Abstract
Background:
Pterygia are common, benign, fibrovascular, and infiltrative processes of the corneo- conjunctival junction of unknown pathogenesis. Cyclooxygenase-2 (COX-2) mediates the rate-limiting step in arachidonic acid metabolism. Extensive evidence indicates that the COX-2 prostanoid pathway is involved in inflammation. The aim of the study was to document the immunohistochemical expression of COX-2 in primary and recurrent pterygia.
Materials and Methods:
In this study, 21 primary pterygia and 12 recurrent pterygia from subjects undergoing pterygium surgery and six normal corneal-scleral tissue specimens were studied immunohistochemically for COX-2 expression.
Results:
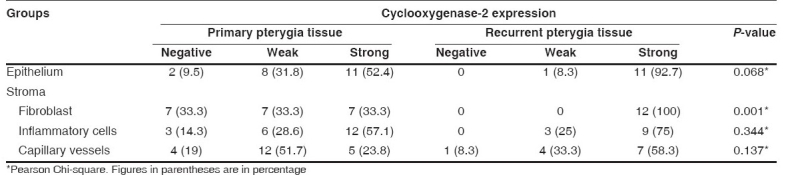
COX-2 was expressed in primary pterygia and recurrent pterygia specimens. There was a statistically significant difference in COX-2 expressions in fibroblasts between primary and recurrent pterygium cases (P = 0.001). There were statistically significant differences in COX-2 expressions in surface epithelium (P = 0.028) and stromal inflammatory cells (P=0.000) between control tissues and primary pterygia tissues. We also detected statistically significant differences in COX-2 expressions in surface epithelium (P=0.000), stromal fibroblasts P=0.000 (stromal fibroblasts and inflammatory cells), vessels (P = 0.027) and inflammatory cells (P=0.001) between control tissues and recurrent pterygia tissues.
Conclusions:
This is the first study to document the expression of COX-2 in primary and recurrent pterygia. In our opinion after excision of pterygia, fibroblastic proliferation continues and this contributes to recurrence.
Keywords: Cyclooxygenase-2, immunohistochemistry, primary pterygium, recurrent pterygium
Pterygia are common, frequently recurring ocular surface lesions characterized by tissue remodeling, cellular proliferation, angiogenesis, and inflammation.1,2 Irritation of th eye by ultraviolet (UV) light in sunny, dry, dusty areas and repeated microtrauma can lead to development of pterygium in susceptible individuals.2 Pterygia are histopathologically fibrovascular proliferations. This expansiveness of fibrovascular proliferation is considered as a convenient morphological index for evaluation of recurrence risk after surgical treatment.1-3 Pterygia usually recur after surgical excision.4 The cause of recurrence may be related to several factors including environmental factors. If etiological and pathogenic differences between recurrent and non-recurrent cases are discovered, the type of treatment can be more easily selected and convenient treatment can be found.1-6
Histopathological findings reveal epithelial hyperplasia and exuberant fibrovascular tissue in the stroma of pterygia.6 Importantly, the extent of fibrovascular proliferation in the stroma has been used as a reliable morphological index for predicting pterygium recurrence following primary excision.7-10 Cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) mediate the rate-limiting step in arachidonic acid metabolism.11 Expression of COX-2 mRNA and protein is often enhanced in various human cell types by inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α).11 Both isoforms of cyclooxygenase, constitutive COX-1 and inducible COX-2, catalyze the production of prostanoids from arachidonic acid.11 COX-2-induced production of prostanoids is often implicated in inflammatory diseases, characterized by edema and tissue injury due to the release of many inflammatory cytokines and chemotactic factors, prostanoids, leukotrienes, and phospholipase.11,12
COX-2 expression is induced by various stimuli, and the over-expression is closely related to the pathogenesis of some degenerative diseases including cancer.13 Several lines of evidence indicate that COX-2 over-expression can be a causal factor for tumor growth and metastasis. Over-expression of COX-2 in vitro promotes cell proliferation in human prostate cancer cells and enhances invasiveness of human bladder, breast and colon cancer cells.14-17 Moreover, the findings regarding the effects of COX-2 in cutaneous tumor formation have also been reported in pterygium, including disruption of apoptosis, limbal epithelial proliferation, abnormal p53 gene expression, and upregulation of basic fibroblast growth factor, vascular endothelial growth factor, and nitric oxide synthase.8,18-21
The aim of the study was to find out the relationship between COX-2 expression and primary as well as recurrent pterygium.
Materials and Methods
Resected pterygium tissue samples from 33 patients of 21 with primary pterygium, 12 with recurrent pterygium and six normal corneo-scleral tissues were included in this study. Primary and recurrent pterygium samples were selected retrospectively from archival material of the department of pathology, Suleyman Demirel University School of Medicine. All pterygia were sectioned along the longitudinal axis to include from the cap (leading edge) to the basal (body) region. Normal corneo-scleral tissues were obtained during forensic autopsy cases with attorney′s permission.
Immunohistochemical analysis for COX-2 was performed on formalin-fixed, paraffin-embedded archival tissue using the streptavidin-biotin-peroxidase technique. For all cases, 4 µm histological section was deparaffinized in xylene and dehydrated in descending dilution of ethanol. For the antigen retrieval, slides were treated by microwave heating in citrate buffer (pH 6.0) for 10 min. Endogenous peroxidase activity was blocked by 20 min of incubation with 0.3% hydrogen peroxidase. Slides were tested with COX-2 antibody (1:100 Epitope specific rabbit antibody, Lab Vision). Sections were tested with streptavidin-biotin-peroxidase kit (Ultra Vision Large Volume Detection System Anti-polyvalent, HRP, Lab Vision, USA), and after incubation the reaction product was detected using diaminobenzidine (DAB). Finally, the sections were counterstained with Mayer′s hematoxylin, and mounted with mounting medium. Tissue of colon cancer from a human served as the positive control in the COX-2 immunostaining.
Two observers analyzed the staining for COX-2. Immunohistochemical analysis of the primary and recurrent pterygium was performed with anti-COX-2 antibody. The mean overall intensity of the immunostaining in the surface epithelium and stromal cells was scored in the analyzed sections of primary and recurrent pterygium samples as follows: 0 = absent immunostaining; 1 = weak immunostaining (few cells being positive focal or scattered); 2 = strong immunostaining (diffuse staining throughout the tissue). The histology was checked using slides stained with Hematoxylin Eosin.
For statistical evaluation, the SPSS software version 13.0 was used. The Fisher exact and chi-square tests were used to analyze the distribution of COX -2 positive cases according to several histopathological features. P-value < 0.05 was considered as significant.
Results
Our group consisted of 23 (59%) men and 16 (41%) women. Ages of the patients ranged from 36 to 80 (median 54 years). Eleven of 21 primary pterygium cases were males and 10 of them were females. Eight of the recurrent pterygium cases were males and four were females. Four of the six healthy conjunctiva cases were males and two of them were females.
COX-2 immunostaining was observed mainly in the cytoplasm of surface epithelium, stromal inflammatory cells, capillary vessels and fibroblasts. There were statistically significant differences in COX-2 expressions in the surface epithelium (P = 0.028) and stromal inflammatory cells (P=0.000) between control tissues and primary pterygia tissues.
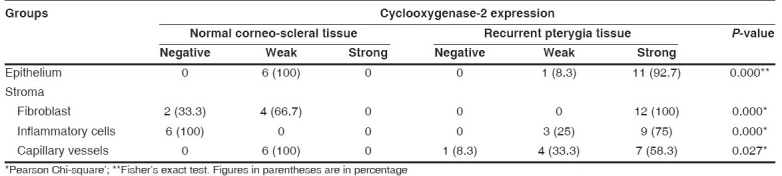
We also detected statistically significant differences in COX-2 expressions in surface epithelium (P=0.000), stromal fibroblasts P=0.000 (stromal fibroblasts and inflammatory cells), vessels (P = 0.027) and inflammatory cells (P=0.001) between control tissues and recurrent pterygia tissues.
Statistical significance was not observed in epithelial staining of primary and recurrent pterygium cases (P = 0.06). In 14 primary pterygium cases and in all recurrent pterygium cases, COX-2 expressions were detected in fibroblasts. In all the recurrent pterygium cases COX-2 showed strong expression. There was a statistically significant difference in COX-2 expression in fibroblasts between primary and recurrent pterygium cases (P = 0.001).
COX-2 expressions in both epithelium and stroma of normal corneo-scleral tissue, primary and recurrent pterygia are shown in Figs. 1-3. The relation between COX-2 expressions in epithelium and stroma of normal corneo-scleral tissue, primary and recurrent pterygia are shown in Tables 1-Table 3.
Figure 1.
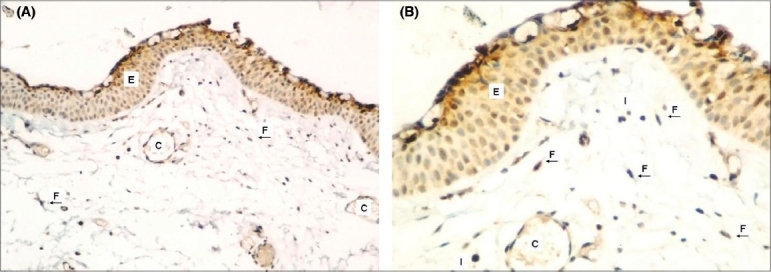
Weak COX-2 expression in epithelium and stroma of normal corneo-scleral tissue (A) (COX- X100), (B) (COX-2X200)
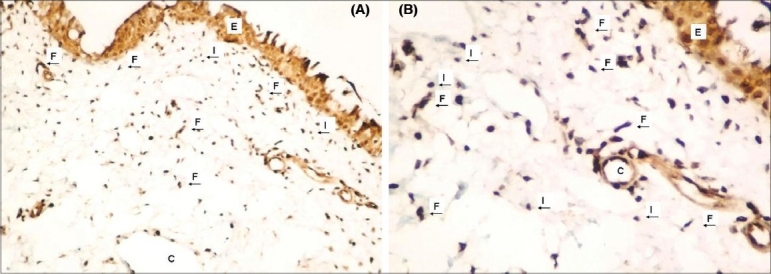
Figure 3.
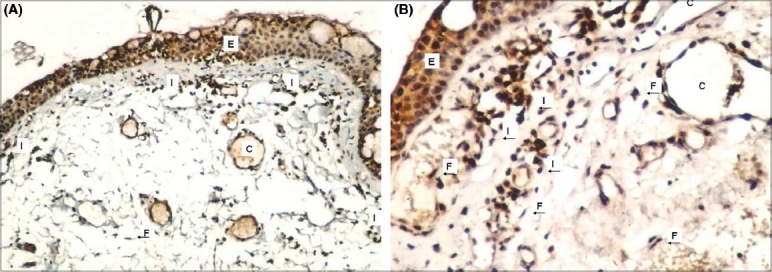
Strong COX-2 expression in epithelium and stroma of recurrent pterygium tissue (A) (COX-2X100), (B) (COX-2X200) Epithelium (E), fibroblasts (F), capillaries (C) and infl ammatory cells (I) were shown in figures with arrows
Table 1.
Cyclooxygenase-2 expressions in epithelium and stroma of normal corneo-scleral tissue and primary pterygia
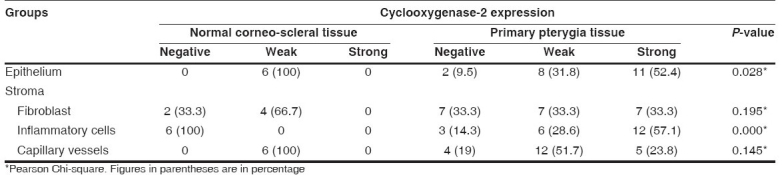
Table 3.
Cyclooxygenase-2 expressions in epithelium and stroma of primary and recurrent pterygia
Discussion
Pterygium has long been considered to be a chronic degenerative condition. However, after over-expression of the p53 protein was found in the epithelium of pterygium, some researchers began to feel that pterygium was an ultraviolet (UV)-related tumor rather than a degenerative disease.18,19
Although the pathogenesis of pterygia is still poorly understood, epidemiologic evidence suggests that environmental stress may have a role.1,3 Recently, several cytokines such as TNF-α, fibroblast growth factor (FGF), and transforming growth factor-β (TGF-β), have been localized to both resident and inflammatory cells in pterygia.2,8 Kria et al.,8 recently reported that pterygium fibroblasts express potent fibroangiogenic factors such as basic fibroblast growth factor (β-FGF), platelet derived growth factor (PDGF), TGF-β and TNF-α, suggesting that they may have a possible role in pterygium pathogenesis.
COX-2 is an inducible immediate-early gene which is up- regulated by various stimuli, including mitogens, cytokines, growth factors, and tumor promoters.11 Extensive evidence indicates that COX-2 prostanoid pathway is involved in inflammation.12 COX-2 modulates angiogenesis by increasing the production of angiogenic factors, such as vascular endothelial growth factor.11,12 Chiang et al.,22 investigated the expression of COX-2 in pterygium and found 75 (83.3%) specimens stained positive for COX-2 in the pterygium group. The staining was limited to the cytoplasm of the epithelial layer and predominantly over the basal epithelial layer. No substantial staining was visible in the subepithelial fibrovascular layers. All specimens were negative in the normal conjunctiva and limbus group. In our study we detected COX-2 expression both in normal corneo-scleral tissues, primary pterygia and recurrent pterygia in superficial epithelium and also in stromal cells with immunohistochemical methods. We also found that COX-2 expressions were significantly more intense in stromal fibroblasts of recurrent pterygia compared to primary pterygia. This finding manifests that fibroblastic proliferation is more developed in recurrent pterygia compared to primary pterygia.
Also, in all the recurrent pterygia there were COX-2 expressions detected in stromal inflammatory cells. Although we observed that COX-2 expression is more excessive in recurrent pterygia compared to primary pterygia, this was not statistically significant. It may be due to our small series of recurrent pterygia.
Pterygium is now considered to be a result of uncontrolled cellular proliferation, similar to that in tumors, in which there is damage to cellular regulation and control of the cell cycle.23 Mitogenity, construction of a new vascular net and remodeling of the extracellular matrix were observed in pterygia. Altogether they create a new vascular and fibrotic tissue, which has an aggressive way of growing to and over the cornea.24
Changes in the proteoglycans are known to induce edematous changes, which in turn cause fibroblast proliferation. It is not yet clear how the tissue alterations are initiated and kept in progress.25,26 Controversial factors include the mutation of limbus stem cells, modification of stromal fibroblasts, and immunological and other processes in the matrix. In new models of pterygial pathogenesis, interactions between epithelial cells, fibroblasts, and matrix are considered as well as the denaturing action of the matrix metalloproteinases.27
The management of pterygia is still a challenge for the ophthalmologist. Many surgical procedures have been proposed for pterygium removal.28 However, surgery alone is not sufficient and cannot prevent a high incidence of recurrence.28 There are theories that the causes considered as etiological agents in recurrence development in pterygia are inflammations besides UV-rays and other environmental factors.2 In our opinion after excision of pterygia, fibroblastic proliferation continues and this contributes to recurrence.
However if a fibroblastic cell invasion of the cornea is considered to be the essential pathology, then a recurrence could be ascribed to the same cause, namely, an accelerated reinvasion of the cornea. As Cameron29 indicated, the recurrence would appear to be due to an accelerated fibroblastic proliferation produced by the trauma of operation. In our opinion fibroblastic proliferation explains the clinical appearance and behavior of a recurrent pterygium. As a result, in the light of these findings, we think that after pterygium excision, selective COX-2 inhibitors may be helpful to prevent recurrence.
Figure 2.
Moderate COX-2 expression in epithelium and stroma of primary pterygium tissue (A) (COX-2X100), (B) (COX-2X200)
Table 2.
Cyclooxygenase-2 expressions in epithelium and stroma of normal corneo-scleral tissue and recurrent pterygia
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Coroneo MT, Girolamo N, Wakefield D. The pathogenesis of pterygia. Curr Opin Ophthalmol. 1999;10:282–8. doi: 10.1097/00055735-199908000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Hill JC, Maske R. Pathogenesis of pterygium. Eye. 1989;3:218–26. doi: 10.1038/eye.1989.31. [DOI] [PubMed] [Google Scholar]
- 3.Degrassi M, Piantanida A, Nucci P. Unexpected histological findings in pterygium. Optom Vis Sci. 1993;70:1058–60. doi: 10.1097/00006324-199312000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Avisar R, Arnon A, Avisar E. Primary pterygium recurrence time. Isr Med Assoc J. 2001;3:836–7. [PubMed] [Google Scholar]
- 5.Marvovich A, Morad Y, Sandbank J. Angiogenesis in pterygium: Morphometric and immunohistochemical study. Curr Eye Res. 2002;25:17–22. doi: 10.1076/ceyr.25.1.17.9959. [DOI] [PubMed] [Google Scholar]
- 6.Tan D, Chee SP, Dear K, Lim AS. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol. 1997;115:1235–40. doi: 10.1001/archopht.1997.01100160405001. [DOI] [PubMed] [Google Scholar]
- 7.Touhami A, Di Pascuale MA, Kawatika T, Del Valle M, Rosa RH, Jr, Dubovy S al, et al. Characterisation of myofibroblasts in fibrovascular tissues of primary and recurrent pterygia. Br J Ophthalmol. 2005;89:269–74. doi: 10.1136/bjo.2004.050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kria L, Ohira A, Amemiya T. Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor and tumor necrosis factor in a primary pterygium. Acta Histochem. 1996;98:195–201. doi: 10.1016/s0065-1281(96)80038-9. [DOI] [PubMed] [Google Scholar]
- 9.Di Girolamo N, Coreneo MT, Wakefield D. UVB-Elicited induction of MMP-1 expression in human ocular surface epithelial cells is mediated through the ERK1/2 MAPK- dependent pathway. Invest Ophthalmol Vis Sci. 2003;44:4705–14. doi: 10.1167/iovs.03-0356. [DOI] [PubMed] [Google Scholar]
- 10.Marcovich A, Morad Y, Sandbank J. Angiogenesis in pterygium: Morphometric and immunohistochemical study. Curr Eye Res. 2002;25:17–22. doi: 10.1076/ceyr.25.1.17.9959. [DOI] [PubMed] [Google Scholar]
- 11.Williams CS, Mann M, DuBois RN. The role of cyclooxyigenases in inflammation, cancer and development. Oncogene. 1999;18:7908–16. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 12.Kuwano T, Nakao S, Yamamoto H, Tsuneyoshi M. Cyclooxygenase 2 is a key enzyme for inflammatory cytokine-induced angiogenesis. FASEB J. 2004;18:300–10. doi: 10.1096/fj.03-0473com. [DOI] [PubMed] [Google Scholar]
- 13.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–60. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 14.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336–40. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujita H, Koshida K, Keller ET, Takahashi Y, Yoshimito T, Namiki M, et al. Cyclooxygenase-2 promotes prostate cancer progression. Prostate. 2002;53:232–40. doi: 10.1002/pros.10152. [DOI] [PubMed] [Google Scholar]
- 16.Li G, Yang T, Yan J. Cyclooxygenase-2 increased the angiogenic and metastatic potential of tumor cells. Biochem Biophys Res Commun. 2002;299:886–90. doi: 10.1016/s0006-291x(02)02707-9. [DOI] [PubMed] [Google Scholar]
- 17.Singh B, Berry JA, Shoher A, Ramakrishnan V, Lucci A. COX-2 overexpression increases motility and invasion of breast cancer cells. Int J Oncol. 2005;26:1393–9. [PubMed] [Google Scholar]
- 18.Tan DT, Lim AS, Goh HS, Smith DR. Abnormal expression of the p53 tumor suppressor gene in the conjunctiva of patients with pterygium. Am J Ophthalmol. 1997;123:404–5. doi: 10.1016/s0002-9394(14)70141-2. [DOI] [PubMed] [Google Scholar]
- 19.Tan DT, Tang WY, Liu YP, Goh HS, Smith DR. Apoptosis and apoptosis related gene expression in normal conjunctiva and pterygium. Br J Ophthalmol. 2000;84:212–6. doi: 10.1136/bjo.84.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dushku N, John MK, Schultz GS, Reid TW. Pterygia pathogenesis: Corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol. 2001;119:695–706. doi: 10.1001/archopht.119.5.695. [DOI] [PubMed] [Google Scholar]
- 21.Lee DH, Cho HJ, Kim JT, Choi JS, Joo CK. Expression of vascular endothelial growth factor and inducible nitric oxide synthase in pterygia. Cornea. 2001;20:738–42. doi: 10.1097/00003226-200110000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Chiang CC, Cheng YW, Lin CL, Lee H, Tsai FJ, Tseng SH, Tsai YY. Cyclooxygenase 2 expression in pterygium. Mol Vis. 2007;13:635–8. [PMC free article] [PubMed] [Google Scholar]
- 23.Perra MT, Maxia C, Corbu A, Minerba L, Demurtas P, Colombari R, et al. Oxidative stress in pterygium: Relationship between p53 and 8-hydroxydeoxyguanosine. Mol Vis. 2006;12:1136–42. [PubMed] [Google Scholar]
- 24.Solomon AS. Pterygium. Br J Ophthalmol. 2006;90:665–6. doi: 10.1136/bjo.2006.091413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakoonwatanyoo P, Tan DT, Smith DR. Expression of p63 in pterygium and normal conjunctiva. Cornea. 2004;23:67–70. doi: 10.1097/00003226-200401000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Saw SM, Tan D. Pterygium: Prevalence, demography and risk factors. Ophthalmic Epidemiol. 1999;6:219–28. doi: 10.1076/opep.6.3.219.1504. [DOI] [PubMed] [Google Scholar]
- 27.Seifert P, Eckert J, Spitznas M. Topological-histological investigation of the pterygium. Graefes Arch Clin Exp Ophthalmol. 2001;239:288–93. doi: 10.1007/s004170100262. [DOI] [PubMed] [Google Scholar]
- 28.Hirst LW. Treatment of pterygium. Aust N Z J Ophthalmol. 1998;26:269–70. doi: 10.1111/j.1442-9071.1998.tb01328.x. [DOI] [PubMed] [Google Scholar]
- 29.Cameron ME. Histology of pterygium: An electron microscopic study. Br J Ophthalmol. 1983;67:604–8. doi: 10.1136/bjo.67.9.604. [DOI] [PMC free article] [PubMed] [Google Scholar]


