Abstract
Objective
Our aim was to estimate the prevalence of HIV, HBV and HCV among the general population of the Philippines using data sources outside of the limited existing active surveillance network.
Methods
We analysed aggregate HIV, HBV and HCV test results for hospital-based blood donors (BDs) and overseas Filipino worker candidates (OFWCs) that had been reported from licensed laboratories to the National STD/AIDS Cooperative Central Laboratory in Manila between 2002 and 2004.
Findings
From over 144 000 blood-screening results, the HIV prevalence was 0.006% in BDs and 0.001% in OFWCs; that of HBV was 4.2% in both groups; and that of HCV was 0.3% in BDs and 0.9% in OFWCs. Males were at increased risk of both HBV and HCV; among OFWCs, younger women were at increased risk. Laboratories that tested sequentially but stopped testing after the first positive result were far less likely to detect HCV, indicating that sequential testing protocols may underestimate HCV and HIV prevalence. OFWCs were at low risk of HIV, and the risk of testing positive for these viruses was not increased among OFWCs applying for a repeated work visa, compared with first time-applicants.
Conclusion
Based on these data, we conclude that HIV is rare in the Philippines. In contrast with prior reports, we found no evidence that OFWCs constitute a high-risk group for HIV. Further research is needed to understand why younger women are at increased risk of acquiring HBV.
Résumé
Objectif
Notre objectif était d’estimer la prévalence du VIH, du VHB et du VHC parmi la population générale des Philippines en utilisant des sources de données extérieures au réseau de surveillance active existant, pour le moment limité.
Méthodes
Nous avons analysé des résultats agrégués de tests pratiqués en milieu hospitalier chez des donneurs de sang et des demandeurs philippins de permis de travail à l’étranger, en vue de dépister les virus VIH, VHB et VHC, et rapportés au Laboratoire central national commun STD/SIDA de Manille entre 2002 et 2004 par des laboratoires homologués.
Résultats
D’après l’analyse du corpus de plus de 144 000 résultats de dépistage sanguin, la prévalence du VIH était de 0,006% chez les donneurs de sang et de 0,001% chez les demandeurs de permis de travail ; celle du VHB était de 4,2% dans les deux groupes et celle du VHC de 0,3% chez les donneurs de sang et de 0,9% chez les demandeurs de permis. Les hommes présentaient un risque accru d’être contaminés à la fois par le VHB et le VHC et parmi les demandeurs de permis de travail, le risque de contamination était plus élevé chez les jeunes femmes. Les laboratoires ayant pratiqué des tests séquentiels de dépistage, mais ayant arrêté les tests après le premier résultat positif, avaient une probabilité bien moindre de détecter le VHC, d’où la possibilité que les protocoles de test séquentiels sous-estiment la prévalence du VHC et du VIH. Chez les demandeurs de permis de travail, le risque d’avoir contracté le VIH était faible et le risque de résultat positif n’augmentait pas entre les demandeurs sollicitant un permis pour la première fois et ceux renouvelant leur demande.
Conclusion
D’après ces données, nous avons conclu que le VIH était rare aux Philippines. A la différence des rapports antérieurs, nous n’avons relevé aucun élément indiquant que les demandeurs de permis de travail constitueraient un groupe à risque pour le VIH. Des travaux de recherche supplémentaires sont nécessaires pour comprendre les raisons de la valeur plus élevée du risque de contamination par le VHB chez les jeunes femmes.
Resumen
Objetivo
Decidimos estimar la prevalencia del VIH, el VHB y el VHC en la población general de Filipinas empleando fuentes de datos distintas de la limitada red de vigilancia activa existente.
Métodos
Analizamos los resultados globales de las pruebas del VIH, el VHB y el VHC a que se sometió a donantes de sangre (DS) en hospitales y a filipinos candidatos a trabajar en el extranjero (FCTE) sobre los que laboratorios autorizados informaron al Laboratorio Central Nacional de Cooperación en materia de ETS/SIDA en Manila entre 2002 y 2004.
Resultados
Los resultados de las más de 144 000 pruebas de cribado de sangre realizadas revelaron que la prevalencia de infección por VIH era del 0,006% entre los DS y del 0,001% entre los FCTE; la prevalencia del VHB era del 4,2% en los dos grupos; y la del VHC era del 0,3% entre los DS y del 0,9% entre los FCTE. Los hombres presentaban un mayor riesgo de infección tanto por VHB como por VHC; entre los FCTE, el riesgo era mayor entre las mujeres más jóvenes. Los laboratorios que realizaban las pruebas de manera secuencial pero las interrumpían tras obtener el primer resultado positivo tenían muchas menos probabilidades de detectar el VHC, lo que lleva a pensar que posiblemente los protocolos de pruebas secuenciales subestiman la prevalencia del VHC y el VIH. Los FCTE presentaban un bajo riesgo de infección por el VIH, y la probabilidad de dar positivo para esos virus no aumentaba entre los FCTE que repetían su solicitud de un visado de trabajo, en comparación con quienes presentaban la solicitud por vez primera.
Conclusión
Se desprende de estos datos que la infección por VIH es infrecuente en Filipinas. A diferencia de otros trabajos anteriores, éste no aporta indicio alguno de que los FCTE constituyan un grupo de alto riesgo de infección por VIH. Habrá que realizar nuevas investigaciones para averiguar por qué las mujeres jóvenes presentan un mayor riesgo de adquisición del VHB.
ملخص
الەدف
إن الغرض من ەذە الدراسة ەو تقدير معدل انتشار فيروس الإيدز، وفيروس التەاب الكبد CوBبين عامة السكان في الفلبين، بالاعتماد على مصادر معطيات أخرى خارج النطاق الضيق للشبكة الحالية للترصد الفعال.
الطريقة
قمنا بتحليل نتائج الاختبارات المجمعة لفيروس الإيدز، وفيروس التەاب الكبد CوBالخاصة بالمتبرعين بالدم في المستشفيات، والعاملين الفلبينيين المرشحين للعمل في الخارج. وقت قامت المختبرات المجازة بتبليغ ەذە النتائج للمختبر الوطني التعاوني المركزي المعني بمرض الإيدز والأمراض المنقولة جنسياً في مانيلا في المدة من 2002 إلى 2004.
الموجودات
أظەرت نتائج تحري عينات الدم لما يربو على 000 144 شخص، أن معدل انتشار فيروس الإيدز يبلغ نحو 0.006% بين المتبرعين بالدم و0.001% بين الفلبينيين طالبي تأشيرات العمل بالخارج. وبلغت نسبة انتشار فيروس التەاب الكبد B نحو 4.2% في المجموعتين. أما نسبة انتشار فيروس التەاب الكبد C فبلغت 0.3% بين المتبرعين بالدم و0.9% بين الفلبينيين طالبي تأشيرات العمل بالخارج. وكان الرجال أكثر عرضة لخطر العدوى بفيروس التەاب الكبد BوC. أما بالنسبة لمجموعة الفلبينيين طالبي تأشيرات العمل بالخارج فكانت الفتيات أكثر اختطاراً. بالنسبة للمختبرات التي تقوم بإجراء الاختبارات بشكل تتابعي، ثم أوقفت الاختبار بعد أول نتيجة إيجابية، فإنەا تكون أقل قدرة على اكتشاف فيروس التەاب الكبد C، مما يشير إلى أن بروتوكول الاختبار التتابعي قد يەون من شأن معدل انتشار فيروس الإيدز وفيروس التەاب الكبد C. فخطر العدوى بفيروس الإيدز ضئيلة بين الفلبينيين طالبي تأشيرات العمل بالخارج، كما لم يزد معدل الاختبارات الإيجابية لەذە الفيروسات بين من يعاود طلب التأشيرات للعمل بالخارج، بالمقارنة بالمتقدمين لأول مرة.
الاستنتاج
أظەرت ەذە المعطيات ندرة انتشار فيروس الإيدز بين الفلبينيين. وعلى عكس ما أظەرت التقارير السابقة، فلا توجد بينات على أن طالبي تأشيرات العمل بالخارج يمثلون مجموعات عالية الاختطار لفيروس الإيدز. وإن كنا بحاجة إلى إجراء المزيد من البحوث لفەم سبب زيادة خطر تعرض الفتيات لاكتساب العدوى بفيروس الكبد الوبائي البائي.
Introduction
Limited surveillance data exist in the Philippines for the three bloodborne viruses (BBVs) of greatest importance: human immunodeficiency virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV). As of December 2005, only 2410 HIV-positive patients had been reported to the national HIV/AIDS registry.1 In addition to the HIV/AIDS registry, the Philippines conducts active surveillance on HIV and other sexually transmitted infections (STIs) among high-risk groups such as female sex workers (FSWs), men who have sex with men and injecting drug users,2,3 but has no surveillance system outside these high-risk groups by which to estimate BBV trends in the general population. The Philippines Department of Health was advised to conduct sentinel surveys for blood banks and overseas Filipino workers, which could contribute to the identification of new high-risk populations.4
In this study, we investigated the prevalence of BBVs from two informal data sets, both collected for reasons other than disease surveillance. The first source was hospital-based blood banks. WHO/UNAIDS currently recommends testing of blood donors (BDs) as a reasonably low-cost approach for HIV surveillance in countries with low rates of HIV infection.5 The second source was persons applying for foreign work visa permits. These overseas Filipino worker candidates (OFWCs) are an important demographic because Filipino migrant workers contribute tremendously to the economy of the Philippines,6,7 and because it is believed that returning workers may be an important source of HIV infections in the country. Since 1999, more than 40% of HIV/AIDS registered cases have previously been overseas.1 Several overseas employees have responded to this by making HIV testing, and occasionally testing for other infections, a systematic requirement of their employer and/or host countries.
Our goals were to estimate the prevalence of HIV, HBV and HCV among these populations, to contrast the prevalence of these viruses between the two source groups, to explore epidemiologic risk factors for BBV positivity; and, because these sets represent convenience samples, to probe where possible for evidence of systematic biases that might impact our interpretation of the sets.
Methods
Data source
We analysed blood-screening data for HIV, HBV and HCV among BDs and OFWCs from 2002–04. These data are reported to the STD/AIDS Cooperative Central Laboratory (SACCL) in Manila. This network of laboratories submits quarterly aggregate results to SACCL on the number of tests performed and positive cases, categorized by sex and age.8 While the potential sources of data were quite large, actual reporting was far lower. Of the over 130 accredited laboratories for the OFWCs screening test, and over 150 licensed as blood banks,9 the number of laboratories with eligible data for this study that actually reported to SACCL was only 17, 13 and 11 in successive years for BDs; and 14, 16 and 9 for OFWCs for the years 2002, 2003 and 2004, respectively.
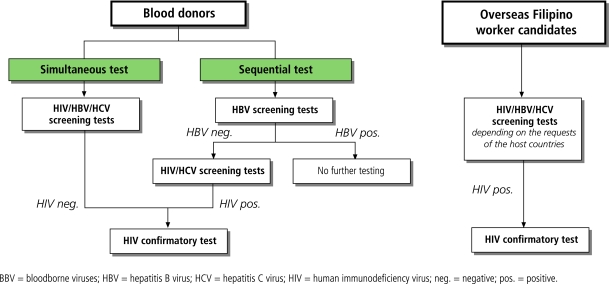
BBV test results for BDs derived from hospital-based blood banks. Procedures for blood screening varied between laboratories, with some adopting either sequential10 or simultaneous testing protocols for the three BBVs. In the sequential testing system, a blood unit would be rejected after the first virus found positive, with no further testing done. Simultaneous testing laboratories generate results for a given BBV, irrespective of the results for the other two BBVs. Because we were usually unable to determine from the laboratories or from SACCL directly whether a given laboratory followed a sequential versus simultaneous testing protocol, we presumptively classified laboratories as either simultaneous or sequential testers by examining the total number of tests for each virus. Because HBV was the most common virus detected, if the number of HIV or HCV samples tested was approximately equal to the number of HBV tested minus the number of HBV positive results we considered them to be sequential testers. If the numbers of tests done on each virus were approximately equal, we considered them to be simultaneous testers. Fig. 1 shows a flowchart of the BBV tests for BDs and OFWCs.
Fig. 1.
Algorithm for BBV tests for blood donors and OFWCs
For OFWCs, we collected data from accredited OFWC clinics/hospitals. In contrast with blood banks, where screening protocols are dictated by hospital policy, the decision as to which viruses to screen for in OFWCs — or the decision to screen at all — depended on the visa requirements for the host countries to which OFWCs were applying.
Laboratory methods
For HIV, the initial anti-HIV-1 and HIV-2 screening tests were conducted at the laboratories, with confirmatory tests done at SACCL for OFWCs and at the Research Institution for Tropical Medicine for BDs, using immunofluoresecent antibody assays and Western blotting. Since HBV screening was through detection of hepatitis B surface antigen (HBsAg), the test only detects acute or chronic active viral hepatitis B. HCV was screened via anti-HCV antibody. The screening test kits varied between laboratories, although they were evaluated by the Department of Health.
Analytic methods
Because the data are reported to SACCL only in aggregate form, we were unable to perform statistical analyses at the level of individual patients. We calculated BD and OFWC positivity rates as the number of positive samples divided by the total number screened. For comparisons of HIV, HBV and HCV infections between groups of people, we calculated relative risks (RR) and 95% confidence intervals (95% CI).
Results
Our final data set consisted of approximately 144 000 blood samples. The precise number of patients could not be ascertained from the aggregate data, but based on the most common test performed (HBsAg) would have been at least 144 624. The majority of samples were collected from large cities. Among 63 249 HIV tests for BDs, 17 326 (27%) were reported from the National Capital Region, 10 589 (17%), 10 345 (16%), and 9714 (15%) from Southern Mindanao, Southern Tagalog and the Central Visayas, respectively. Of the 69 123 HIV tests for OFWCs, 64 764 (94%) were from the National Capital Region.
Table 1 shows prevalence ratios among BDs and OFWCs. The sex ratio differed substantially between the two groups, with 92% of BDs being male versus ca. 42% for OFWCs. Because HIV cases were few and the prevalence of HCV and HBV were similar across the three years of data collection, we combined the data from 2002–2004 in our analyses.
Table 1. HIV/HBV/HCV prevalence among blood donors and OFWCs.
| Anti-HIV Ab |
HBsAg |
Anti-HCV Ab |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number tested | Number positive | % | Number tested | Number positive | % | Number tested | Number positive | % | |||
| Blood donors | |||||||||||
| Male | 58 169 | 4 | 0.007 | 59 740 | 2 551 | 4.27 | 58 021 | 203 | 0.35 | ||
| Female | 5 080 | 0 | 0 | 5 214 | 153 | 2.93 | 5 145 | 5 | 0.10 | ||
| All | 63 249 | 4 | 0.006 | 64 954 | 2 704 | 4.16 | 63 166 | 208 | 0.33 | ||
| OFWCs | |||||||||||
| Male | 27 771 | 1 | 0.004 | 30 484 | 1 374 | 4.51 | 4 660 | 43 | 0.92 | ||
| Female | 41 352 | 0 | 0 | 49 186 | 1 952 | 3.97 | 6 354 | 60 | 0.94 | ||
| All | 69 123 | 1 | 0.001 | 79 670 | 3 326 | 4.17 | 11 014 | 103 | 0.94 | ||
Ab, antibody; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; OFWCs, overseas Filipino worker candidates.
HIV rates were extremely low in both BDs and OFWCs. HIV was detected in only 4 of 63 249 BDs screened (prevalence 0.006%) and only 1 of 69 123 OFWCs (prevalence 0.001%). All 5 positive cases were men.
HBsAg was detected in 6030 out of 144 624 screened (2704 of 64 954 BDs [prevalence, 4.2%] and 3326 of 79 670 OFWCs [prevalence, 4.2%]). The HBV rates were similar between BDs and OFWCs (RR, 1.00 [95% CI: 0.95–1.05]). The risk was higher in males for both BDs (male prevalence, 4.3% and female prevalence, 2.9%; RR 1.45 [95% CI: 1.24–1.71]), and OFWCs (4.5% versus 4.0%, RR 1.14 [95% CI: 1.06–1.22]). Among the groups tested, age proved to be an important risk factor only in the case of hepatitis B among female OFWCs. In this group, HBV prevalence was inversely related to age, with a consistent gradient of risk when comparing each age group to that above it (Table 2). Women under 19 years were far more likely to test positive for HBV when compared with any of the higher age categories. Similarly, women aged 19–29 years and women aged 30–39 years were also more likely to test positive for HBV compared with the higher age categories.
Table 2. Risks of HBV/HCV positivity among BDs and OFCWs.
| Risk for HBsAg + |
Risk for HCV+ |
||||
|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | ||
| Testing group | |||||
| BDs | 1.00 | 0.95–1.05 | 0.35 | 0.28–0.45 | |
| OFWCs | Reference category | Reference category | |||
| Blood donors | |||||
| Male | 1.45 | 1.24–1.71 | 3.60 | 1.48–8.74 | |
| Female | Reference category | Reference category | |||
| OFWCs | |||||
| Male | 1.14 | 1.06–1.22 | 0.97 | 0.66–1.45 | |
| Female | Reference category | Reference category | |||
BDs, blood donors; CI, confidence interval; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; OFWCs, overseas Filipino worker candidates; RR, relative risk.
HCV was detected in 311 of 74 180 screened (208 of 63 166 BDs [prevalence, 0.3%] and 103 of 11 014 OFWCs [prevalence, 0.9%]). HCV prevalence in BDs was almost one-third in OFWCs (RR 0.35 [95% CI: 0.28–0.45]). The HCV prevalence was significantly higher among male BDs than female BDs (RR 3.60 [95% CI: 1.48–8.74]), but did not differ significantly between male OFWCs and female OFWCs (RR 0.97 [95% CI: 0.66–1.45]).
The effect of sequential testing versus simultaneous testing among BDs
Given that the HCV rates among BDs were far lower than among OFWCs, whereas the HBV rates were similar for each group, we considered whether some of this difference could be an artefact of the sequential testing approach used at many blood banks, a bias that should not apply to the OFWCs. Therefore, we performed a sensitivity analysis in which we contrasted the rates of HBV and HCV depending on whether the laboratory used a sequential versus a simultaneous testing protocol for the BDs. Because HBV is the first virus screened for (due to the prevalence of the disease and the low cost of the assay relative to HIV or HCV), we hypothesized that this would have the effect of systematically under reporting the prevalence of HCV and HIV. As shown in Table 3, 8 blood banks reported or appeared to be performing simultaneous testing, whereas 18 blood banks conducted sequential testing. Approximately 5% fewer tests were submitted for HIV and HCV than for HBV among the sequential testing laboratories. Compared with simultaneous testing laboratories, sequential testers (Table 4) were far less likely to detect HCV infections (RR, 0.33 [95% CI: 0.22–0.41]), whereas no such effect was seen for HBV infections (RR, 0.99 [95% CI: 0.92–1.06]). The HIV prevalence was also lower among the sequential testing laboratories, though this difference was not statistically significant (RR, 0.30 [95% CI: 0.03–2.85]).
Table 3. Effect of age on HBsAg positivity among female OFWCs.
| Relative risk for HBsAg + (95% CI) | ||||
|---|---|---|---|---|
| Reference category by age (years) | 19–29 | 30–39 | 40–49 | > 49 |
| < 19 | 1.65 (1.23–2.22) | 1.94 (1.44–2.61) | 1.93 (1.42–2.63) | 2.32 (1.39–3.86) |
| 19–29 | – | 1.17 (1.06–1.30) | 1.17 (1.03–1.33) | 1.40 (0.92–2.15) |
| 30–39 | – | – | 1.00 (0.87–1.15) | 1.20 (0.78–1.84) |
| 40–49 | – | – | – | 1.20 (0.78–1.86) |
HBsAg, hepatitis B surface antigen; OFWCs, overseas Filipino worker candidates.
Table 4. Effect of sequential versus simultaneous testing protocol on risk of HBV/HIV/HCV positivity.
| HBsAg |
Anti-HIV Ab |
Anti-HCV Ab |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Testing protocol | Number tested | Number positive | % | Number tested | Number positive | % | Number tested | Number positive | % | ||
| Sequential | |||||||||||
| Male | 32 826 | 1 418 | 4.32 | 31 255 | 1 | 0.003 | 31 107 | 52 | 0.17 | ||
| Female | 2 350 | 38 | 1.62 | 2 216 | 0 | 0 | 2 281 | 0 | 0 | ||
| All | 35 176 | 1 456 | 4.14 | 33 471 | 1 | 0.003 | 33 388 | 52 | 0.16 | ||
| Simultaneous | |||||||||||
| Male | 26 914 | 1 133 | 4.21 | 26 914 | 3 | 0.01 | 26 914 | 151 | 0.56 | ||
| Female | 2 864 | 115 | 4.02 | 2 864 | 0 | 0 | 2 864 | 5 | 0.17 | ||
| All | 29 778 | 1 248 | 4.19 | 29 778 | 3 | 0.01 | 29 778 | 156 | 0.52 | ||
| RR = 0.99a 95% CI: 0.92–1.06 | RR = 0.30a 95% CI: 0.03–2.85 | RR = 0.33a 95% CI: 0.22–0.41 | |||||||||
Ab, antibody; CI, confidence interval; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; RR, relative risk. a Risk is for sequential versus simultaneous.
The prevalence of BBVs among first-time versus repeat OFWCs
Given the presumption that OFWCs constituted a higher-risk group for BBVs, we hypothesized that OFWCs applying for a visa for a second or subsequent trip should have an increased risk of BBVs compared with those applying for their first trip abroad.
We analysed data from the 17 OFWC testing clinics/hospitals that reported data separately for first-time and repeat OFWCs. Contrary to our expectations, the prevalence of HBV did not vary between the two groups as a function of whether the OFWC was a first-time or a repeat applicant (Table 4, RR, 1.05 [95% CI: 0.97–1.14]). In the case of HCV, first-time OFWCs were actually more likely to test positive than repeated OFWCs (RR, 1.53 [95% CI: 1.03–2.28]).
Discussion
In the published literature, our study was the first to examine the effect of first-time versus repeat visa application as a risk factor for BBVs among OFWCs, and the first to contrast directly the BBV prevalence between BDs and OFWCs. Our analysis showed that HIV was extremely uncommon among this population of BDs and OFWCs, whereas HBV was very common and the prevalence of HCV was between the two. Significantly, our data suggested a far lower prevalence of HIV among OFWCs than previous estimates of 0.01%.11 In contrast with previously identified high-risk groups, such as injecting drug users, men who have sex with men and FSWs, our analysis showed a negligible risk for HIV among those applying for foreign work visa permits — only a single case of HIV among about 70 000 OFWC samples tested. Moreover, the risk of all three viruses among OFWCs applying for their second or subsequent permits to work abroad, among whom the prevalence of BBVs should logically have been higher if prior overseas work was a risk factor for infection, was no different for HIV and HBV. For HCV infection, the risk was lower among repeat OFWCs (Table 5).
Table 5. Effect of first-time versus repeat applicant status on risk of HIV/HBV/HCV seropositivity among overseas foreign worker candidates.
| Anti-HIV Ab |
HBsAg |
Anti-HCV Ab |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number tested | Number positive | % | Number tested | Number positive | % | Number tested | Number positive | % | |||
| First-time | |||||||||||
| Male | 8 715 | 0 | 0 | 10 245 | 451 | 4.40 | 1 197 | 16 | 1.34 | ||
| Female | 13 549 | 0 | 0 | 15 260 | 596 | 3.91 | 1 830 | 22 | 1.20 | ||
| All | 22 264 | 0 | 0 | 25 506 | 1 047 | 4.10 | 3 027 | 38 | 1.26 | ||
| Repeated | |||||||||||
| Male | 13 556 | 0 | 0 | 16 548 | 692 | 4.18 | 3 433 | 27 | 0.79 | ||
| Female | 18 682 | 0 | 0 | 19 036 | 698 | 3.67 | 4 490 | 38 | 0.85 | ||
| All | 32 238 | 0 | 0 | 35 584 | 1 390 | 3.91 | 7 923 | 65 | 0.82 | ||
| NA | RR = 1.05a 95% CI: 0.97–1.14 | RR = 1.53a 95% CI: 1.03–2.28 | |||||||||
Ab, antibody; CI, confidence interval; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; NA, not applicable; RR, relative risk. a Risk is for positive result of first-time versus ‘repeat’ candidate.
How can we reconcile our findings with previous reports on the prevalence of BBVs among workers returning from overseas? One possible explanation is the manner in which HIV cases are registered in the Philippines. To date, overseas Filipino workers have been the largest single demographic group in the HIV/AIDS registry.1 However, this could largely be due to ascertainment bias, given that the visa testing requirements imposed on OFWCs do not apply to the general population. Because the OFWCs are oversampled in the national registry, they may appear more important than they really are, principally because these totals do not consider the denominator that represents the population from which they were drawn.
Likewise, HBV seroprevalence was comparable to the 2–5% among the general population in previous studies, but far lower than a WHO report of 8–10% overall, 10% among males, and 10% in injecting drug users.12–15 The HCV prevalence in the study was slightly lower than previous data of 2–4% for the general population, but far lower than the 70% reported for injecting drug users.14,16,17
We observed higher rates for HBV and HCV infection among males than females. Why this might be the case cannot be explained using the aggregate data that was available to us. However, we hypothesize that it could represent different patterns of sexual activity or higher rates of injecting drug use among males. The finding that among OFWCs younger women were at higher risk of HBV might also represent high-risk sexual behaviour in this group. In partial support of this theory, a recent study showed that the Chlamydia infection rate of antenatal women was significantly higher among younger women.18
Our report sheds light on the limitations of sequential testing among BDs. Among sequential testing groups, HIV and HCV tests were done only for blood units that tested negative for HBsAg. Because it is unlikely that co-infection rates for HBV and HCV and/or HIV occur in a random fashion, this would lead to a proportionally greater underestimation of these infections. Previous studies have shown that 7–18% of those with HBV infection were co-infected with HCV.19–22 Also, a significant percentage of HIV-infected patients were co-infected with HBV and/or HCV.23–26 To provide safe blood within limited budgets, many blood banks have adopted the sequential testing approach to contain costs associated with HIV and HCV tests. This ongoing testing system for donated blood misses these co-infection cases. Given the residual uncertainty regarding whether laboratories were truly using simultaneous versus sequential testing, our analysis is vulnerable to misclassification. However, such misclassification would actually underrepresent the true bias from sequential testing.
Our analysis has several limitations. First, there is a sampling bias. Our data were not randomly collected from all licensed laboratories but from a comparatively small number of laboratories. Because of aggregate data reporting, we were unable to assess co-infection rates, or to do patient-level analysis to control for the effect of repeat donors. Also, the reliability of the data could not be confirmed, and was frequently suspect. For example, some reports lacked information on age, sex and the purpose for testing, and had unreadable handwriting and obvious errors (e.g. the number of positive cases was greater than total tested), and as such, they were rejected from our analysis. Similarly, there was no mechanism in this analysis for quality control of the test results themselves. The effect of this would be to underestimate the prevalence of the BBVs to some degree. Perhaps most importantly, while our data set was extremely large, it nevertheless represents a ‘convenience sample’ in that it was collected for non-research purposes and was not collected systematically. Consequently, there could easily be undetectable biases in the data that reflect the non-random manner in which patients chose to become (or were rejected from becoming) blood donors or to seek overseas visas.
We also identify several points at which the prevalence of the BBVs could be underestimated. The first is the bias introduced by sequential testing protocols, for which we provide empirical evidence. In addition, BBV prevalence in BDs may be underrepresented due to the frequent practice of excluding those considered to be at high risk, such as those with tattoos or a remote history of IDU.11 Similarly, the practice believed to be used at some laboratories of pretesting for HBV with a rapid test before doing a formal screen would also underrepresent the total number of patients identified with BBVs, though such patients would not be included in the denominator of those tested in this report.
Nevertheless, we feel confident in concluding that the prevalence of HIV in the Philippines is extremely low, since for the aggregate effect of these biases to increase the prevalence of HIV from our observed level of 0.0038% (5 positives of 132 372 samples tested) to a range more comparable with that found in neighbouring south-east Asian countries (ca. 1%)27 would require the biases to underestimate the prevalence by 2–3 orders of magnitude, which is very unlikely. This conclusion is further strengthened by a significant proportion of the donors in the Philippines often being not anonymous volunteers but replacement donors, meaning donors recruited from among a patient’s family or acquaintances, who may paradoxically be at increased risk of HIV or other diseases.28,29
In conclusion, we suggest that the prevalence of the three BBVs among BDs, and possibly OFWCs as well, probably offers a reasonable if imperfect source for monitoring prevalence. Our analyses raise several additional questions, such as why males overall had higher rates of HBV and HCV, and why younger female OFWCs appeared to be at higher risk of HBV. Our surprising result was that OFWCs were not at increased risk of HIV or other viruses, a finding that contrasts with previous reports and could be explained by ascertainment bias in the national registry statistics. Our analysis suggests the possibility that the national statistics oversampled OFWCs, leading to an overestimation of the importance of this contribution of this group to overall numbers of HIV infections. However, our analysis cannot estimate the magnitude of the effect of any such sampling bias. We provide empirical evidence that the sequential testing procedures introduce under reporting bias, and suggest that this practice be abandoned in favour of simultaneous testing. This issue will likely grow in importance as the HIV epidemic increases in the future. We provide additional evidence that HIV remains extremely uncommon in the Philippines — a fascinating and somewhat surprising observation given the rising rates of HIV in neighbouring south-east Asian nations. While our data set cannot explain the reason for this, this evidence at least prompts inquiry into the very relevant issue of what cultural, biologic, geographic or political factors have so far left the Philippines comparatively unscathed by the HIV pandemic. ■
Footnotes
Competing interests: None declared.
References
- 1.Department of Health, Philippines. HIV/AIDS Registry, December 2005. National Epidemiology Center.
- 2.Department of Health, National Epidemiology Center. The 2003 Technical Report of the National HIV/AIDS /STI Surveillance System. Status and Trends of HIV/AIDS in the Philippines. 2003. [Google Scholar]
- 3.Mateo R Jr., Sarol JN Jr., Poblete R. HIV/AIDS in the Philippines. AIDS Education & Prevention 2004; 16 Suppl A: 43-52. [DOI] [PubMed]
- 4.United States Agency for International Development, Assessment of the Philippines National HIV/AIDS Sentinel Surveillance System, 2005.
- 5.World Health Organization and Joint United Nations Programme on HIV/AIDS. Working Group on Global HIV/AIDS and STI Surveillance. Guidelines for Second Generation HIV Surveillance. WHO/CDS/CSR/EDC/2000.5. UNAIDS 2000;03E.
- 6.National Statistics Office. Philippines. Press release on the 2004 Survey on Overseas Filipinos. http://www.census.gov.ph/data/pressrelease/2005/of04tx.html
- 7.Bangko Sentral ng Pilipinas. Statistics: Overseas Filipino Workers’ Remittances by Country and by Type of Worker. http://www.bsp.gov.ph/statistics/spei/tab11.htm
- 8.STD/AIDS Cooperative Central Laboratory. Quarterly Census Form.http://www.doh.gov.ph/saccl/pdf%20file/quarterly%20report.PDF
- 9.Department of Health. Philippines. Hospital Statistics: List of Accredited OFW Medical Clinics/Hospitals (as of December 2004). List of licensed blood banks 2003. [Google Scholar]
- 10.Gibney L, Choudhury P, Khawaja Z, et al. HIV/AIDS in Bangladesh: an assessment of biomedical risk factors for transmission. Int J STD AIDS. 1999;10:338–46. doi: 10.1258/0956462991914087. [DOI] [PubMed] [Google Scholar]
- 11.Consensus report on STI, HIV and AIDS Epidemiology, Philippines, 2002. World Health Organization Regional Office for the Western Pacific. Department of Health, Philippines. 2002.
- 12.Western Pacific Regional Plan to improve hepatitis B control through Immunization. Manila, Philippines: World Health Organization Regional Office for the Western Pacific, WP/ICP/EPI/5.2/001-E, January 2003.
- 13.Family Health International. RTI/STI Prevalence in Selected Sites in the Philippines, 2002.
- 14.Agdamag DM, Kageyama S, Alesna ET, et al. Rapid spread of hepatitis C virus among injecting-drug users in the Philippines: implications for HIV epidemics. J Med Virol. 2005;77:221–6. doi: 10.1002/jmv.20439. [DOI] [PubMed] [Google Scholar]
- 15.Arguillas MO, Domingo EO, Tsuda F, et al. Seroepidemiology of hepatitis C virus infection in the Philippines: a preliminary study and comparison with hepatitis B virus infection among blood donors, medical personnel, and patient groups in Davao, Philippines. Gastroenterol Jpn. 1991;26:170–5. doi: 10.1007/BF02779292. [DOI] [PubMed] [Google Scholar]
- 16.Hepatitis C prevalence rate based on published reports, by country/area. Wkly Epidemiol Rec. 1999;74:421–8. [Google Scholar]
- 17.Katayama Y, Barzaga NG, Alipio A, et al. Genotype analysis of hepatitis C virus among blood donors and inmates in Metro Manila, the Philippines. Microbiol Immunol. 1996;40:525–9. doi: 10.1111/j.1348-0421.1996.tb01104.x. [DOI] [PubMed] [Google Scholar]
- 18.Aplasca-De Los Reyes MR. Prevalence of Sexually Transmitted Infection among Selected Population Groups in the Philippines, 1999.
- 19.Di Marco V, Lo Iacono O, Camma C, et al. The long-term course of chronic hepatitis B. Hepatology. 1999;30:257–64. doi: 10.1002/hep.510300109. [DOI] [PubMed] [Google Scholar]
- 20.Gaeta GB, Stornaiuolo G, Precone DF, et al. Epidemiological and clinical burden of chronic hepatitis B virus/hepatitis C virus infection. A multicenter Italian study. J Hepatol. 2003;39:1036–41. doi: 10.1016/S0168-8278(03)00470-7. [DOI] [PubMed] [Google Scholar]
- 21.Sato S, Shigetoshi F, Tanaka M, et al. Coinfection of hepatitis C virus in patients with chronic hepatitis B infection. J Hepatol. 1994;21:159–66. doi: 10.1016/S0168-8278(05)80389-7. [DOI] [PubMed] [Google Scholar]
- 22.Crespo J, Lozano JL, de la Cruz F, et al. Prevalence and significance of hepatitis C viremia in chronic active hepatitis B. Am J Gastroenterol. 1994;89:1147–51. [PubMed] [Google Scholar]
- 23.Dimitrakopoulos A, Takou A, Haida A, et al. The prevalence of hepatitis B and C in HIV-positive Greek patients: relationship to survival of deceased AIDS patients. J Infect. 2000;40:127–31. doi: 10.1053/jinf.1998.0636. [DOI] [PubMed] [Google Scholar]
- 24.Miller CL, Wood E, Spittal PM, et al. The future face of coinfection: prevalence and incidence of HIV and hepatitis C virus coinfection among young injection drug users. J Acquir Immune Defic Syndr. 2004;36:743–9. doi: 10.1097/00126334-200406010-00012. [DOI] [PubMed] [Google Scholar]
- 25.Sterling RK. Triple infection with human immunodeficiency virus, hepatitis C virus, and hepatitis B virus: a clinical challenge. Am J Gastroenterol. 2003;98:2130–4. doi: 10.1111/j.1572-0241.2003.07720.x. [DOI] [PubMed] [Google Scholar]
- 26.De Luca A, Bugarini R, Lepri AC, et al. Italian Cohort Naive Antiretrovirals Study Group. Coinfection with hepatitis viruses and outcome of initial antiretroviral regimens in previously naive HIV-infected subjects. Arch Intern Med. 2002;162:2125–32. doi: 10.1001/archinte.162.18.2125. [DOI] [PubMed] [Google Scholar]
- 27.Joint United Nations Programme on HIV/AIDS and World Health Organization. AIDS epidemic update, December 2005. UNAIDS 2005;19E.
- 28.Global database on blood safety. Summary report 1998-1999. Geneva, Switzerland: World Health Organization, HTP/BCT/Blood Transfusion Safety; 2001.
- 29.Sedyaningsih-Mamahit E, Schinaia N, Lazzari S, et al. The use of blood donor data for HIV surveillance purposes. AIDS. 2004;18:1849–51. doi: 10.1097/00002030-200409030-00016. [DOI] [PubMed] [Google Scholar]