Intraocular pressure, RNFL - Retinal nerve fibre layer, D - Diopter, WWP - White on white perimetry, CCT - Central corneal thickness, DVT - Diurnal variation test, POAG - Primary open angle glaucoma, OHT - Ocular hypertension, NTG - Normal tension glaucoma, HVS - Humphrey visual field, SWAP - Short wave automated perimetry
Applanation tonometry: On its own, applanation tonometry has a poor sensitivity and specificity for the diagnosis of glaucoma.11 Repeat IOP and diurnal measurements have more value. IOP in combination with other signs can help in diagnosis. The Schiotz tonometer is considered outdated and has at best a very limited role to play in modern glaucoma management.
Gonioscopy: The diagnosis of POAG is one of exclusion made after gonioscopy using indentation lenses, under standard testing conditions.12 Gonioscopy helps to rule out angle closure and secondary causes of glaucoma. Gonioscopy is a dynamic procedure and may need to be repeated at regular intervals.
Automated perimetry: Automated white-on-white perimetry (WWP) is the gold standard for the diagnosis of functional defects in glaucoma.
Optic disc and RNFL examination with stereo biomicroscopy: Clinically, RNFL loss and optic disc changes are best appreciated using stereo-biomicroscopy with a 60-90 D indirect lens; red free illumination is needed for examination of the RNFL. If the disc is suspicious, a contact lens examination may reveal additional information. Stereo photographs of the optic disc are the current gold standard but have some disadvantages. Optic disc drawings and or descriptions are less acceptable but provide valuable information to an experienced examiner.
Imaging techniques: The Association of International Glaucoma Societies (AIGS) consensus meeting does not support the routine use of newer optic disc imaging technique such as Heidelberg retinal tomography (HRT), scanning laser polarimetry (GDx VCC), optical coherence tomography (OCT) for all patients but suggests a role for them in decision-making when used by experts for selected cases.13 We use the sensitivity, specificity and likelihood ratios of test parameters on these imaging techniques to calculate the probability of glaucoma in an individual patient.14,15
It is important to remember that temporal progression of findings compared to the baseline values (increase in baseline IOP, disc changes, field and imaging parameters), even if they remain within the normal range, can be suggestive of an early disease.
Establish A Good Baseline IOP
IOP is the only known causal and treatable risk factor.16,17 A one-time IOP recording is likely to be misleading and is a poor indicator for diagnosis and treatment. Knowledge of diurnal variation of IOP in an individual provides information about the peak IOP as well as fluctuation. It helps to set the target IOP and decide on the group of drugs to be used to initiate treatment.
Ideally, we would like a formal diurnal curve on all patients. In routine clinical practice, we measure several IOP recordings at different times of the day. Any ″high″ reading is confirmed by repeat IOP measurement. If not possible on 1 day, an estimate is obtained by measuring IOP at different times during office visits. If treatment is initiated or changed, the process is repeated. For patients who present with high IOP (30 or more), we confirm the IOP and initiate medical management without multiple IOP readings. In such patients, we obtain the multiple IOPs on treatment. While a 24-h diurnal IOP for all patients (IOP readings taken every three-hourly over a period of 24 h referred to as diurnal variation test (DVT)) is desired, we usually obtain such a 24-h DVT at least once in all suspected NTG patients (CCT corrected) who might otherwise undergo unnecessary systemic investigations and in those who are progressing despite ″well controlled″ office hours IOP.
Goldmann applanation tonometer records false high IOP in thick corneas (except in corneal edema) and falsely low IOPs in thin corneas. If not in all cases, CCT should at least be measured in those with suspected OHT and NTG. While no formula for correction of CCT is universally accepted, we use Ehlers correction.18
Set A Target IOP
The Early-Manifest Glaucoma Treatment Study showed that IOP reduction by at least 25% reduced progression from 62 to 45% in the treated group compared to an untreated group.19 The Collaborative Initial Glaucoma Treatment Study (CIGTS) lowered the IOP by 35%, demonstrated equivalence of medical and surgical treatment, and decreased disease progression to less than 15%.20
IOP lowering needs to be individualized with the goal of preventing any decrease in the QOL during the patient′s lifetime. That in essence is the target IOP. There is, however, no hard evidence for the concept or the methods used to determine the target. The following factors should be considered at the time of presentation to customize the target IOP:21
Structural damage: optic disc and RNFL
Functional damage on WWP
Baseline IOP at which the damage occurred (correlate the above two with baseline IOP).
Age
Presence of additional risk factors
Target IOP has to be individualized based on patient′s clinical profile. This can be calculated using tables, graphs or formulae. The formula used by CIGTS is shown below. This is similar to that published by Jample et al.22
Formula for target IOP =
![]()
While we can formally calculate the target IOP in this manner or using graphs or tables, the rule of thumb is to reduce the IOP by at least 20% in mild, 30% in moderate and more than 40% in severe glaucoma. Generally, the formulae and other methods will provide similar values.
The higher the IOP, the larger the reduction required. If a patient has a starting IOP of 40 mmHg, we would opt for a larger percentage reduction. A 20% reduction from 40 mmHg would bring the IOP into the 30s; which is not good enough even for pre-perimetric glaucoma. In an advanced glaucoma (evident by structural and functional damage), in a young or middle-aged patient, one may choose to reduce IOP by 50% from the baseline. However, for the same clinical findings in a very old patient, the target may be set to a higher level so as to minimally hamper the QOL for that individual.
There are limitations to the target IOP approach. There is no sure-fire method of estimating it and no hard evidence that it works. Also, we do not know what aspect of the IOP actually causes the damage (peak IOP, fluctuations, short spikes, etc). Currently, we monitor the patient using visual fields; we need a more sensitive outcome measure to monitor the patient to reset the target if necessary. At the moment, however, it is a good concept to manage the patient.
A word of caution, there is a real danger of using the target IOP approach. Despite popular belief that ″lower is better″, not every patient requires the IOP to be lowered to a mean of 12 mmHg. Also, the target IOP is just a guideline, not a number to be strictly adhered to; it is better to use a range rather than a single number. Using a range of IOP provides safety from unnecessary aggressive therapy.
The target IOP is not a fixed magic number. Neither is it a static number, but changes depending on the results on long- term monitoring. If a patient is progressing on the target IOP we have set, we may need to lower it further. If a patient is stable on our target IOP, it may well be that it could be readjusted higher; we may try to withdraw some treatment.
Initiate Therapy and Attempt to Lower IOP to Target
The goal of medical treatment is to obtain ″24-h″ IOP control with the minimum concentration and number of medications, as well as minimal local and systemic side effects. We routinely explain and demonstrate instillation of drops and encourage our patients to perform eyelid closure and punctum occlusion after instilling every antiglaucoma medication.
The selection of initial drug depends on the target IOP. The factors to keep in mind while prescribing a drug include: efficacy, compliance, safety, persistence and last, but not the least, affordability. If the drug is cost-effective and dosage is convenient, compliance should improve. Introduction of fixed combination drugs has helped improve compliance and cut down the costs.
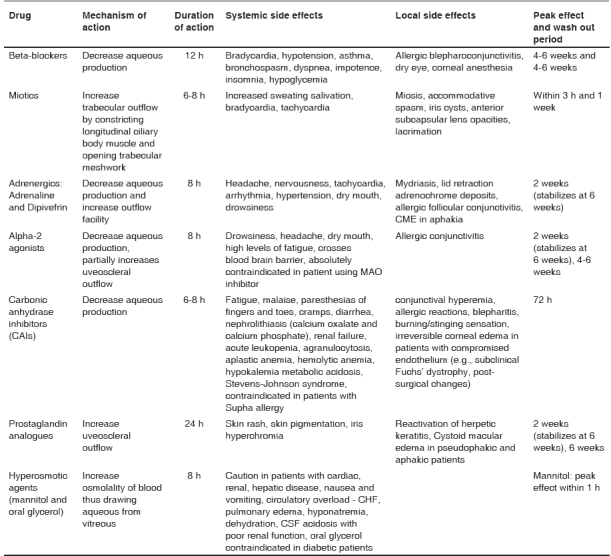
Table 1 shows the list of available antiglaucoma medications with their mechanism of action and side effects.
Table 1.
Main classes of ocular hypotensive drugs and their mechanisms
Once initiated, glaucoma therapy is usually lifelong. This involves considerable expense and inconvenience. Accordingly before initiating therapy, we must be sure of the diagnosis and reasonably sure that the medication works. To establish the efficacy of a drug, we perform a unilateral drug trial. Such a drug trial determines the efficacy of a single or combined therapy in one eye so as to decide if the drug works. The efficacy of the components of a combination must be tested separately.
We usually start the therapy in worse eye (usually with higher IOP/more structural and functional damage) first. After the peak IOP reduction ability of that drug is reached, we again repeat day DVT (IOP reading at every 2½ h from 8 AM to 6 PM) to look for reduction in IOP and fluctuation. Table 1 shows the time to peak effect of common antiglaucoma drugs. If the drug achieves our target IOP, it is continued and started in the second eye. If drug fails to reduce IOP by at least 15% IOP from baseline or produces severe side effects, we do not prescribe that drug; and go on to a unilateral drug trial with our second option. However, if the first drug does reduce IOP more than 15% from baseline but not to our target IOP level, we have at least established that the medication works. We now have a choice of adding a second medication or changing to a new medication. Usually, we will try to keep the tested drug as a reserve medicine to be used in combination if other options also do not provide adequate IOP control.
In an ideal world (not considering cost), we would like to use a prostaglandin analogue in most glaucoma patients as a first line. Cost is, however, a consideration and if our target IOP is around 20% IOP reduction from the baseline, beta-blockers could be the first line of drug. Systemic beta-blocker have the ability to achieve 80% of the topical drop′s effect on IOP.23 If the patient is already on systemic beta-blockers for hypertension, IOP reduction ability of topical beta-blockers will likely be reduced significantly; it should perhaps be avoided as a first- line drug in this instance. They are, however, worth trying as a second line of management if necessary.
If our target is 30-35% IOP reduction from the baseline, Prostaglandin analogues (PGA) like Latanoprost (0.005%), Bimatoprost (0.03%) or Travatoprost (0.004%) are preferable. Some patients may not respond to one brand of PGA, but may respond to another. In a non-responder, it is worthwhile trying another brand.
If 30% or more IOP lowering is required and either a prostaglandin does not achieve this or there is a cost problem, combination of beta-blockers with α-2 agonists like brimonidine P 0.15% or topical carbonic anhydrase inhibitors may suffice. Combination drugs have made life easier, but each component must be individually shown to be effective.
Generally, the IOP cannot be lowered below the episcleral venous pressure and once the IOP is lowered by the first medication, the amount it can be further lowered (to the episcleral pressure) is less for the second medication.24 Theoretically, PGA act via the uveoscleral outflow and can reduce IOP below episcleral pressure. Practically, we have not seen any patient (without other co-morbidity) whose IOP has actually been reduced below 11 mmHg on any medication.
Many ophthalmologists are concerned about waiting for the results of a unilateral trial, especially if the IOP is ″high″. Glaucoma is a chronic progressive disease and the patient has probably already had the disease for a long time before detection. Nothing acute or significantly detrimental is likely to happen during the time we do the unilateral trial. However, many patients (and doctors) are uncomfortable with treating only one eye especially if the baseline IOP is high. If baseline IOP is at such a level (usually high 20s and above), we could start the unilateral trial as planned along with systemic acetazolamide for a temporary IOP control for both eyes. As the peak effect and wash period is minimal, we prefer it when situation demands IOP reduction in contra-lateral eye for temporary time period. Discontinuing the systemic acetazolamide 72 h before the next follow-up allows us to assess the effect of the eye drop in one eye without too much risk to the fellow eye.
In situations where the patient is unable to return within 4-6 weeks to assess the results of the unilateral trial, we start treatment in both eyes and do a reverse trial. The patient is asked to discontinue the medication under assessment 4-6 weeks (depending on the wash-off period for that drug) prior to the follow-up visit.
The flowchart [Fig. 2] shows the overview of unilateral drug trial and monitoring of medical management of an individual patient.
Figure 2.
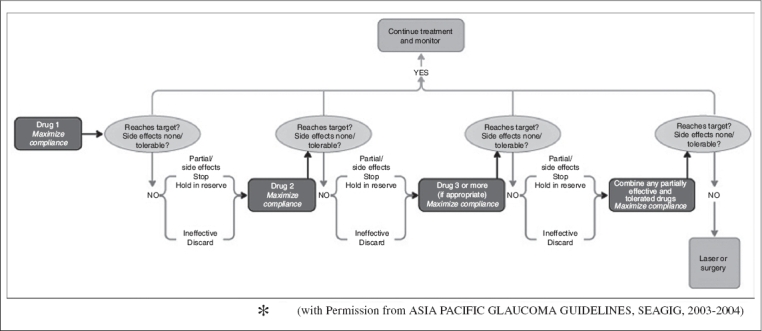
Flowchart showing unilateral drug trial and monitoring of medical management for an individual patient (with Permission from Asia Pacific Glaucoma Guidelines, Seagig, 2003-2004)
Side effects and contra-indications of anti-glaucoma medications
While prescribing medications, common side effects and contra- indications of the drug should be kept in mind. Table 1 shows the reported side effects of the common antiglaucoma drugs. In patients with asthma and heart block, beta-blocker should be avoided. Many older patients have mild chronic obstructive pulmonary disease (COPD) and may feel much better once a beta-blocker is stopped. We avoid PGAs in inflammatory glaucoma, in patients with history of herpetic keratitis and cystoid macular edema with a compromised posterior capsule. It is advisable to make the patient aware of the side effects of PGAs like elongation and hyper-pigmentation of lashes, iris hyperchromia before initiating therapy; more so if the therapy is unilateral. Topical carbonic anhydrase inhibitors (CAIs) should be avoided in patients with poor endothelial status as it can worsen corneal edema in such cases. Alpha-2 agonists can cross blood -brain barrier and are absolutely contra-indicated in infants and patients using MAO inhibitors. Alpha-2 agonist also causes drowsiness that can have major impact on a patient′s QOL. Educating the patient about punctum occlusion and eyelid closure can decrease this side effect. Oral CAI should not be given for a long duration or be given with consent of the patient′s physician.
Whatever therapy we choose, the concept to be kept in mind is that glaucoma therapy is lifelong and directly related to HRQOL; be it cost, side effects or regime of the drug.
Follow-up
Regular follow-up is necessary to detect progression that might require escalation of treatment. Progression in glaucoma is assessed by structure (optic disc and RNFL) and function (visual field testing with WWP) independently or in association. A higher IOP is a risk factor for progression. Structure, function and IOP should be monitored at regular intervals. The follow-up period depends on the stage of the disease and stability.
Individualizing follow-up visits
If the unilateral drug trial has been started, we review patients depending on the peak duration of the drug used. Subsequent follow-up is customized depending on the decision to continue the same drug, add or replace the drug in the same eye and the other eye also.
Once we are sure that IOP is stable (assessed by day DVT or checking IOP at frequent intervals at different hours of the day at different visits), the subsequent follow-up visits of glaucoma patients depend on the severity of glaucoma (amount of disc and field damage) at the time of presentation and follow-up, as well as additional risk factors. The initial visits are used to obtain baseline fields.
Glaucoma (pre-perimetric or early functional damage), with IOP at target might be reviewed in 6 months and then if still stable, at yearly intervals. The yearly follow-ups would include the full CEE as well as several IOPs, visual fields and other imaging tests required.
Stable patients with moderate damage would be examined at 6-month intervals. For severe glaucoma in the better eye, the interval could be 3-4 months. If the IOP is slightly higher than the target level, say 2 mmHg, but the patient has not progressed on other parameters, we would schedule the next visit a few months earlier to measure IOPs and then go back to the routine. If there is confirmed progression in structure or function (repeatable progression of field defect on at least two occasions), even if the office hour IOP is at target level, we repeat DVT to look for undetected IOP peaks or high fluctuations as well as systemic factors like nocturnal dips in blood pressure. While it is controversial, we believe that diurnal fluctuation of 8 mmHg or more is probably an independent risk factor for progression.25
Judging progression: Progression can be judged by documenting structural changes in the optic disc and/or functional changes on the visual fields. IOP and optic disc examinations are mandatory at every routine visit.
Progression in structure: Any new RNFL defect, presence of new disc hemorrhage or change in neuroretinal rim status indicates unstable and progressive glaucoma. Such patients requires a visual field to document functional progression.
Progression on visual fields: In current clinical practice, we rely mainly on WWP to detect progression. In our own clinical routine: we use the ″Overview″ and glaucoma progression analysis (GPA) programs. Eyeballing the overview, we get a feel for whether field deterioration is due to generalized sinking of the hill of vision or whether it is due to glaucoma. GPA is based on the pattern deviation plot and is a better option to diagnose progression.
Progression in the research setting requires confirmation over four to six fields.26 In the clinic, however, the judgment of progression is more ″corroborative″. There are other factors like the IOP (hopefully several, over time, at various times of the day), appearance of the disc, RNFL, hemorrhage, etc. If these suggest progression, eyeballing the overview gives us an idea as to what is going on and we get a GPA. ″Possible″ progression on the GPA corroborates our clinical concern; a repeat field with the message ″Likely″ progression is then probably good enough to take decisions, especially if the deteriorating points correlate with our disc findings. It is possible that GPA might underestimate visual field progression even in cases without evidence of increasing media opacity.27 We feel that consideration of the overview program (total and pattern deviation plot) in combination with the GPA analyses addresses this concern.
Progression on imaging technologies: Imaging technique offers exciting possibilities to detect progression. The HRT has the longest track record in documenting structural change (trend analysis and topographic change analysis).28 GDx and OCT have developed programs more recently. Progression on imaging is more likely to be useful in the earlier stages of the disease, which is an advantage.
If the progression is confirmed on both structure and function or even if only on visual fields, there is no other option but to further reduce our target IOP by adding or substituting therapy.
Everything else being equal, we take more cognizance of optic disc deterioration in the presence of a field defect than without. Some findings like disc hemorrhage, RNFL defect or a rim notch are so predictive of future field defects that we initiate our routine (DVT, detection of IOP dips etc) on this basis and would step up therapy without a field defect.
We treat progression on imaging (trend analysis and topographic change analysis on HRT) in the same manner. Without a field defect progression (unless associated with the disc findings referred to above or ″high″ IOP), we would usually elect to observe the patient more carefully. In the presence of an existing functional defect (early or moderate), the change is more likely to be real and result in functional damage. Accordingly, we consider progression on imaging more sinister in this situation and treat it akin to disc progression in this situation.
Either way, if target IOP was not achieved, then in these cases we may add on or change the therapy.
Some clinical situations merit mention.
Maximum medical therapy: If the IOP is not at target level, we consider adding or substituting the therapy depending upon target IOP required. The need to decrease the IOP to target depends on the amount of damage already present. There is no consensus on how many drugs can be used. This brings us to the concept of maximum medical therapy. Maximal medical therapy can be defined as the minimum number and concentration of drugs (within the combination of different classes of medications) that provides maximum lowering of IOP. It should be tailored to the individual patient.29
If the progression is confirmed but IOP is at target (confirmed with DVT): Revisit other risk factors like nocturnal hypotension, systemic or topical steroid use, recent major surgery or blood loss due to trauma, central corneal thickness. We would also talk to the patient′s physician regarding any hypertension, its control and the avoidance of night-time dips in blood pressure as well as the possibility of sleep apnea. If the patient shows progression with normal IOPs, we would enquire about other habits; yoga, especially ″aasnas″ in the inverted position, use of wind instruments, rapid consumption of large quantities of water or beer. An ″alternative medicine″ prescription of a liter of water in the morning mimics the water drinking provocative test and can cause optic nerve damage. This situation also requires a repeat DVT to see if the IOPs are fluctuating beyond the normal range especially in the early morning hours.
It is also possible that our assessment of the initial target IOP was wrong and we need to readjust it. On other hand, we may have a patient at the opposite end of the spectrum: mild or pre-perimetric glaucoma with stable visual functions at target. In this scenario, it is possible that we may have overestimated the target IOP and we may be overtreating the patient. In this instance, we would try to withdraw unnecessary drugs.
Role of surgery in the management of a glaucoma patient: As this article is primarily about medical management of POAG, this section is small. While surgery is usually considered if the patient is progressing despite maximum medical therapy, socioeconomic and other considerations like age, disease status in both eyes, presence of visually disabling cataract and general health of the patient may dictate primary intervention. We may decide not to operate on an 80-year-old with advanced glaucoma in one eye and very early glaucoma in the other eye. The same holds true for a 45-year-old with terminal malignancy. The decision is tailored to the individual patient. Intervention is only undertaken after a detailed discussion of risks, benefits and patient preferences.
Normal tension glaucoma
Essentially, the approach and treatment is the same as POAG. We only use the term here because it is in common use.
Perform a ″careful″ gonioscopy to rule out primary angle closure.
Perform a CCT to rule out a ″garden variety″ POAG. This is especially true if the IOPs are in the high ″normal″ range.
Twenty-four hour DVT prior to any expensive or invasive investigations. IOP fluctuation of more than six to eight is suggestive of IOP-related risk.
Treatment is usually initiated with a PGA.
Certain NTG cases, those with unilateral disease, pallor of the disc, atypical defect and color defects require appropriate cardiovascular or neurological investigation.
Role of neuroprotection: Currently, there is no evidence for neuroprotection as an isolated strategy. Available options are calcium channel blockers and alpha-2 agonists. Their use could be restricted to those cases in which a strong link to vasospastic disease is present, thought to be the most important causative factor in those specific cases of NTG. They should be used with caution in combination with beta-blockers to avoid severe bradycardia. If a glaucoma patient is also on antihypertensives, we may request the physician to consider a calcium channel blocker for treatment. Alpha-2 agonists are currently prescribed primarily for their IOP lowering effects.
Primary angle closure glaucoma (PACG): The first line of management for chronic primary angle closure (PAC), PACG, is laser iridotomy. The details of other laser and surgical options for PACG are out of the scope of this manuscript, but a few important points are highlighted.
In general, PACG requires closer monitoring than POAG. Once iridotomy is done and angle is open in at least 180°, medical management should be same as POAG. If the angle does not open, consider other measures like laser iridoplasty.
Effect of PGA is inversely proportional to degree of closed angle. The effect of PGA in a totally closed angle is minimal.
If patient is on pilocarpine for whatever reason, the effect of PGA on IOP reduction is minimal and other medication (drugs which work on ciliary body) should be used.
Summary
POAG, a diagnosis of exclusion is usually a chronic, slowly progressive disease. At present, all resources are directed towards reduction of IOP, the only known causal and treatable risk factor for glaucoma, and medical management is frequently the first choice in most cases. The aim is to prevent any reduction in QOL from visual disability with minimum effects on QOL in terms of cost, side effects, treatment regime, follow- up schedules as well as socioeconomic burden.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–93. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster PJ, Johnson GJ. Glaucoma in China: How big is the problem? Br J Ophthalmol. 2001;85:1277–82. doi: 10.1136/bjo.85.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dandona L, Dandona R, Srinivas M, Mandal P, John RK, McCarty CA, et al. Open-angle glaucoma in an urban population in southern India: the Andhra Pradesh Eye Disease Study. Ophthalmology. 2000;107:1702–9. doi: 10.1016/s0161-6420(00)00275-x. [DOI] [PubMed] [Google Scholar]
- 4.Vijaya L, George R, Arvind H, Baskaran M, Paul PG, Ramesh SV, et al. Prevalence of angle-closure disease in a rural southern Indian population. Arch Ophthalmol. 2006;124:403–9. doi: 10.1001/archopht.124.3.403. [DOI] [PubMed] [Google Scholar]
- 5.Vijaya L, George R, Paul PG, Baskaran M, Arvind H, Raju P, et al. Prevalence of open-angle glaucoma in a rural south Indian population. Invest Ophthalmol Vis Sci. 2005;46:4461–7. doi: 10.1167/iovs.04-1529. [DOI] [PubMed] [Google Scholar]
- 6.Wilson MR, Martine JF. Epidemiology of chronic open-angle glaucoma. In: Ritch R, Shield MB, Krupin T, editors. The Glaucomas. 2nd ed. St. Louis: Mosby Yearbook Inc; 1996. [Google Scholar]
- 7.Werner EB. Normal tension glaucoma. In: Ritch R, Shield MB, Krupin T, editors. The glaucomas. 2nd ed. St. Louis: Mosby Yearbook Inc; 1996. p. 770. [Google Scholar]
- 8.Mardin CY, Horn FK, Jonas JB, Budde WM. Preperimetric glaucoma diagnosis by confocal scanning laser tomography of the optic disc. Br J Ophthalmol. 1999;83:299–304. doi: 10.1136/bjo.83.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–42. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allingham RR, Damji KF, Freedman S, Moroi SE, Shafranov G, Shields MB, editors. Shield′s Textbook of Glaucoma. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2005. p. 438. [Google Scholar]
- 11.Sommer A, Tielsch JM, Katz J, Quigley HA, Gottsch JD, Javitt J, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans: The Baltimore Eye Survey. Arch Ophthalmol. 1991;109:1090–5. doi: 10.1001/archopht.1991.01080080050026. [DOI] [PubMed] [Google Scholar]
- 12.Thomas R, Thomas S, Chandrashekar G. Gonioscopy. Indian J Ophthalmol. 1998;46:255–61. [PubMed] [Google Scholar]
- 13.Grave E, Wienereb R, editors. Glaucoma diagnosis: Structure and function. Hague, Netherlands: Kurger Publication; 2004. [Google Scholar]
- 14.Parikh RS, Parikh S, Chandra Sekhar G, Kumar RS, Prabakaran S, Ganesh Babu J, et al. Diagnostic capability of optical coherence tomography (stratus OCT 3) in early glaucoma. Ophthalmology. 2007;114:2238–43. doi: 10.1016/j.ophtha.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Sackett DL, Haynes RB, Guyatt GH, Tugwell P. A Basic Science for Clinical Medicine. New York: Little, Brown and co; 1991. Clinical Epidemiology; pp. 109–67. [Google Scholar]
- 16.Cioffi GA, Liebmann JM. Translating the OHTS results into clinical practice. J Glaucoma. 2002;11:375–7. doi: 10.1097/00061198-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E, et al. Factors for glaucoma progression and the effect of treatment:the early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 18.Ehlers N, Bramsen T, Sperling S. Applanation tonometry and central corneal thickness. Acta Ophthalmol (Copenh) 1975;53:34–43. doi: 10.1111/j.1755-3768.1975.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 19.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, et al. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–79. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 20.Feiner L, Piltz-Seymour JR. Collaborative Initial Glaucoma Treatment Study: A summary of results to date. Curr Opin Ophthalmol. 2003;14:106–11. doi: 10.1097/00055735-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Hodapp E, Parrish RK, 2nd, Anderson DR. St Louis: Mosby and Co; 1993. Clinical decisions in glaucoma; pp. 63–92. [Google Scholar]
- 22.Jampel HD. Target pressure in glaucoma therapy. J Glaucoma. 1997;6:133–8. [PubMed] [Google Scholar]
- 23.Schuman JS. Effects of systemic beta-blocker therapy on the efficacy and safety of topical brimonidine and timolol: Brimonidine Study Groups 1 and 2. Ophthalmology. 2000;107:1171–7. doi: 10.1016/s0161-6420(00)00081-6. [DOI] [PubMed] [Google Scholar]
- 24.Hodapp E, Parrish RK 2nd, Anderson DR, editors. Clinical decisions in glaucoma. St Louis: Mosby and Co; 1993. pp. 93–124. [Google Scholar]
- 25.Asrani S, Zeimer R, Wilensky J, Gieser D, Vitale S, Lindenmuth K. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma. 2000;9:134–42. doi: 10.1097/00061198-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Schulzer M. Errors in the diagnosis of visual field progression in normal tension glaucoma. Ophthalmology. 1994;101:1589–95. doi: 10.1016/s0161-6420(94)31133-x. [DOI] [PubMed] [Google Scholar]
- 27.Artes PH, Nicolela MT, LeBlanc RP, Chauhan BC. Visual field progression in glaucoma:total versus pattern deviation analyses. Invest Ophthalmol Vis Sci. 2005;46:4600–6. doi: 10.1167/iovs.05-0827. [DOI] [PubMed] [Google Scholar]
- 28.Chauhan BC, Blanchard JW, Hamilton DC, LeBlanc RP. Technique for detecting serial topographic changes in the optic disc and peripapillary retina using scanning laser tomography. Invest Ophthalmol Vis Sci. 2000;41:775–82. [PubMed] [Google Scholar]
- 29.Fechtner RD, Singh K. Maximal glaucoma therapy. J Glaucoma. 2001;10:S73–5. doi: 10.1097/00061198-200110001-00026. [DOI] [PubMed] [Google Scholar]
- 30.Asia Pacific Glaucoma Guidelines 2003-04. http://www.seagig.org/guidelines1.php.


