Abstract
Purpose:
To analyze the results of Descemet stripping and endothelial keratoplasty (DSEK) in the first consecutive 75 cases.
Materials and Methods:
Prospective, non-randomized, non-comparative interventional case series. Seventy- five eyes of 75 patients with endothelial dysfunctions of different etiology, scheduled for DSEK, were included in this study. Healthy donor cornea with a cell count of >2000 cells/sq mm was considered for transplantation in each case. Indications, operative problems and postoperative complications were noted. Best corrected visual acuity (BCVA), refractive and keratometric astigmatism, central corneal thickness (CCT) and endothelial cell density (ECD) were analyzed for each patient after a minimum follow-up of three months.
Results:
Main indication was pseudophakic corneal edema and bullous keratopathy in 53 (70.7%) eyes. Seventeen (22.7%) cases had moderate to severe Fuchs′ dystrophy with various grades of cataract; and DSEK was combined with manual small-incision cataract surgery (MSICS) with posterior chamber intraocular lens (PCIOL) in those cases. After three months, BCVA was 20/60 or better in 62 (82.7%) cases. Mean refractive and keratometric astigmatism were 1.10 ± 0.55 diopter cylinder (DCyl) and 1.24 ± 0.92 DCyl. The CCT and ECD were 670.8 ± 0.32 µm and 1485.6 ± 168.6/sq mm respectively. The mean endothelial cell loss after three months was 26.8 ± 4.24% (range: 13.3-38.4%). Dislocation of donor lenticule occurred in six (8.0%) eyes. Graft failure occurred in one case.
Conclusions:
Descemet stripping and endothelial keratoplasty is a safe and effective procedure in patients with endothelial dysfunctions with encouraging surgical and visual outcomes. It can be safely combined with MSICS with PCIOL in patients with moderate to severe Fuchs′ dystrophy with cataract.
Keywords: Descemet stripping and endothelial keratoplasty, endothelial dysfunctions, visual and surgical outcomes
Endothelial dysfunction from surgery or diseases is one of the leading indications for corneal transplantation. The only solution for this dysfunction over the past 100 years has been penetrating keratoplasty (PK).1 Penetrating keratoplasty is considered as the gold standard for treating corneal decompensation from endothelial dysfunctions but its disadvantages are well known.2 They are delayed rehabilitation, long visual recovery time, and poor visual quality due to high and irregular astigmatism. Furthermore, there are surface and suture-related problems, more chance of graft rejection or infection, greatest risk of trauma and wound dehiscence, frequent and long follow-up visits, and problems of long-term use of topical steroids.
In 1998, Melles and coworkers first described a new technique in human subjects and called it ′posterior lamellar keratoplasty′ or PLK.3 Terry and Ouslay performed a series of newly designed similar posterior lamellar transplantation surgery with technical modifications and termed them as ′deep lamellar endothelial keratoplasty′ or DLEK in 2000.4 All this work represents a radical change from the conventional PK technique in which endothelial replacement is performed without disturbing the recipient′s corneal surface.
Francis W Price further modified and simplified the technique in preparation of the recipient′s bed by stripping off the recipient Descemet membrane, now popularly called ′Descemet stripping and endothelial keratoplasty′ or DSEK.5 It has the advantage of being easier for the surgeon to perform than DLEK, and of providing a smoother interface on the recipient side of the visual axis.6 The other advantages of DSEK are faster visual recovery, better visual quality because of less astigmatism and no irregular astigmatism. Furthermore, there is no traumatic rupture of globe, no suture-related surface problems and less graft rejection or infection. It also minimizes the follow-up visits and use of corticosteroids. But it has its own disadvantages like donor dislocation (10-34.7%), higher iatrogenic primary graft failure, longer operating time (45- 60 min) and a steeper learning curve.7,8
The purpose of the present study was to evaluate and report the visual and surgical outcomes of this new technique of DSEK in the first 75 consecutive cases of endothelial dysfunction.
Materials and Methods
It was a prospective, non-randomized, non-comparative interventional surgical case series. The study was approved by the institutional review board, and a special informed written consent was taken from all patients prior to surgery. The first 75 consecutive patients (age ≥18 years with BCVA ≤20/200) with endothelial dysfunction who were enlisted for corneal grafting in our eye bank were included for DSEK. The exclusion criteria were corneal stromal scarring, irregular and deformed anterior chamber (AC), vitreous in AC, aphakia, gross peripheral anterior synechia (PAS), uncontrolled glaucoma, and gross posterior segment pathology detected by ultrasonography B-scan.
The donor prerequisites were healthy young donor tissue preferably below 60 years of age, endothelial count >2000 cells/ sq mm as determined by Eye bank specular microscope (HAI Laboratories, Inc., Lexington, MA, USA) and scleral rim 2 mm all around during in situ corneo-scleral button collection or preparation. Another optical quality donor cornea was always kept ready during the surgery for the initial few cases.
Steps of surgical procedure
All 75 surgeries were performed by a single surgeon (SKB) from July 2006 to September 2007. In each case, surgery was performed under conventional peribulbar anesthesia. Pupillary dilation was required only in cases where it was combined with manual small-incision cataract surgery (MSICS) with posterior chamber intraocular lens (PCIOL) implantation. In other cases, the pupil was constricted by instillation of three drops of 2% pilocarpine eye drops 30 min prior to surgery.
Donor lamellar dissection
Before starting the surgery, the donor cornea with its scleral rim was mounted on a disposable artificial AC (Katena Products, Inc., NJ, USA). The central point and 8.0 to 9.0 mm trephination site were marked with gentian violet. Initial 5 mm corneal incision was first made at the limbus with a 500-micron blade. Manual lamellar dissection was then carried out at approximately two-thirds depth with a crescent blade and innovative spatula by close method. After complete lamellar dissection of whole cornea, the donor tissue was transferred on a Teflon block with endothelial side up, filled up with McCaray-Kaufmann medium and covered for further preparation of appropriate size donor lenticule later on.
Recipient bed preparation
A circular template mark (with a diameter of 8.0, 8.5 or 9.0 mm) with gentian violet was made on the corneal epithelial surface which served as a reference mark for Descemet stripping. In some cases, loose edematous and hypertrophied epithelium was removed before marking.
After making a conjunctival flap and applying wet field cautery, a 5 to 5.5-mm sclero-corneal tunnel was prepared just like making a tunnel in MSICS.
Two 1-mm side-ports were made on either side of the main incision at 10 and 2 o′clock positions. These were to manipulate and unfold the donor lenticule.
Cohesive viscoelastic agent, 1% Sodium hyaluronate (Healon, Advanced Medical Optics, Inc, Uppsala, Sweden) was injected through the side port. No dispersive viscoelastic agent was used during any step of the procedure.
The AC was entered with a 2.8-mm angular keratome in such a way that the internal incision was at the template line. It was enlarged on either side to the same length as the external incision.
In patients who underwent a combined procedure (DSEK and MSICS with PCIOL) the surgery was performed through a 6.0-mm tunnel and irrigating vectis technique was used to remove the nucleus. After cortical cleaning a 6.0-mm single-piece PCIOL was placed in-the-bag. Every attempt was made to overlap the capsulorrhexis margin by 1 mm on the optic of the IOL. The IOL power was calculated from the spectacle history or from the biometry of the same or the other eye. The pupil was then constricted with intracameral pilocarpine (0.5%) injection.
Circular dissection of the Descemet membrane (or Descemet scoring) was carried out with a reverse Sinsky′s hook which corresponded to the 8.0 to 9.0 mm epithelial template mark. Descemet membrane was completely stripped off with the help of the hook, and the diseased tissue was removed. In some cases with severe corneal edema, trypan blue (0.06%) solution was used to stain the diseased endothelium and it was also useful to stain the anterior capsule in the combined procedure.
Viscoelastic agent was washed out thoroughly and carefully with balanced salt solution (BSS) using an irrigation/aspiration cannula and AC was then well formed with BSS.
Transplantation of donor lenticule
The posterior lamellar donor lenticule was punched from the endothelial side on the Teflon block using a same- size disposable trephine (8.0, 8.5 or 9.0 mm). It was then transferred on the recipient′s corneal surface with the endothelial side up.
The endothelial side was coated and protected by a thin layer of Healon. The posterior lamella of the donor tissue was then folded into an asymmetric ′taco-shape′, in a 60:40% ratio with a fine (0.12 mm) forceps grasping only the edge of the donor lenticule. This ′taco′ was gently held at the leading edge with an Utrata forceps with 60%-side up and inserted through the tunnel into the AC like a foldable IOL. The platforms of the Utrata forceps do not oppose thereby minimizing the crush injury to the donor endothelium.
The anterior chamber was filled up by BSS and one could notice the tissue unfolded to 60 to 80 degrees. Air was then injected carefully through a 30-gauze cannula from the left side-port to unfold the donor lenticule completely.
Centering of the donor lenticule was done by massaging over the cornea with an iris repositor or round cannula. More air was injected until a ′golden ring′ was observed around the edge of the donor lenticule. Interface fluid was removed by gentle massage and stroking the corneal epithelial surface with a flat cannula.
The microscope light was turned off and the eye was left undisturbed for a full 10 min.
After 10 min, 30-40% of the AC air bubble was replaced with BSS. The intraocular tension was checked digitally. The conjunctiva was closed by wet field cautery. Patient received sub-conjunctival injection of dexamethasone and gentamycin, and one drop of atropine was put at the end of the procedure. A bandage contact lens was given in selective cases when more than two-thirds of the recipient′s corneal epithelium was removed during the procedure.
The eye was patched and the patient was instructed to lie supine for at least 4 to 6 h.
The patients were discharged after 24 to 36 h. Postoperatively, the patients received topical antibiotics four times; timolol maleate (0.5%) twice; and cyclopentolate twice daily for the first 10 days. In addition, they received topical betamethasone eight times daily for one week and then six times daily for the next three weeks. After one month, the dose of topical betamethasone was gradually tapered over six months and finally discontinued. The same regimen was employed in patients with DSEK and manual SICS with PCIOL.
The patients were followed up at one week, one month and three months after surgery. Then they were asked to visit after six months and further yearly. All the patients who had completed the first three months′ follow-up were included in this report. Uncorrected visual acuity (UCVA), best corrected visual acuity (BCVA), refractive and keratometric astigmatism, and clinical specular microscopy (Topcon, SP 2000P, Japan) for corneal thickness (CCT) and endothelial cell density (ECD), were performed after three months in each case.
Best corrected visual acuity, refractive and keratometric astigmatism were tested by trained optometrists and they were masked in conducting the tests. Corneal thickness and specular microscopy were performed by a trained technician and he was also masked in conducting the test. He did not know the preoperative endothelial count of the donor cornea which was transplanted in a particular patient. The data were analyzed statistically using SPSS software (SPSS version 14.0, Chicago, IL). A P-value of <0.05 was considered statistically significant.
Results
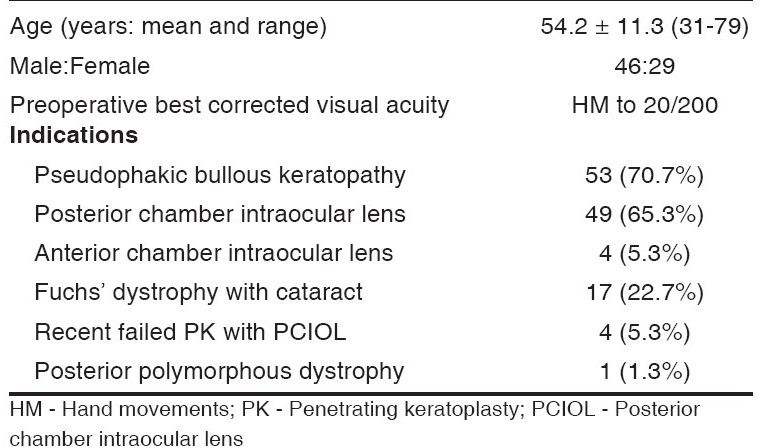
Seventy-five eyes of 75 consecutive patients included in this study were analyzed. There were 46 males and 29 females with a mean age of 54.2 ± 11.3 years (range: 31 to 79 years). All patients had clinically significant stromal edema, microcystic epithelial edema or frank bullous keratopathy. Eighteen eyes were phakic and 57 were pseudophakic. Fifty-eight (77.3%) of them had frank bullous keratopathy and 13 (17.3%) patients had epithelial hypertrophy. Seventeen (22.7%) of the patients had moderate to severe Fuchs′ dystrophy with different grades of cataract, and were treated by combined DSEK and MSICS with PCIOL. One young patient had posterior polymorphous dystrophy and was treated by DSEK alone. Preoperatively, in all cases, the BCVA in the affected eye was between hand movement (HM) to ≤20/200 [Table 1]. The preoperative refractive errors data were obtained from spectacle history in some cases, and the IOL power was calculated from the spectacle history or from the biometry of the same or other eye. The IOL power was selected to aim 0.5 D myopia as there is a hyperopic shift in ′DSEK triple procedure′ due to the increased thickness of the postoperative cornea.5,9
Table 1.
Patients' profile and preoperative data (n = 75)
The results in this series were highly encouraging in different types of endothelial dysfunctions - pseudophakic bullous keratopathy with PC IOL (Figs. 1A, 1B, 1C) as well as with anterior chamber intraocular lens (Figs. 2A, 2B, 2C and in combined cases in Fuchs′ dystrophy with cataract (Figs. 3A, 3B, 3C). After three months 62 (82.7%) patients regained 20/60 or better vision with small spherical and cylindrical corrections. The details of the results are shown in Table 2. There were some co-morbid conditions in eight (10.7%) cases, detected after the operation, like glaucomatous cupping in two cases, epiretinal membrane in two cases and age-related macular degeneration in four patients.
Figure 1A.
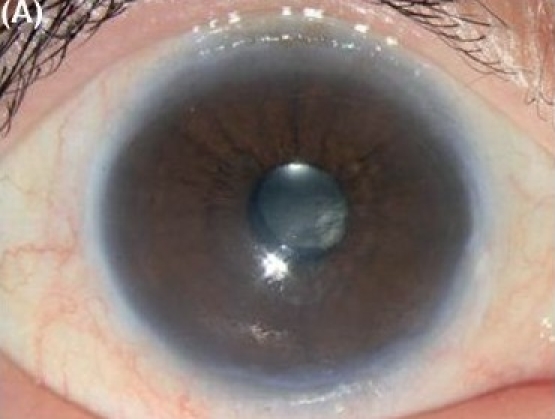
Descemet stripping endothelial keratoplasty in early pseudophakic bullous keratopathy. (A) Preoperative
Figure 1B.
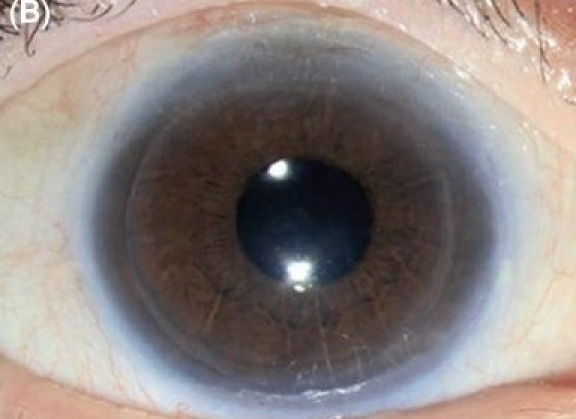
Descemet stripping endothelial keratoplasty in early pseudophakic bullous keratopathy. (B) Postoperative after three months (diffuse illumination)
Figure 1C.
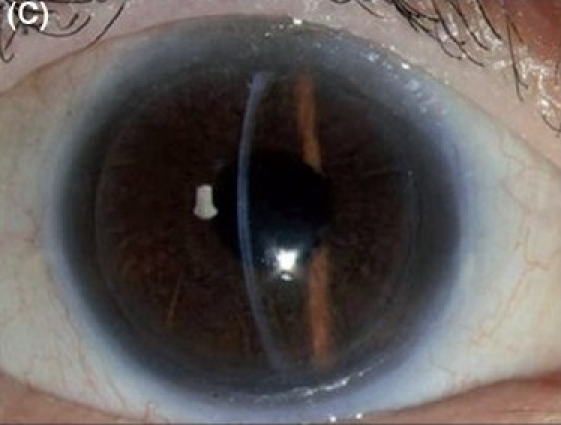
Descemet stripping endothelial keratoplasty in early pseudophakic bullous keratopathy. (C) Postoperative after three months (slit-beam illumination)
Figure 2A.
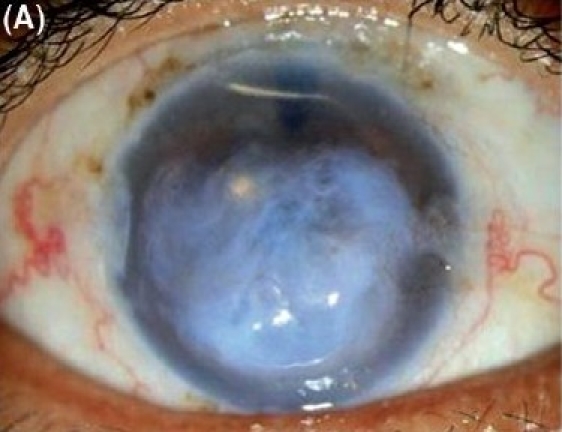
Descemet stripping endothelial keratoplasty in severe pseudophakic bullous keratopathy with epithelial hypertrophy in anterior chamber intraocular lens. (A) Preoperative.
Figure 2B.
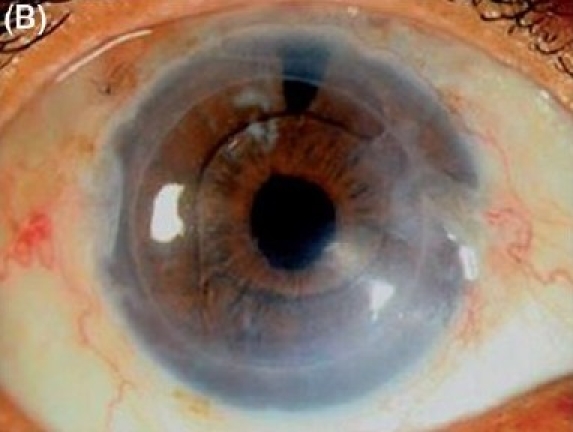
Descemet stripping endothelial keratoplasty in severe pseudophakic bullous keratopathy with epithelial hypertrophy in anterior chamber intraocular lens. (B) Postoperative after three months (diffuse illumination).
Figure 2C.
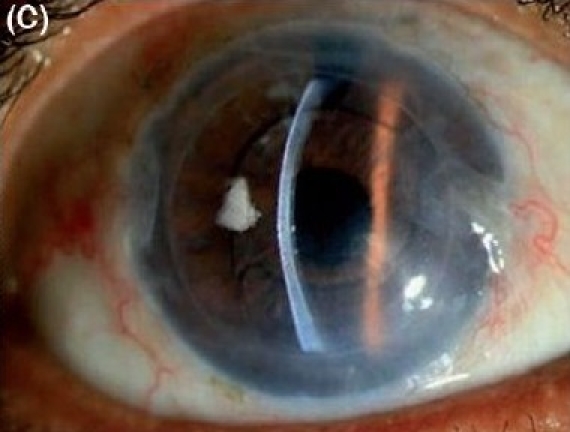
Descemet stripping endothelial keratoplasty in severe pseudophakic bullous keratopathy with epithelial hypertrophy in anterior chamber intraocular lens. (C) Postoperative after three months (slit-beam illumination)
Figure 3A.
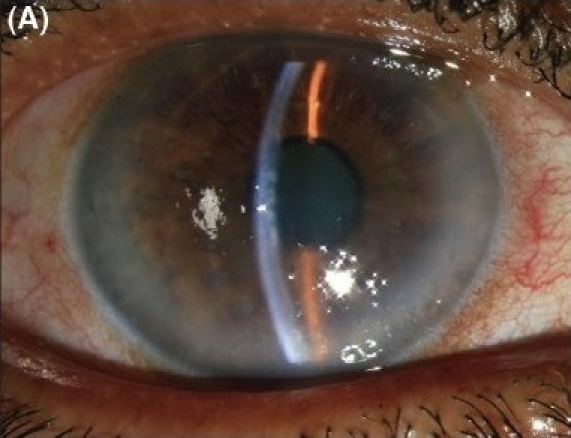
Combined Descemet stripping endothelial keratoplasty and manual small incision cataract surgery with posterior chamber intraocular lens implantation in severe Fuchs' dystrophy with cataract. (A) Preoperative
Figure 3B.
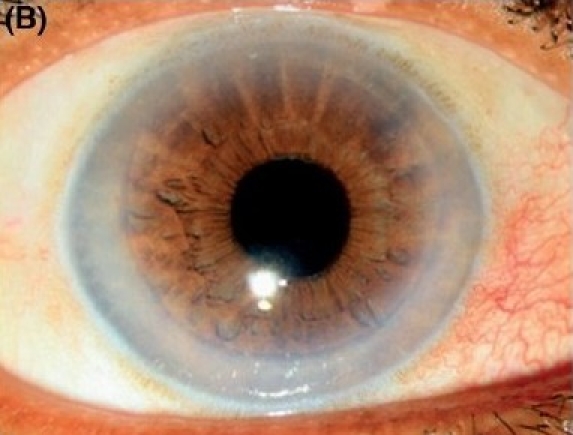
Combined Descemet stripping endothelial keratoplasty and manual small incision cataract surgery with posterior chamber intraocular lens implantation in severe Fuchs' dystrophy with cataract. (B) Postoperative after three months (diffuse illumination).
Figure 3C.
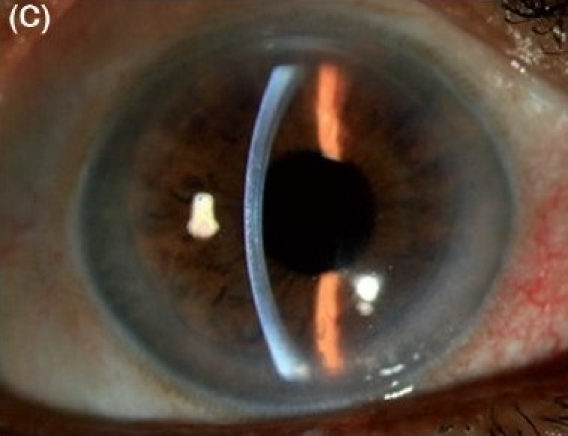
Combined Descemet stripping endothelial keratoplasty and manual small incision cataract surgery with posterior chamber intraocular lens implantation in severe Fuchs' dystrophy with cataract. (C) Postoperative after three months (slit-beam illumination)
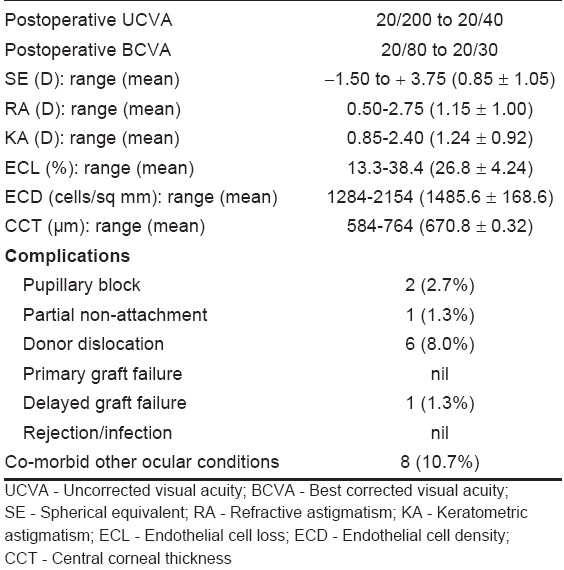
Table 2.
Overall visual and surgical results after three months (n = 75)
There was no problem during donor button preparation in the artificial AC except there was a little variation in the depth of dissection. Temporal approach was chosen in 14 (18.7%) cases because of scarring of the conjunctiva superiorly. The unfolding of the donor lenticule was difficult in 18 (24.0%) cases, but there was no occurrence of reverse unfolding (endothelial-side up against the recipient′s stroma). A 30-gauze reverse bent needle was used in 11 (14.7%) cases for proper centration of the donor lenticule. In three cases (4.0%), additional sutures (one or two with 10-0 monofilament nylon) were required to secure the tunnel.
The average spherical equivalent refractive error was 0.85 ± 0.95 D after three months. The mean refractive astigmatism and the mean keratometric astigmatism were 1.15 ± 1.0 DCyl and 1.24 ± 0.92 DCyl respectively. These changes were not statistically significant. The average CCT and ECD were 670.8 ± 0.32 µm and 1485.6 ± 168.6/sq mm after three months. The mean endothelial cell loss after three months was 26.8 ± 4.24% (range: 13.3-38.4%) which was statistically significant (P < 0.05).
There was no primary graft failure during the first three months. Pupillary block occurred in two cases on the first postoperative day and settled by full dilation of the pupil and head positioning. Partial non-attachment of graft occurred in one case which was resolved after three weeks. Donor dislocation occurred in six (8%) cases within 72 h of surgery and all were reattached after rebubbling, which was done on the same day on emergency basis. There was no graft rejection or infection within the follow-up period of three months. However, one patient developed graft failure after three months due to intractable secondary glaucoma.
Discussion
Descemet stripping and endothelial keratoplasty represents the most preferred type of posterior lamellar keratoplasty in recent times. It allows selective replacement of diseased host endothelium with a suitable and healthy donor posterior lamella. The major advantage of posterior lamellar or endothelial keratoplasy procedure in comparison to PK is that the normal corneal surface of the recipient is retained because of the avoidance of surface corneal incisions and sutures.3-5,10 This surgery may be manual (DSEK), automated (DSAEK) or Femto-second laser assisted (FS-DSEK) depending upon the method of preparation of the posterior lamellar donor lenticule.8,11 Among these, manual dissection in DSEK is the least expensive and may be the more appropriate procedure in the Indian scenario.
The UCVA in most of the cases in this series is better than conventional PK because of smooth ocular surface.12 The BCVA is comparable in most situations, but unlike with PK as mentioned in other studies, patients undergoing DSEK do not experience suture-induced astigmatism and surface- related problems.7,13,14 The regular refractive astigmatism is comparable with results described in other studies. In the present series, the mean refractive astigmatism was 1.65 ± 0.56 D. This astigmatism was 1.15 ± 1.35 D and 1.56 ± 0.46 D in other studies after three to six months.4,6 Like in other studies, all DSEK patients in this series received their final postoperative spectacle correction by three months. In contrast, PK patients typically undergo suture removal after one year and then receive final spectacle correction.6,7
Fortunately, there was no significant operative problem in this series. Only in cases of bullous keratopathy with ACIOL, the introduction of ′taco′ and subsequently unfolding of donor lenticule were little difficult compared to PCIOL cases. The following precautionary measures were taken in those cases. Before insertion of ′taco′, the AC must be well formed with BSS. The ′taco′ should not touch the IOL during insertion. During unfolding of the donor lenticule, BSS jet was used gently rather than by strong jet for gradual unfolding. Air was also used in a controlled manner to float the lenticule against host cornea for giving tamponade. Care was taken that injected air should not go posteriorly into the vitreous cavity.
Postoperatively, donor dislocation occurred in six cases (8.0%) within 72 h after surgery and all of them were successfully reattached by rebubbling immediately on diagnosis. This dislocation rate is the same or lower than other studies.7,8 One patient developed donor failure after three months due to uncontrollable steroid-induced secondary glaucoma. Other minor postoperative complications were similarly comparable with other reports and settled easily. The mean endothelial cell count after three months was 1485.6 ± 168.5 cell/sq mm and the endothelial cell loss was between 26.8 ± 4.24% (range: 13.3 to 38.4%) which is again comparable with other series.7,15
Interestingly, in the Western world, donor selection criteria are stricter than that in India: there the donor cell count must be ≥2500 cells/sq mm preoperatively.6 In India, as we do not get enough tissues with this kind of higher cell count, we kept our preoperative donor cell count criteria >2000 cells/sq mm. In this series, the donor used had an endothelial cell count range between 2086 to 2740 cells/sq mm (mean 2284.7 cells/sq mm).
Recently, 53 patients of this series have completed their six months′ follow-up. Now the cell loss is approximately 32.1% as compared to 26.8% after three months. This was again comparable to PK and also with Terry′s study.15,16 The patients are now comfortable and happy, and some patients with PK done earlier in the other eye are more happy and comfortable with their DSEK-operated eye.
Still, there are some questions to be addressed with this kind of surgery on a long-term basis, like interface problems, graft rejection rate (theoretically it should be less than PK, 7.5% in DSEK versus 13% in PK17), long-term endothelial cell loss in comparison to conventional PK, correct method to check intraocular pressure (as the corneal thickness is increased), effect of increased corneal thickness in relation to the angle of the AC, and cataract surgical method in phakic eyes in future. The techniques for DSEK are still evolving and will continue to offer new and exciting options for surgeons and patients and will address these questions in future.2
In conclusion, DSEK provides rapid visual recovery with minimum astigmatism for the patients with enthdothelial dysfunctions. It can be safely combined with MSICS with PCIOL in patients with moderate to severe Fuchs′ dystrophy with cataract. This technique maintains the structural integrity of the globe with preservation of excellent ocular surface.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Sugar A, Sugar J. Techniques in penetrating keratoplasty: A quarter century of development. Cornea. 2000;19:603–10. doi: 10.1097/00003226-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Patel SV. Keratoplasty for endothelial dysfunction. Ophthalmology. 2007;114:627–8. doi: 10.1016/j.ophtha.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Melles GR, Eggink FA, Lander F, Pels E, Rietveld FJ, Beekhuis WH, et al. A surgical technique for posterior lamellar keratoplasty. Cornea. 1998;17:618–26. doi: 10.1097/00003226-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Terry MA, Ouslay PJ. Endothelial replacement without surface corneal incisions or sutures: Topography of the deep lamellar endothelial keratoplasty procedure. Cornea. 2001;20:14–8. doi: 10.1097/00003226-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Price FW, Price MO. Descemet stripping with endothelial keratoplasty in 50 eyes: A refractive neutral corneal transplant. J Refract Surg. 2005;21:339–45. doi: 10.3928/1081-597X-20050701-07. [DOI] [PubMed] [Google Scholar]
- 6.Price FW, Price MO. Descemet′s stripping with endothelial keratoplasty in 200 eyes: Early challenges and techniques to enhance donor adherence. J Cataract Refract Surg. 2006;32:411–8. doi: 10.1016/j.jcrs.2005.12.078. [DOI] [PubMed] [Google Scholar]
- 7.Price MO, Price FW. Descemet′s stripping with endothelial keratoplasty. Curr Opin Ophthalmol. 2007;18:290–4. doi: 10.1097/ICU.0b013e3281a4775b. [DOI] [PubMed] [Google Scholar]
- 8.Koenig SB, Covert DJ. Early results of small-incision Descemet′s stripping and automated endothelial keratoplasty. Ophthalmology. 2007;114:221–6. doi: 10.1016/j.ophtha.2006.07.056. [DOI] [PubMed] [Google Scholar]
- 9.Koenig SB, Covert DJ, Dupps WJ., Jr Visual acuity, refractive error and endothelial cell density six month after Descemet stripping and automated endothelial keratoplasty (DSAEK) Cornea. 2007;26:670–4. doi: 10.1097/ICO.0b013e3180544902. [DOI] [PubMed] [Google Scholar]
- 10.Fogla R, Padmanabhan P. Initial results of small incision deep lamellar endothelial keratoplasty (DLEK) Am J Ophthalmol. 2006;141:346–51. doi: 10.1016/j.ajo.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Cheng YY, Pels E, Nuijts RM. Femtosecond-laser-assisted Descemet′s stripping endothelial keratoplasty. J Cataract Refract Surg. 2007;33:152–5. doi: 10.1016/j.jcrs.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 12.Panda A, Bageshwar LM, Ray M, Singh JP, Kumar A. Deep lamellar keratoplasty versus penetrating keratoplasty for corneal lesions. Cornea. 1999;18:172–5. doi: 10.1097/00003226-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Heidemann DG, Dunn SP, Chow CY. Comparison of deep lamellar endothelial keratoplasty and penetrating keratoplasty in patients with Fuchs endothelial dystrophy. Cornea. 2008;27:161–7. doi: 10.1097/ICO.0b013e31815b8304. [DOI] [PubMed] [Google Scholar]
- 14.Christo CG, van Rooij J, Geerards AJ, Remeijer L, Beekhuis WH. Suture-related complications following keratoplasty: A 5-year retrospective study. Cornea. 2001;20:816–9. doi: 10.1097/00003226-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 15.van Dooren BT, Mulder PG, Nieuwendaal CP, Beekhuis WH, Melles GR. Endothelial cell density after posterior lamellar keratoplasty: Five to seven year follow-up. Am J Ophthalmol. 2007;144:471–3. doi: 10.1016/j.ajo.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Terry MA, Wall J, Hoar KL, Ousley PJ. A prospective study of endothelial cell loss during the 2 years after deep lamellar endothelial keratoplasty. Ophthalmology. 2007;114:631–9. doi: 10.1016/j.ophtha.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 17.Allan BD, Terry MA, Price FW, Jr, Price MO, Griffin NB, Claesson M. Corneal transplant rejection rate and severity after endothelial keratoplasty. Cornea. 2007;26:1039–42. doi: 10.1097/ICO.0b013e31812f66e5. [DOI] [PubMed] [Google Scholar]


