Abstract
Objective
We sought to describe a method that explicitly considers both a health-care programme’s cost-effectiveness and its affordability. For illustration, we apply the method to the programme to vaccinate infants against hepatitis B in the Gambia.
Methods
We synthesized selected data and developed a computer-based model from the societal and payer perspectives to evaluate the cost-effectiveness of routine infant vaccination against hepatitis B in the Gambia compared with no vaccination. The primary outcome measure was cost per averted disability-adjusted life year (DALY), which was expressed in 2002 US dollars. We used Monte Carlo methods for uncertainty analysis to examine the affordability of the programme from the payer’s perspective, and we derived an affordability curve and cost-effectiveness affordability curves for the programme.
Findings
In the Gambia, vaccinating infants against hepatitis B is highly cost-effective. Compared with offering no intervention, the vaccination programme would cost US$ 28 per DALY averted from the societal perspective or US$ 47 per DALY averted from the payer’s perspective. The programme also has the potential to be affordable, starting at a relatively low budget of US$ 160 000 per year. Combining the two dimensions of the outcome measure, the probability that vaccinating infants would be both cost-effective and affordable is 40% at an annual programme budget of US$ 182 000 (the estimated total programme cost from the payer’s perspective), given a threshold cost-effectiveness value of US$ 47 per DALY averted.
Conclusion
In the face of uncertainties about both the health and economic consequences of a vaccine programme, as well as the availability and magnitude of resources needed to fund the programme, cost-effectiveness affordability curves can provide information to decision-makers about the probability that a programme will be both cost-effective and affordable: these are distinct but equally relevant considerations in resource-poor settings.
Résumé
Objectif
Nous avons recherché une méthode permettant de prendre en compte explicitement à la fois le rapport coût/efficacité d’un programme et son accessibilité économique. A titre illustratif, nous avons appliqué cette méthode au programme de vaccination des nourrissons contre l’hépatite B en Gambie.
Méthodes
Nous avons fait la synthèse de données sélectionnées et mis au point un modèle informatique pour évaluer le rapport coût/efficacité pour la société d’une part et pour ceux qui financent le programme d’autre part de la vaccination systématique des nourrissons contre l’hépatite B en Gambie par rapport à l’absence de vaccination. La principale mesure des résultats programmatiques est le coût par année de vie corrigée de l’incapacité évitée (DALY), exprimé en US$ de 2002. Dans le cadre de l’analyse d’incertitude, nous avons étudié l’accessibilité économique du programme pour ceux qui le financent par des méthodes de Monte Carlo, ce qui nous a permis d’établir une courbe d’accessibilité économique et des courbes rapport coût/efficacité en fonction de l’accessibilité économique pour ce programme.
Résultats
En Gambie, le rapport coût/efficacité de la vaccination des nourrissons contre l’hépatite B est très bon. Par comparaison avec la situation en l’absence d’intervention, ce programme vaccinal devrait coûter US$ 28 par DALY évitée pour la société ou US$ 47 par DALY évitée pour ceux qui le financent. Ce programme pourrait aussi être abordable économiquement en débutant avec un budget relativement faible de US$ 160 000 par an. Si l’on combine ces deux dimensions de la mesure de résultats, la probabilité que cette vaccination des nourrissons soit à la fois d’un bon rapport coût/efficacité et abordable économiquement est de 40% pour un budget annuel du programme de US$ 182 000 (coût total estimé du programme pour ceux qui le financent), sachant que la valeur seuil du rapport coût/efficacité est de US$ 47 par DALY évitée.
Conclusion
Face aux incertitudes quant aux conséquences tant sanitaires qu’économiques d’un programme de vaccination et quant à la disponibilité et à l’ampleur des ressources nécessaires pour financer ce programme, les présentes courbes coût/efficacité fonction de l’accessibilité économique peuvent fournir aux décideurs des indications sur la probabilité qu’il soit à la fois d’un bon rapport coût/efficacité et abordable, ces deux aspects étant distincts, mais tout aussi pertinents l’un que l’autre dans les pays à faible revenu.
Resumen
Objetivo
Describir un método que considerase explícitamente tanto la costoeficacia de un programa de atención sanitaria como los recursos disponibles para llevarlo a cabo. A modo de ejemplo, se aplicó el método al programa de vacunación de los lactantes contra la hepatitis B en Gambia.
Métodos
Se sintetizaron datos seleccionados y se elaboró un modelo computadorizado desde las perspectivas de la sociedad y del pagador para evaluar la costoeficacia de la vacunación sistemática de los lactantes contra la hepatitis B en Gambia en comparación con la ausencia de vacunación. La medida de resultado principal fue el costo por año de vida ajustado en función de la discapacidad (AVAD) evitado, que se expresó en US$ de 2002. Se utilizaron métodos de Monte Carlo de análisis de incertidumbre para examinar la capacidad de pago del programa desde la perspectiva del pagador, y se obtuvieron una curva de la capacidad de pago y curvas de costoeficacia/capacidad de pago para el programa.
Resultados
En Gambia, la vacunación de los lactantes contra la hepatitis B es muy costoeficaz. En comparación con la no intervención, el programa de vacunación costaría US$ 28 por AVAD evitado desde el punto de vista de la sociedad o US$ 47 por AVAD evitado desde la perspectiva del pagador. El programa puede también ser asequible, comenzando por un presupuesto relativamente bajo de US$ 160 000 al año. Combinando las dos dimensiones de la medida de resultados, la probabilidad de que la vacunación de los lactantes sea tanto costoeficaz como asequible es del 40% para un presupuesto programático anual de US$ 182 000 (costo total estimado del programa desde la perspectiva del pagador), considerando un valor de costoeficacia umbral de US$ 47 por AVAD evitado.
Conclusión
Ante las incertidumbres asociadas a las consecuencias sanitarias y económicas de un programa de vacunación, así como a la disponibilidad y magnitud de los recursos necesarios para financiar el programa, las curvas de costoeficacia/capacidad de pago pueden aportar a los responsables de la toma de decisiones información de interés sobre la probabilidad de que un programa sea tanto costoeficaz como asequible, conceptos éstos distintos pero igualmente pertinentes en los entornos de recursos escasos.
ملخص
الەدف
بحثنا عن وصف طريقة تأخذ باعتبارەا وبشكل صريح كلاً من فعَّالية برامج الرعاية الصحية لقاء التكاليف والقدرة على دفع التكاليف. وللتوضيح، طبقنا الطريقة على برنامج لتلقيح الأطفال ضد التەاب الكبد البائي في غامبيا.
الطريقة
قمنا بإنشاء المعطيات المنتقاة مع إعداد نموذج يستند على الحاسوب من حيث الجوانب الاجتماعية والدافعين للتكاليف، وذلك لتقيـيم الفعالية لقاء التكاليف للتلقيح الروتيني للرضَّع ضد التەاب الكبد البائي في غامبيا ومقارنتە مع عدم التلقيح. وقد كانت حصيلة القياس الرئيسية التكاليف لكل سنة من سنوات العمر المصحَّحة باحتساب مدد العجز التي يمكن توقِّيەا، ومعبِّراً عنەا بالدولارات الأمريكية وفق عام 2002. وقد استخدمنا طريقة مونت كارلو لتحليل الارتياب لدراسة القدرة على دفع تكاليف البرنامج من وجەة نظر الدافعين للتكاليف؛ وتوصلنا إلى منحنيات للقدرة على دفع التكاليف والفعالية لقاء التكاليف، لەذا البرنامج.
الموجودات
إن تلقيح الأطفال ضد التەاب الكبد البائي في غامبيا مرتفع الفعالية لقاء التكاليف، وبمقارنتە مع عدم التلقيح قد يكلف برنامج التلقيح 28 دولاراً أمريكياً لكل سنة من سنوات العمر المصحَّحة باحتساب مدد العجز التي يمكن توقِّيەا من منظور اجتماعي و47 دولاراً أمريكياً لكل سنة من سنوات العمر المصحَّحة باحتساب مدد العجز التي يمكن توقِّيەا من منظور الدافعين للتكاليف. ويتمتَّع البرنامج أيضاً بإمكانية دفع تكاليفە، وذلك بدءاً من ميزانية منخفضة نسبياً لا تزيد على 000 160 دولار أمريكي كل عام. وعند ضم البعدين الخاصين لقياس الحصيلة إلى بعضەما يصبح احتمال أن يؤدِّي تلقيح الرضَّع فعَّالاً لقاء التكاليف ويمكن دفع تكاليفە 40% وذلك بميزانية سنوية للبرنامج لا تتعدَّى 000 182 دولار أمريكي، (وەي التكاليف المقدَّرة الإجمالية للبرنامج من منظور الدافعين)، مع اعتبار عتبة قيمة الفعالية لقاء التكاليف 47 دولاراً أمريكياً لكل سنة من سنوات العمر المصحَّحة باحتساب مدد العجز المتوقَّاة.
Introduction
Hepatitis B virus (HBV) infection remains a global public health challenge that causes significant morbidity and mortality,1 and the burden of disease is especially high in less-developed countries.2 Since 2000, the GAVI Alliance (formerly the Global Alliance for Vaccines and Immunisation) and the Vaccine Fund have accelerated the introduction of HBV vaccines in low-income countries by providing five years of funding for new and underused vaccines, including HBV vaccines.3 As of April 2005, mainly as a result of these efforts, 158 of 192 WHO Member States had adopted routine infant or childhood vaccination against HBV (GAVI Working Group, unpublished data, 2005). Moreover, building on the success of phase 1 of the programme (2000–2005), more resources were mobilized, and the GAVI Alliance decided to extend its support for the 72 poorest countries for phase 2 (2006–2015).4
However, there is no guarantee of long-term support for HBV vaccines, and each recipient country is required to cofinance its immunization programme, gradually increasing its contribution to a self-sustainable level at the end of the current grant.4 Therefore, despite the GAVI Alliance’s extended support, each recipient country needs to be conscious of the financial sustainability of its HBV vaccination programme and, accordingly, each country will need information on the total amount of resources required to fund the programme. The introduction of other new and underused vaccines – for example, those against Haemophilus influenzae type b (Hib) and yellow fever – in an increasing number of countries may intensify competition for limited resources. Finally, there will inevitably be uncertainty surrounding the real-world benefits of vaccination (for example, the ability to achieve adequate vaccine coverage) and the resources required to implement and sustain programmes,5 particularly in settings with weak health infrastructure, which will further complicate budget-related decisions.
In this context, decision-makers will likely benefit from additional information about whether an HBV vaccination programme is affordable and cost-effective. In addition, decision-makers will benefit from an explicit recognition that the information will be uncertain and from analyses that take into account this uncertainty. In part, this has been addressed by summarizing cost-effectiveness results using cost-effectiveness acceptability curves; these curves show the probability that a programme will be cost-effective as a function of different thresholds for acceptable cost–effectiveness ratios.6 However, one limitation of this approach is that the total amount of resources required to fund a programme is not considered.7 The cost-effectiveness affordability curve proposed by Sendi and Briggs overcomes this limitation because it presents the probabilities that a programme is simultaneously cost-effective and affordable as a function of both the threshold cost–effectiveness ratio and budgetary constraints.8 To illustrate the usefulness of providing decision-makers with information on affordability and cost-effectiveness while formally accounting for uncertainty, we applied this approach to a real-world policy example: the programme to vaccinate infants against HBV in the Gambia.
Methods
Analytical overview
Using the example, we first evaluated the programme’s cost-effectiveness and derived cost-effectiveness acceptability curves. The cost-effectiveness of a programme was benchmarked in reference to specified thresholds, such as per-capita gross domestic product (GDP). A cost-effectiveness acceptability curve, constructed in the context of a multivariate uncertainty analysis, presents the probability that a programme will be cost-effective in relation to a range of different cost-effectiveness thresholds.
We next assessed affordability at country level in terms of the annual expected cost for the vaccination programme compared with a specified programme budget. We considered a range of circumstances under which the programme might be assigned a single fixed budget. An affordability curve – also derived within a multivariate uncertainty analysis – presents the probability that a programme will be affordable under various programme budgets.
The cost-effectiveness analysis was conducted from both a societal perspective and a payer perspective; the affordability analysis was conducted from the payer’s perspective. The societal perspective was chosen so that the cost-effectiveness results would be comparable with other studies; the payer perspective was chosen in order to explicitly consider affordability, since the payer is likely to play an important part in making decisions about the budget.
Finally, we simultaneously considered both cost-effectiveness and affordability, combining these results graphically in cost-effectiveness affordability curves. These curves depict the probabilities that a programme will be both cost-effective and affordable under different annual budgets and at different threshold values of cost-effectiveness.
A policy example
The Gambia – which has a per capita income of around US$ 300 and high HBV endemicity – first introduced the HBV vaccine in 1990, depending heavily on external aid (Gambian government, unpublished data, 2001) and eventually began offering it routinely to all infants. Since 2003, the Gambia has been receiving support from the Vaccine Fund for HBV vaccines and two other new and underused vaccines, Hib and yellow fever (Gambian government, unpublished data, 2004); nonetheless, the Gambia faces potential financial challenges. Extending earlier work,9 we assessed the cost-effectiveness of the routine vaccination of infants against HBV compared with no vaccination, reflecting recent changes in cost and new data on long-term vaccine efficacy and the incidence of liver cancer. We followed published guidelines for conducting cost-effectiveness analyses.10–12
We developed a computer-based model for a birth cohort of 56 000 Gambian infants born in 2002 and simulated the health and economic consequences associated with HBV infection. The model takes into account the mother’s HBV infection status, the risk of transmission to the infant and the consequences resulting from HBV infection over the course of the cohort’s lifetime. The model’s outcomes include lifetime costs, cases of new HBV infection, primary liver cancer, disease-specific deaths and disability-adjusted life years (DALYs). A technical appendix (available from the authors upon request) includes details of the model schematic and assumptions, the natural history model of HBV infection and data sources. Tables 1 and 2 present baseline values, ranges and imposed distributions of epidemiological parameters.
Table 1. Assumptions about hepatitis B vaccine coverage and efficacy, and epidemiology of infection with hepatitis B virus (HBV) used in the model evaluating the cost-effectiveness and affordability of vaccinating infants against infection.
| Parameters | Baseline | Rangea | Assumedb distribution | Sources |
|---|---|---|---|---|
| Hepatitis B vaccine coverage (%) | 94 | 85–100 | Triangular | Unpublished datac |
| Vaccine efficacy (3 doses) | ||||
| Initial protective immunity against infection (%) | 95 | 90–100 | Triangular | – |
| Breakthrough infection after 6 years among those vaccinated (%)d,e | 4.5 | 1.3–10.7 | Beta | 21,22 |
| Chronic carriage after breakthrough infection (%) | 1.1 | 0–2.9 | Beta | 21,22 |
| Epidemiological assumptions | ||||
| Prevalence of pregnant women who are hepatitis B surface antigen positive (HBsAg+) (%) | 10.6f | 7.4–15.0g | Logit-normal | 23 |
| Prevalence of mothers who are hepatitis B early antigen positive (HBeAg+) among HBsAg+ mothers (%) | 11.6f | 4.9–24.8g | Logit-normal | 23 |
| Perinatal risk of infection (%) | ||||
| Babies born to HBeAg+ mothers | 27.9f | 9.2–59.6g | Logit-normal | 23 |
| Babies born to mothers negative for hepatitis B early antigen (HBeAg-) | 8.0f | 1.8–28.7g | Logit-normal | 23 |
| Cumulative prevalence of HBV infection (%) | ||||
| At age 5 years | 50 | 35–55 | Triangular | 21,24 |
| At age 15 years | 90 | 63–95 | – | – |
| lifetime risk | 95 | 80–99 | – | – |
a The ranges of parameters are for univariate sensitivity analyses and were chosen to be as inclusive as possible based on the literature. b The technical appendix (available from the authors) provides details on the choice of distributional forms and parameterization. c Gambian Government, unpublished data, 2003. d Annual rate of breakthrough infection was calculated using this value, assuming that immunity wanes exponentially over time. e Annual rate per person. f Values specific to the sub-Saharan region. g 95% confidence interval for the sub-Saharan region.
Table 2. Further assumptions about the natural history of hepatitis B infection and disability weights used in the model evaluating the cost-effectiveness and affordability of vaccinating infants against hepatitis B virus (HBV).
| Parameters | Baseline | Rangea | Assumedb distribution | Sources |
|---|---|---|---|---|
| Transition probabilities | ||||
| Acute infection | ||||
| Symptomatic cases (%) | 1–30 | 0–40 | – | 23,25 |
| Fulminant cases among symptomatic infection (%) | 0.1–0.6 | 0–0.6 | – | 26 |
| Death among fulminant cases (%) | 70 | 60–80 | Triangular | 25 |
| Risk of developing chronic hepatitis (%) | ||||
| ≤ 0.5 years | 0.885 | 0.84–0.93c | Normal | 27 |
| > 0.5 years | exp(–0.645 × age0.455) | – | 27 | |
| Chronic hepatitis | ||||
| Transferring into inactive carrier stated | ||||
| < 25 years | 0.02–0.16 | 0–0.163 | – | 28 |
| ≥ 25 years | 0.10 | 0.083–0.163 | Triangular | 29 |
| Rate of developing compensated cirrhosisd | ||||
| < 25 years | 0.0001–0.0012 | – | – | 30 |
| ≥ 25 years | 0.015 | 0.01–0.057 | Triangular | 26,31 |
| Rate of developing hepatocellular carcinoma | 0–0.0027 | – | – | 30 |
| Inactive carrier | ||||
| Risk of relapsing to chronic hepatitisd | ||||
| < 25 years | 0.0043 | 0.003–0.0065 | Triangular | 32 |
| ≥ 25 years | 0.03 | 0.029–0.073 | Triangular | 29 |
| Rate of resolutiond | ||||
| < 25 years | 0.0056 | 0.0005–0.01 | Triangular | 28 |
| ≥ 25 years | 0.01 | 0.0005–0.015 | Triangular | 26 |
| Compensated cirrhosis | ||||
| Risk of developing decompensated cirrhosisd | 0.035 | 0.032–0.046 | Triangular | 26,31 |
| Risk of developing hepatocellular carcinoma | 0–0.066 | – | – | 26,31 |
| Risk of premature deathd | 0–0.0056 | – | – | 33 |
| Decompensated cirrhosis | ||||
| Risk of developing hepatocellular carcinomad | 0–0.003 | – | – | 31,33 |
| Risk of premature deathd | 0–0.0056 | – | – | 33 |
| Hepatocellular carcinoma | ||||
| Risk of premature deathd | ||||
| < 25 years | 0–0.00015 | – | – | 33 |
| ≥ 25 years | 0.081–0.5 | – | – | 30 |
| Disability weights for liver diseases | 34 | |||
| Episode of acute infectione | 0.075 | 0.075–0.212 | Triangular | |
| Cirrhosis | 0.33 | 0.31–0.495 | Triangular | |
| Liver cancerf | 0.59 | 0.59–0.73 | Triangular |
a The ranges of parameters are for univariate sensitivity analyses and were chosen to be as inclusive as possible based on the literature. b The technical appendix (available from the authors) provides details on the choice of distributional forms and parameterization. c 95% confidence interval for the sub-Saharan region. d Annual rate per person. e Assuming a duration of 0.17 years, the model used an annual average weight of 0.013. f Liver cancer was assumed to progress in three consecutive phases: (1) diagnosis/primary therapy/waiting; (2) metastasis; and (3) terminal. Disability weights for each stage were 0.20 (range: 0.2–0.43), 0.75 (range: 0.75–0.83), and 0.81 (range: 0.81–0.93). The average weight of 0.59 was calculated assuming that each stage lasted approximately the same duration.
For the analysis from a societal perspective, we estimated the direct medical costs – including programme costs (vaccine, injection supplies, delivery) and averted costs of medical treatment – and direct non-medical costs (travel, parents’ time spent immunizing children, patients’ time). In our analysis from the payer’s perspective, only programme costs were included. We adjusted for inflation using the Gambian GDP deflators,13 and expressed costs in 2002 US dollars. Table 3 summarizes the assumptions on costs (further detail is given in the technical appendix). The primary outcome measure was cost- effectiveness, expressed as the incremental cost per DALY averted for vaccination compared with no intervention. For the base case, all outcomes were discounted at 3% per year based on WHO guidelines,10 although other rates were used in the sensitivity analyses.
Table 3. Assumptions on resource utilizationa used in the model of the cost-effectiveness and affordability of vaccinating infants against hepatitis B virus (HBV).
| Parameters | Baseline | Rangeb | Assumedc Distribution | Sources |
|---|---|---|---|---|
| Programme costs | ||||
| Vaccines per dosed,e | 0.32 | 0.23–0.43 | Triangular | 35 |
| Injection supplies per dosed,e | ||||
| Auto-disable syringes | 0.068 | – | – | 35 |
| Safety boxes | 0.0056 | – | – | 35 |
| Delivery costs per dosef | 0.63 | 0.21–0.84 | Triangular | Estimated |
| Wastage rates (%) | ||||
| Vaccines | 20 | 10–50 | Triangular | 36 |
| Auto-disable syringes | 10 | 0–20 | Triangular | 36 |
| Safety boxes | 50 | 10–100 | Triangular | 36 |
| Treatment costs of HBV-related diseases | ||||
| Average no. lifetime outpatient visits for each of HBV infection-related diseasesg | 1 | 0.5–2 | Triangular | 37 |
| Average cost per outpatient visit | 0.86 | 0.60–1.27 | Triangular | 10 |
| Average no. days hospitalized per lifetimeh | 8.4i | 7.0–9.5 | Triangular | 37 |
| Average cost per inpatient day | 4.15 | 2.08–6.23 | Triangular | 10 |
| Travel and time costsj | ||||
| Average transportation costs per travel | 0.31 | 0.20–0.50 | Triangular | 37,38 |
| Average time for travel/waiting/treatment at public health facilities (hours) | 2.3 | 0.96–2.76 | Triangular | 39 |
| Average hourly wage | 0.155 | 0.12–0.19 | Triangular | Unpublished datak |
a All cost estimates are expressed in 2002 US$ at an official exchange rate of 19.91825 Gambian dalasi = US$ 1.00 (source: World Development Indicators Online, World Bank. Available at: http://web.worldbank.org/WBSITE/EXTERNAL/DATASTATISTICS/0,,contentMDK:20398986~menuPK:64133163~pagePK:64133150~piPK:64133175~theSitePK:239419,00.html). b The ranges of parameters are for univariate sensitivity analyses and were chosen to be as inclusive as possible based on the literature. c Because most sources for costs did not provide enough information, we assumed triangular forms for all cost parameters. d UNICEF’s 2002 average unit prices were used for the vaccine (a 10-dose vial) and injection supplies. e In the model, the unit costs for the vaccine and injection supplies were adjusted for the corresponding wastage rates and were further adjusted for the average freight cost rates (6% for vaccines and 15% for injection supplies) to incorporate the costs incurred for international transport. f Total delivery costs included all incremental non-recurrent (capital) and recurrent (operational) programme costs other than vaccine and injection supply costs required to deliver HBV vaccines under the current Gambian immunization system. Delivery costs per dose were adjusted for coverage. See Appendix 6 in the technical appendix (available upon request) for details. g It was assumed that over a lifetime HBV infection entails an average of one outpatient visit each for symptomatic acute infection, chronic hepatitis, cirrhosis and primary liver cancer. h It was assumed that fulminant cases and HBV infections developing into chronic status entail hospitalization and that these inpatient costs are incurred during the year of death. i Data obtained from representative sub-Saharan African countries. j Patients’ time-costs for treatment of HBV-related disease were calculated by multiplying the average time for travel, waiting and treatment at public health facilities (obtained from the Gambian government document, reference 43) by average hourly wage. Parents’ time and travel costs for bringing their children to immunization facilities were computed using the direct allocation method to divide costs between different vaccines administered together in the Gambian vaccination schedule under an average costing approach. k Gambian Government, unpublished data, 2003.
To explore parameter uncertainty, we conducted univariate sensitivity analyses (reported in the technical appendix) and multivariate probabilistic sensitivity analysis. For the latter, we specified distributions around uncertain parameters (Tables 1, 2 and 3, and technical appendix) and performed 1000 Monte Carlo simulations for each perspective. Results of the probabilistic sensitivity analyses were used in the subsequent derivation of cost-effectiveness acceptability curves and affordability curves.
Assumptions for the affordability consideration
We intentionally made simplifying assumptions to demonstrate the application of our analytical framework in the most transparent manner possible. These include the assumption that coverage for traditional vaccines used in the Expanded Programme on Immunization (measles, diphtheria–tetanus–pertussis, oral polio vaccine and bacille Calmette–Guérin) is already high (> 90% as of 2002) in the Gambia, so that investment in new and underused vaccines (HBV, Hib and yellow fever) are justified (Gambian government, unpublished data, 2003). Also, each routine infant vaccination programme is indivisible – meaning vaccination cannot be administered to only a fraction of infants – based on arguments that this would be inequitable. If there were a deficit in the funding for the national immunization programme, less-expensive monovalent vaccines would be used; and based on previous analyses,14,15 the childhood vaccination programmes would be assigned priority in the following order: traditional vaccines plus tetanus toxoid, HBV vaccine, other new and underused vaccines.
Derivation of an affordability curve and cost-effectiveness affordability curves
We evaluated programme affordability based on the joint distribution of simulated incremental health gains and the costs of HBV vaccination from the payer’s perspective. If we plot the simulated outcomes on a cost-effectiveness plane (which has net costs on the vertical axis and net health outcomes on the horizontal), an affordability curve captures the proportion of points in this plane that fall below the horizontal lines corresponding to different budget levels.8 A set of cost-effectiveness affordability curves combines the information from a cost-effectiveness acceptability curve and an affordability curve to capture the proportion of points that fall below both the diagonal line representing a particular cost-effectiveness threshold and the horizontal line representing a particular budget level.8
Findings
Table 4 presents the incremental cost–effectiveness ratio of vaccinating infants against HBV compared with no vaccination from the payer’s perspective. The point estimate provides information on the cost-effectiveness of the HBV programme, allowing the programme’s value-for-money to be compared with various benchmarks. For example, the programme’s incremental cost–effectiveness ratio of US$ 47 per DALY averted is lower than the Gambia’s per-capita GDP (around US$ 300), a measure often used as a surrogate indicator of the cost-effectiveness threshold below which a programme would be considered highly cost-effective.16 However, the point estimate does not consider uncertainty around the programme’s costs and effects.
Table 4. Base case results discounted at 3% for a birth cohort of 56 000 infants, assuming 94% coverage of hepatitis B virus (HBV) vaccine.
| Strategy | Total no. cases of new infectionsa | Total no. cases of primary liver cancera | Total no. cases of premature deathsa | Costb | Incremental costb | Effectivenessc | Incremental effectivenessd | Incremental cost–effectiveness ratioe |
|---|---|---|---|---|---|---|---|---|
| Societal perspective | ||||||||
| No vaccination | 41 245 | 155 | 161 | 134 400 | – | 295 266 | – | – |
| Routine infant vaccination | 12 119 | 21 | 29 | 243 600 | 109 760 | 291 385 | 3 881 | 28 |
| Payer’s perspective | ||||||||
| No vaccination | 41 245 | 155 | 161 | 0 | – | 295 266 | 0 | |
| Routine infant vaccination | 12 119 | 21 | 29 | 182 000 | 182 000 | 291 385 | 3 881 | 47 |
a Epidemiological outcomes do not vary by perspective. b All costs in 2002 US$. c Effectiveness measured in disability-adjusted life years. d Incremental effectiveness measured in disability-adjusted life years averted. e The incremental cost–effectiveness ratio is the ratio of dollars per disability-adjusted life year averted.
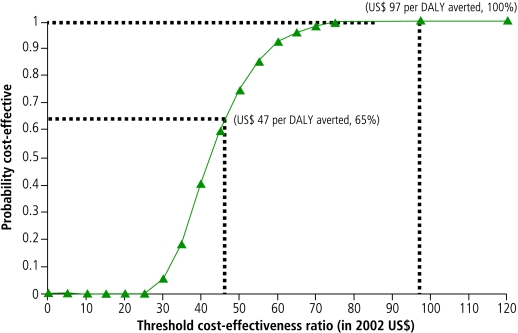
The cost-effectiveness acceptability curve in Fig. 1 incorporates such uncertainty and presents the probability that the HBV vaccination programme is cost-effective in relation to different threshold values for cost-per-DALY ratios. The curve was constructed by plotting the proportion of simulated outcomes on a cost-effectiveness plane that fall below the diagonal lines through the origin of which slopes represent various cost-effectiveness cut-off points. The curve shows that the programme would not be considered cost-effective at thresholds < US$ 25 per DALY averted but would always be considered cost-effective at thresholds > US$ 97 per DALY averted. The curve also indicates that if the programme’s payer is willing to pay US$ 47 per DALY averted, there is a 65% probability the programme will be cost-effective.
Fig. 1.
Cost-effectiveness acceptability curve
DALY, disability-adjusted life year.
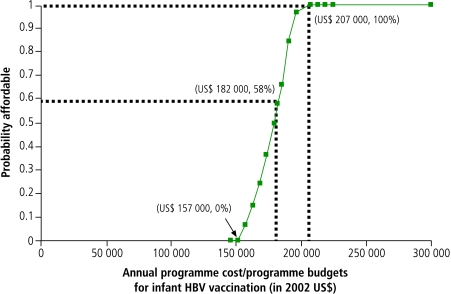
A cost-effectiveness acceptability curve provides an intuitive visual summary of uncertainty about cost-effectiveness, but it does not explicitly account for potential constraints on total resources.7,8 On the other hand, an affordability curve does account for this constraint in presenting the probability that the total costs of a programme fall below a specified budget level. In our policy example (Fig. 2), the probability that the HBV programme is affordable is 0% up to a budget of US$ 157 000 but increases as the budget increases, reaching 100% when the budget increases to US$ 207 000. Accordingly, US$ 157 000 can be considered the lower boundary and US$ 207 000 the upper boundary of the amount of resources required for the programme. The curve also indicates that if the programme budget were set at US$ 182 000 per year based on the estimated average programme costs (Table 4), the probability that the programme will be affordable is only 58% because of the uncertainty surrounding the programme’s costs.
Fig. 2.
This affordability curve shows the probability that the HBV vaccination programme would be affordable under various programme budgets. The US$ 182 000 budget is the point estimate of the projected annual programme costs
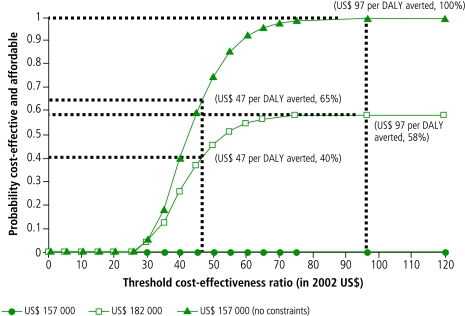
Fig. 3 combines the information provided by the cost-effectiveness acceptability curve and the affordability curve and presents a set of cost-effectiveness affordability curves for the three different levels of programme budget examined in Fig. 2. In the graph, each curve represents the probability that the HBV programme is both cost-effective and affordable at each combination of a cost-effectiveness threshold (horizontal axis) and a programme budget (indicated by legends for each curve). The bottom curve indicates that under programme budgets < US$ 157 000 (the lower bound of the resources required), the probability that the programme is simultaneously cost-effective and affordable is 0% regardless of the levels of willingness-to-pay since the probability that the programme is affordable is 0% under such low budgets. The middle curve shows that under the US$ 182 000 budget (the average programme cost), the probability that the programme is both affordable and cost-effective would increase as the cost-effectiveness threshold increases, reaching a maximum of 68% at the threshold of US$ 97 per DALY averted (the minimum threshold above which the probability that the programme is cost-effective is 100%). Similarly, the upper curve shows that the corresponding probability under the US$ 207 000 budget (the upper bound of the resources required) would reach 100% at the same threshold of US$ 97 per DALY averted. The curve under “no budget constraint” is identical to the curve under the US$ 207 000 budget. This implies that setting a programme budget above the upper bound of the resources required for the programme (US$ 207 000) would not add any further health benefits. It is also noteworthy that the curve under “no budget constraint” is by definition identical to the cost-effectiveness acceptability curve shown in Fig. 1. From a different angle, with a cost-effectiveness threshold set at US$ 47 per DALY averted (the point estimate of the incremental cost–effectiveness ratio) the probabilities that the programme is simultaneously cost effective and affordable are 0% under an annual budget of US$ 157 000, 40% under a budget of US$ 182 000, and 65% under a budget of US$ 207 000.
Fig. 3.
Cost-effectiveness affordability curves show the probabilities that HBV vaccination is both cost effective and affordable under different annual budgets at varying threshold cost-effectiveness values
DALY, disability-adjusted life year.
Discussion
We found from the policy example that vaccinating infants against HBV reduces the burden of HBV-related diseases by > 80%, and it is a highly cost-effective health intervention in the Gambia (Table 4). Our findings also showed that the programme has the potential to be affordable even with a relatively low annual budget of US$ 160 000 (Fig. 2).
Our example illustrates how cost-effectiveness affordability curves can enhance the information provided by traditional analyses of cost-effectiveness. While cost-effectiveness acceptability curves provide a valuable heuristic for summarizing the distribution of expected health and economic consequences in the setting of multivariate uncertainty, they do not distinguish between joint distributions of costs and effects that share the same correlations between these two dimensions but differ in scale.7,8 Cost-effectiveness affordability curves provide one way to address this limitation. When all points in the joint distribution of costs and benefits are positive on both dimensions (as is likely with most childhood vaccines), the consideration of budget constraints in addition to cost-effectiveness thresholds in analyses of multivariate uncertainty enhances the information available to guide real-world decisions.
Limitations
There are several limitations to this analysis. First, our modelling of a single birth cohort for the example does not reflect the indirect effects of vaccination on the force of infection (the rate at which susceptible individuals become infected) over time, and estimated benefits may be higher. Second, because our analysis is intended to demonstrate the framework of how one may add the dimension of affordability to cost-effectiveness analyses, we focused on the affordability of the HBV programme. We did not evaluate the affordability of all competing interventions, so this example provides only qualitative insight into the potential impact of the affordability of the HBV programme. We cannot draw conclusions that depend on the affordability of other programmes delivering new and underused vaccines that are competing for resources. Finally, we could not take into account the potential impact of technological changes that may considerably affect the programme’s cost-effectiveness, such as the introduction of pre-filled auto-disable devices or multivalent vaccines.
Implications
Despite the favourable cost-effectiveness profile of HBV vaccination programmes, without adequate long-term funding when the GAVI Alliance’s support is terminated, low-income countries such as the Gambia may face difficult decisions over how to set priorities and allocate limited resources among different childhood vaccines, and in particular new vaccines. In this context, the importance of examining affordability as well as cost-effectiveness when considering introducing a new vaccine should be increasingly emphasized.17,18 Few studies have explicitly examined the affordability of new vaccines under such circumstances, and no specific guidelines for evaluating affordability have been suggested.
In this regard, our study provides guidance relevant to policy-making in two respects. First, in the face of uncertainties about both the health and economic consequences of a programme, as well as the availability and magnitude of resources needed to fund the programme, policy-makers can use affordability curves to discern the probability that a new programme will be affordable under a specified budget or can project the consequences of assigning different amounts of funding to the programme over a range of programme budgets. Although economic studies that compare a programme’s annual per-capita cost with annual per-capita government health expenditure or compare a programme’s estimated annual average cost with its annual budget are useful, the approach suggested here takes into account uncertainty in the estimates of costs and benefits.19 Second, the cost-effectiveness acceptability curves may serve as tools to communicate results of complex probabilistic cost-effectiveness analyses to policy-makers. When policy-makers need information on affordability in addition to the cost-effectiveness of a new vaccine, a single illustration that combines the cost-effectiveness and affordability profile of the vaccine may allow for easier interpretation.
The usefulness of cost-effectiveness affordability curves is limited when a resource allocation problem needs to be addressed generally because it focuses on a single new programme’s fixed budget. In our example, we assumed that before support from the GAVI Alliance ended, low-income countries would be likely to secure sufficient resources to fund the traditional vaccines and might want to set a separate budget for the increased use of new and underused vaccines. We also assumed that there may not be sufficient resources available to fund all of the relatively expensive new and underused vaccines, and that if so, HBV vaccines would be assigned priority, based on the cost-effectiveness profiles reported in studies.14,15 If this is not the case, fully evaluating the HBV vaccination programme’s affordability requires a more comprehensive approach that explicitly considers the affordability of other competing vaccination programmes under a shared budget. For example, for the case in which the budget constraint is the total portfolio cost, Sendi and colleagues propose calculating the probability that each portfolio would be affordable over a range of budget levels by iterating Monte Carlo simulations of all relevant interventions and then calculating the joint distribution of the total portfolio costs and effectiveness through summation of each programme’s cost and effectiveness.20 However, if HBV vaccines are to be used in multivalent presentations (for example, diphtheria–tetanus–pertussis with HBV vaccine, or diphtheria–tetanus–pertussis with Hib and HBV vaccine) then an optimal mix of antigens should be identified, taking into account the fact that the cost of a combined vaccine would not be the sum of the costs of each monovalent vaccine. Additional research is needed to solve resource allocation problems and it should consider all relevant programmes under a shared budget and incorporate complicated constraints.
Nevertheless, given that such research would require an enormous amount of effort in a real-world setting, and that HBV vaccines might reasonably satisfy the assumptions necessary for the existence of a fixed budget based on the vaccines’ favourable cost-effectiveness profiles, an approach that considers cost-effectiveness, affordability and uncertainty can serve as a practical tool to provide valuable information to decision-makers in low-income countries who might face severe budget constraints for their HBV programmes or programmes for other new and underused vaccines. ■
Acknowledgements
We are grateful to Milton Weinstein and Tracy Lieu for their valuable suggestions regarding the analysis. We also greatly appreciate helpful comments from anonymous reviewers.
Footnotes
Funding and competing interests: Sun-Young Kim was supported in part by a Harvard Graduate Society Fellowship. The funding source did not have any involvement in conducting the study or deciding to submit the manuscript for publication.
References
- 1.Beutels P. Economic evaluations of hepatitis B immunization: a global review of recent studies (1994-2000). Health Econ. 2001;10:751–74. doi: 10.1002/hec.625. [DOI] [PubMed] [Google Scholar]
- 2.Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis. 2002;2:395–403. doi: 10.1016/S1473-3099(02)00315-8. [DOI] [PubMed] [Google Scholar]
- 3.Martin JF, Marshal J. New tendencies and strategies in international immunization: GAVI and The Vaccine Fund. Vaccine. 2003;21:587–92. doi: 10.1016/S0264-410X(02)00564-9. [DOI] [PubMed] [Google Scholar]
- 4.GAVI. the Vaccine Fund. Progress and challenges 2004. Geneva: GAVI; 2004. [Google Scholar]
- 5.Brisson M, Edmunds WJ. Impact of model, mathodological, and parameter uncertainty in the economic analysis of vaccination programmes. Med Decis Making. 2006;26:434–46. doi: 10.1177/0272989X06290485. [DOI] [PubMed] [Google Scholar]
- 6.Briggs AH, O’Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health. 2002;23:377–401. doi: 10.1146/annurev.publhealth.23.100901.140534. [DOI] [PubMed] [Google Scholar]
- 7.Groot KB, Hunink MGM, Stijnen T, Hammitt JK, Kuntz KM, Weinstein MC. Limitations of acceptability curves for presenting uncertainty in cost-effectiveness analysis. Med Decis Making. 2007;27:101–11. doi: 10.1177/0272989X06297394. [DOI] [PubMed] [Google Scholar]
- 8.Sendi PP, Briggs AH. Affordability and cost-effectiveness: decision-making on the cost-effectiveness plane. Health Econ. 2001;10:675–80. doi: 10.1002/hec.639. [DOI] [PubMed] [Google Scholar]
- 9.Hall AJ, Roberston RL, Crivelli PE, Lowe Y, Inskip H, Snow SK, et al. Cost-effectiveness of hepatitis B vaccine in The Gambia. Trans R Soc Trop Med Hyg. 1993;87:333–6. doi: 10.1016/0035-9203(93)90154-I. [DOI] [PubMed] [Google Scholar]
- 10.Edejer TT, Baltussen R, Adam T, Hutubessy R, Acharya A, Evans DB, et al., editors. Making choices in health: WHO guide to cost-effectiveness analysis. Geneva: WHO; 2003. [Google Scholar]
- 11.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 12.Beutels P, Edmunds WJ, Antonanzas F, De Wit GA, Evans D, Feilden R, et al. Economic evaluation of vaccination programmes: a consensus statement focusing on viral hepatitis. Pharmacoeconomics. 2002;20:1–7. doi: 10.2165/00019053-200220010-00001. [DOI] [PubMed] [Google Scholar]
- 13.Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al. Disease Control Priorities Project. In: Disease control priorities in developing countries. 2nd edition. New York: Oxford University Press; 2006. [Google Scholar]
- 14.Miller MA, McCann L. Policy analysis of the use of hepatitis B, Haemophilus influenzae type b, Streptococcus pneumoniae-conjugate and rotavirus vaccines in national immunization schedules. Health Econ. 2000;9:19–35. doi: 10.1002/(SICI)1099-1050(200001)9:1<19::AID-HEC487>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 15.Shepard DS, Walsh JA, Kleinau E, Stansfield S, Bhalotra S. Setting priorities for the Children’s Vaccine Initiative: a cost-effectiveness approach. Vaccine. 1995;13:707–14. doi: 10.1016/0264-410X(94)00063-S. [DOI] [PubMed] [Google Scholar]
- 16.Commission on Macroeconomics and Health. Macroeconomics and health: investing in health for economic development. Report of the Commission on Macroeconomics and Health. Geneva: WHO; 2001. [Google Scholar]
- 17.Cost effectiveness of rotavirus vaccines and other interventions for diarrhoeal diseases: meeting report 2006. Wkly Epidemiol Rec. 2006;81:350–3. [PubMed] [Google Scholar]
- 18.Adding a vaccine to a national immunization programme: decision and implementation. Geneva: WHO; 2005 (WHO/IVB/05.18). Available at: http://www.who.int/vaccines-documents/DocsPDF05/777_screen.pdf
- 19.Walker D, Fox-Rushby JA. Economic evaluation of communicable disease interventions in developing countries: a critical review of the published literature. Health Econ. 2000;9:681–98. doi: 10.1002/1099-1050(200012)9:8<681::AID-HEC545>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 20.Sendi P, Gafni A, Birch S. Opportunity costs and uncertainty in the economic evaluation of health care interventions. Health Econ. 2002;11:23–31. doi: 10.1002/hec.641. [DOI] [PubMed] [Google Scholar]
- 21.Whittle H, Jaffar S, Wansbrough M, Mendy M, Dumpis U, Collinson A, et al. Observational study of vaccine efficacy 14 years after trial of hepatitis B vaccination in Gambian children. BMJ. 2002;325:569. doi: 10.1136/bmj.325.7364.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuen MF, Lim WL, Chan AO, Wong DK, Sum SS, Lai CL. 18-year follow-up study of a prospective randomized trial of hepatitis b vaccinations without booster doses in children. Clin Gastroenterol Hepatol. 2004;2:941–5. doi: 10.1016/S1542-3565(04)00384-2. [DOI] [PubMed] [Google Scholar]
- 23.Edmunds WJ, Medley GF, Nokes DJ, O’Callaghan CJ, Whittle HC, Hall AJ. Epidemiological patterns of hepatitis B virus (HBV) in highly endemic areas. Epidemiol Infect. 1996;117:313–25. doi: 10.1017/s0950268800001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edmunds WJ, Medley GF, Nokes DJ. The transmission dynamics and control of hepatitis B virus in The Gambia. Stat Med. 1996;15:2215–33. doi: 10.1002/(SICI)1097-0258(19961030)15:20<2215::AID-SIM369>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Margolis HS, Coleman PJ, Brown RE, Mast EE, Sheingold SH, Arevalo JA. Prevention of hepatitis B virus transmission by immunization. An economic analysis of current recommendations. JAMA. 1995;274:1201–8. doi: 10.1001/jama.274.15.1201. [DOI] [PubMed] [Google Scholar]
- 26.de Franchis R, Hadengue A, Lau G, Lavanchy D, Lok A, McIntyre N, et al. EASL international consensus conference on hepatitis B. 13-14 September, 2002 Geneva, Switzerland. Consensus statement (long version). J Hepatol. 2003;39(Suppl 1):S3–25. [PubMed] [Google Scholar]
- 27.Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC. The influence of age on the development of the hepatitis B carrier state. Proc R Soc Lond B Biol Sci. 1993;253:197–201. doi: 10.1098/rspb.1993.0102. [DOI] [PubMed] [Google Scholar]
- 28.Chang MH. Decreasing incidence of hepatocellular carcinoma among children following universal hepatitis B immunization. Liver Int. 2003;23:309–14. doi: 10.1034/j.1478-3231.2003.00865.x. [DOI] [PubMed] [Google Scholar]
- 29.Wong JB, Koff RS, Tine F, Pauker SG. Cost-effectiveness of interferon-alpha2b treatment for hepatitis B e antigen-positive chronic hepatitis B. Ann Intern Med. 1995;122:664–75. doi: 10.7326/0003-4819-122-9-199505010-00004. [DOI] [PubMed] [Google Scholar]
- 30.Broderick AL, Jonas MM. Hepatitis B in children. Semin Liver Dis. 2003;23:59–68. doi: 10.1055/s-2003-37585. [DOI] [PubMed] [Google Scholar]
- 31.Lin X, Robinson NJ, Thursz M, Ronsenberg DM. Chronic hepatitis B virus infection in the Asia-Pacific region and Africa: Review of disease progression. J Gastroenterol Hepatol. 2005;20:833–43. doi: 10.1111/j.1440-1746.2005.03813.x. [DOI] [PubMed] [Google Scholar]
- 32.Bortolotti F, Jara P, Crivellaro C, Hierro L, Cadrobbi P, Frauca E, et al. Outcome of chronic hepatitis B in Caucasian children during a 20-year observation period. J Hepatol. 1998;29:184–90. doi: 10.1016/S0168-8278(98)80002-0. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein ST, Zhow F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34:1329–39. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- 34.Mathers CD, Stein C, Ma Fat D, Rao C, Inoue M, Tomijima N, et al. Global Burden of Disease 2000: version 2 methods and results. Geneva: WHO; 2002. Available at: http://www.who.int/healthinfo/paper50.pdf
- 35.UNICEF. 2002Vaccine projections: quantities and pricing. Available at: http://www.unicef.org/supply/files/2002_Vaccine_Projection.pdf
- 36.Kou U. Guidelines for estimating costs of introducing new vaccines into the national immunization system. Geneva: World Health Organization; 2002. [Google Scholar]
- 37.Ekwueme DU, Weniger BG, Chen RT. Model-based estimates of risks of disease transmission and economic costs of seven injection devices in sub-Saharan Africa. Bull World Health Organ. 2002;80:859–70. [PMC free article] [PubMed] [Google Scholar]
- 38.Fox-Rushby J, Foord F. Costs, effects and cost-effectiveness analysis of a mobile maternal health care service in West Kiang, The Gambia. Heatlh Policy. 1996;35:123–43. doi: 10.1016/0168-8510(95)00774-1. [DOI] [PubMed] [Google Scholar]
- 39.Government of the Gambia. Health map: final report. Banjul: The Republic of The Gambia; 1999. [Google Scholar]