Abstract
Objective
To determine the role of vector control in further decreasing the transmission of bancroftian filariasis achieved by mass drug administration and the long-term impact on filariometric indices.
Methods
Three rounds of annual mass drug administration, with diethylcarbamazine and ivermectin, were complemented by vector control (mainly using polystyrene beads) in villages of Tirukoilur, south India, during 1995–99. Subsequently, drug administration is being carried out with diethylcarbamazine and albendazole or diethylcarbamazine alone. We evaluated the impact of mass drug administration used alone or in conjunction with vector control (from 1995 to 2005) on vector transmission indices (such as transmission intensity index, monthly biting rate, monthly transmission potential and annual transmission potential). We analysed data on filarial infection in the community to estimate the prevalence of microfilaraemia and antigenaemia using χ² analysis and Fisher’s exact test.
Findings
Vector density greatly decreased in villages where vector control was used as an adjunct to mass drug administration and almost no infective mosquitoes were found in the small numbers still remaining. Filarial antigenaemia was low and continued to decrease significantly in the age group 15–25 years in villages receiving mass drug administration with vector control in contrast to villages receiving only mass drug administration.
Conclusion
The gains of mass drug administration were sustained only with the integration of vector control measures. We advocate the incorporation of vector control in the Global Programme to Eliminate Lymphatic Filariasis as it can potentially decrease the time required for eliminating lymphatic filariasis.
Résumé
Objectif
Déterminer le rôle de la lutte antivectorielle dans l’abaissement des niveaux de transmission de la filariose à W. bancrofti déjà obtenus grâce au traitement médicamenteux de masse et son effet à long terme sur les indices filariométriques.
Méthodes
Trois tournées annuelles de traitement médicamenteux de masse par la diéthylcarbamazine et l’ivermectine ont été complétées par une lutte antivectorielle (billes de polystyrène principalement) dans des villages du Tirukoilur au sud de l’Inde, de 1995 à 1999. On a appliqué par la suite un traitement constitué de diéthylcarbamazine et d’albendazole ou de diéthylcarbamazine seule. On a évalué l’effet du traitement médicamenteux de masse seul ou en association avec la lutte antivectorielle (de 1995 à 2005) sur les indices de transmission vectorielle (comme l’indice d’intensité de la transmission, le taux d’agressivité mensuel, le potentiel de transmission mensuel et le potentiel de transmission annuel). On a analysé les données sur l’infection filarienne dans la communauté pour estimer la prévalence de la microfilarémie et de l’antigénémie au moyen de l’analyse de χ² et du test exact de Fisher.
Résultats
La densité vectorielle a sensiblement diminué dans les villages où des mesures de lutte antivectorielle complétaient le traitement médicamenteux de masse et chez les rares moustiques qui subsistaient, l’infestation avait presque entièrement disparu. L’antigénémie filarienne était faible et elle a continué à diminuer considérablement parmis la tranche d’âge 15-25 ans dans les villages bénéficiant à la fois du traitement et de la lutte antivectorielle par rapport aux villages qui ne bénéficiaient que du traitement.
Conclusion
Les avantages du traitement médicamenteux de masse n’ont pu être durablement maintenus qu’en intégrant des mesures de lutte antivectorielle. Il est donc conseillé d’incorporer de telles mesures au Programme mondial d’élimination de la filariose lymphatique car elles offrent un moyen susceptible de réduire le délai d’élimination.
Resumen
Objetivo
Determinar la contribución de la lucha antivectorial a la reducción de la transmisión de la filariasis de Bancroft lograda mediante la administración masiva de medicamentos y su impacto a largo plazo en los índices filariométricos.
Métodos
Se procedió a complementar tres rondas de administración anual masiva de dietilcarbamazina e ivermectina con medidas de lucha antivectorial (principalmente microesferas de poliestireno) en aldeas de Tirukoilur, en el sur de la India, durante 1995-1999. Posteriormente ha proseguido la administración de medicamentos, utilizando conjuntamente dietilcarbamazina y albendazol, o bien sólo dietilcarbamazina. Evaluamos el impacto de la administración masiva de medicamentos por separado o unida a medidas de lucha antivectorial (entre 1995 y 2005) en los índices de transmisión vectorial (como el índice de intensidad de transmisión, la tasa de picaduras al mes, el potencial de transmisión mensual y el potencial de transmisión anual). A partir de los datos sobre la infección filárica en la comunidad se estimó la prevalencia de microfilaremia y antigenemia usando la prueba de ji cuadrado y la prueba exacta de Fisher.
Resultados
La densidad de vectores disminuyó considerablemente en las aldeas donde se recurrió a medidas de lucha antivectorial como complemento de la administración masiva de medicamentos, y entre los escasos mosquitos supervivientes apenas se hallaron ejemplares infecciosos. La antigenemia filárica fue baja y siguió disminuyendo de forma significativa en el grupo de edad de 15 a 25 años en las aldeas donde la administración masiva de medicamentos se combinó con la lucha antivectorial, a diferencia de las aldeas en que sólo se hizo lo primero.
Conclusión
Los beneficios conseguidos mediante la administración masiva de medicamentos sólo pudieron mantenerse integrando un componente de control de los vectores. Preconizamos la incorporación de la lucha antivectorial en el Programa Mundial de Eliminación de la Filariasis Linfática, pues ello permitiría reducir el tiempo requerido para eliminar esa enfermedad.
ملخص
الەدف
التعرف على دور مكافحة نواقل المرض في إحراز خفضٍ أكثر لسراية داء الفلاريات البنكروفتية تلو إعطاء الأدوية، والتعرف على التأثير على مَناسِب القياسات الفلارية على المدى الطويل.
الطريقة
استكملت ثلاث دورات من الإعطاءٍ الجموعي لدوائَيْ الإيفيرميكتين ودي إيثيل كاربامازين وذلك بمكافحة نواقل المرض (وبشكل رئيسي باستخدام حبات البولي ستيرين في قرى تيروكويلور في جنوب الەند في الفترة بين عامي 1995 و1999). وەكذا فإن إعطاء الأدوية قد نُفِّذ بتوزيع دي إيثيل كاربامازين مع الألبندازول أو بتوزيع دي إيثيل كاربامازين لوحدة. وأجرينا تقيـيماً لتأثير الإعطاء الجموعي للأدوية لوحدە أو مصحوباً بمكافحة نواقل المرض (منذ عام 1995 وحتى 2005) على مَناسِب سراية نواقل المرض (مثل مَنْسَب شدة السراية، ومُعدَّل اللسع الشەري، وإمكانية السراية الشەرية وإمكانية السراية السنوية) وقد أجرينا تحليلاً للمعطيات حول العدوى بالفلاريات في المجتمع لتقدير معدل انتشار وجود الفلاريات في الدم ووجود مستضدەا في الدم، وذلك باستخدام تحليل خي مربع واختبار الدقة لفيشر.
الموجودات
لقد نقصت كثافة نواقل المرض بشكل واضح في القرى التي أجريت فيەا مكافحة نواقل المرض إلى جانب الإعطاء الجموعي للأدوية، ولم يكشف عن أي بعوضة تحمل العدوى في الأعداد الضئيلة الباقية منەا على قيد الحياة. وقد كان معدل وجود المستضدات الفلارية في الدم منخفضاً، وتواصل انخفاضە بشكل واضح في الفئات العمرية بين 15 – 25 عاماً في القرى التي أعطي فيەا الدواء جموعياً مع مكافحة نواقل المرض، وذلك بعكس القرى التي لم تـتلق سوى الإعطاء الجموعي للأدوية.
الاستنتاج
لا يمكن ضمان استدامة المكاسب من الإعطاء الجموعي للأدوية إلا باستكمال وسائل مكافحة نواقل المرض. وينصح بإدراج مكافحة نواقل المرض ضمن البرنامج العالمي للتخلص من داء الفلاريات اللمفية، فذلك قد يؤدي لتقصير الوقت اللازم للتخلص منە.
Introduction
Lymphatic filariasis is a major cause of acute and chronic morbidity among humans in tropical and subtropical areas of Asia, Africa, the western Pacific and some parts of the Americas. Of the estimated 128 million cases of lymphatic filariasis, 91% are caused by Wuchereria bancrofti.1 The Global Programme to Eliminate Lymphatic Filariasis (GPELF) was launched in 2000 based on the principles of interruption of transmission, and alleviation and prevention of disability due to lymphatic filariasis.2 Currently, the GPELF depends largely on mass drug administration (MDA) to interrupt the transmission of W. bancrofti. This strategy is based on the evidence that single annual doses of antifilarial drugs (diethylcarbamazine (DEC) with or without ivermectin (IVR) or albendazole (ALB)) can suppress microfilaraemia for prolonged periods, and the cumulative effect is expected to lead towards the elimination of lymphatic filariasis.3,4
Globally, the majority of W. bancrofti is transmitted by Culex quinquefasciatus, which typically breeds in stagnant and organically polluted water.5 It seems unlikely that MDA would be sufficient for sustained interruption of transmission in areas of Culex transmission of lymphatic filariasis, due to their high vectorial efficiency.6 Therefore, vector control would be an important supplement to sustain the interruption of transmission in some epidemiological settings.7 In Makunduchi, Zanzibar, the Culex mosquito population decreased by about 98% after applying expanded polystyrene (EPS) beads to all the wet pit latrines, without any change in a nearby untreated community.8 One round of MDA with DEC resulted in decreasing the proportion of mosquitoes with third-stage larvae (L3) causing an overall 99.7% decrease in the number of infective bites per year in the treated area. In this area, microfilaraemia also remained low for 10 years, whereas in another Zanzibari community where only one round of MDA was given, microfilaraemia reemerged after five years.
In the present study, we aimed to determine the role of vector control (with EPS beads in soakage pits and larvivorous fishes in unused wells) when used as adjunct to MDA given annually (not just for one year, as in United Republic of Tanzania) in reinforcing the effects of annual MDA on antigenaemia and microfilaraemia.
Methods
Study area
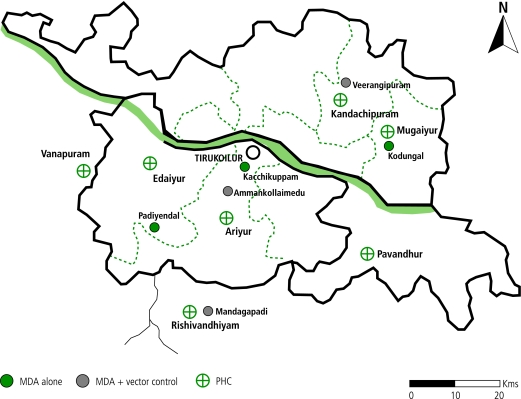
The study area was located in the filaria endemic villages of Tirukoilur (latitude: 11º57’00”; longitude: 79º12’00”) of Villupuram district, Tamil Nadu state, south India, 40–80 km inland from Pondicherry on the east coast (Fig. 1). Most of the annual rainfall (mean = 1125 mm) occurs during the north-east monsoon months of October–December. Agriculture is the predominant occupation of the study population with the majority being landless labourers depending on agriculture and livestock husbandry for their survival. The population depends mainly on primary health centres for health care.
Fig. 1.
Map of the study area showing six filarial endemic villages of Tirukoilur, India
Study design
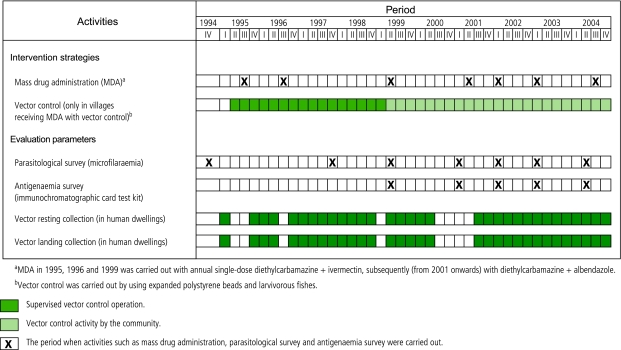
We have been conducting lymphatic filariasis control studies in nine villages of south India from 1995 to 1999 and in six villages since 2000.9,10 The nine villages were randomly allocated to three groups; one group of three villages received MDA (DEC + IVR) in 1995 and 1996; a second group of three villages received a combined approach of MDA (DEC + IVR) with vector control in 1995 and 1996; and a third group of three villages was the placebo group until 1999, for comparison. In 1999, as the placebo group also received antifilarial drugs, we confined our analyses to six villages — those receiving MDA only versus those receiving MDA with vector control. From 2001, these six villages were included in the GPELF programme with the community carrying out vector control activities in villages receiving MDA with vector control (Fig. 2). The institutional ethical committee has approved this study.
Fig. 2.
Time schedule of entomological and parasitological evaluation following intervention strategies in the filaria endemic villages of Tirukoilur, India
Intervention strategies
Mass drug administration
We conducted three rounds of MDA with DEC + IVR in all the six villages during 1995–99. Of the total residents eligible for treatment more than 90% took the drugs. We carried out MDA through door-to-door visits. The Government of Tamil Nadu included these villages in the GPELF in 2001 using DEC alone in one village and DEC + ALB in the other two (Fig. 2). In villages receiving MDA with vector control, one village received DEC + ALB while two villages received DEC alone.
Vector control activities
In urban areas of India Culex breeds heavily in blocked drains. But in rural areas, where our study was carried out, the soakage pits in the backyard of each household rendered an environment suitable for breeding of the vector. Unused wells were the other primary breeding habitat in this area. We undertook vector control operations in all three villages by modifying all the soakage pits and subsequently applying EPS beads @ 350–400 g/m² water surface area between 1995 and 1999.10 We carried out the cleaning of soakage pits, bead expansion and application with active involvement of the community. During the initial period, we introduced larvivorous fishes in the unused wells. We monitored the vector breeding habitats until October 1997, after which the community assumed responsibility for it.
Monitoring and evaluation
Vector transmission parameters
We monitored the vectors every month in the six villages by collecting adult Culex quinquefasciatus “resting” in 16 houses between 09:00 and 11:00 hours, spending 15 minutes in each house. We also collected mosquitoes “landing” on human volunteers from 18:00 to 06:00 hours, from one village in each intervention strategy, i.e. MDA alone and MDA with vector control, every month. After the MDA in 2001, landing collections were made every quarter. Mosquitoes from the resting and landing collections were identified in the laboratory and we dissected all female mosquitoes to determine the parity and filarial infection status. We calculated infection rates by including all mosquitoes found to have microfilariae and/or other filarial larval stages. Infectivity rates were based on mosquitoes with third-stage larvae (L3; infective stage). From these data we estimated vector density, infection and infectivity rates, the transmission intensity index (TII) and annual transmission potential (ATP).10 ATP is the sum of all monthly transmission potentials. ATP was calculated based on an estimate of the annual biting rate from the mean landing rate per hour multiplied by 12 hours of the night and 365 nights of the year. In contrast, TII was based on the number of catches of resting mosquitoes.11
Filarial infection in the community
We measured the microfilaraemia status in the community in 1994 (pre-treatment), in 1997 (one year after two annual MDAs) and in 1999 (before the 1999 MDA). We used the conventional finger prick–thick smear method for determining microfilaraemia by collecting 20 µl blood between 21:00 and 00:00 hours from 10% of the randomly selected population.10 During the 1999 survey, the antigenaemia (filarial antigen) prevalence (AGP) was also estimated from 100 µl of blood placed on a immunochromatographic card test (ICT) kit, from the same individuals who were screened for microfilaraemia.9 We observed the antigenaemia and microfilaraemia status in subsequent parasitological surveys (2001–04) where only age groups 2–5 years and 15–25 years were screened. While the 2001 survey was carried out before MDA in 2001, in subsequent years the surveys were carried out one year after each MDA.
Data analyses
We analysed the data on filarial infection obtained from humans as well as mosquito vectors until 2005. We tested the significance of the prevalence of microfilaraemia and antigenaemia in the two treatment arms (before and after MDA) using χ² analysis and Fisher’s exact test. Geometric mean intensities of microfilaraemia were calculated as antilog [Σ log (x+1)/n] –1, with ‘x’ being the number of microfilarae/20 µl of blood and ‘n’ the number of individuals examined, including microfilaraemia-negative individuals. We attached binomial confidential intervals to the proportions of the vector infection and infectivity rates.12
Findings
Vector transmission parameters
We found a drastic decrease in the vector density in villages receiving MDA with vector control (Table 1 and Table 2) and this was sustained throughout the study period.
Table 1. Entomological parameters estimated from the indoor resting Culex quinquefasciatus, Tirukoilur, India.
| Treatment in village | Period | Mosquitoes caught per worker hour | Number dissected | Number infected (%) with 95% CIa | Number infective (%) with 95% CI | Mean number of third-stage larvae (L3) | Transmission intensity indexb |
|---|---|---|---|---|---|---|---|
| Mass drug administration (MDA) alone | July 1999–March 2002 | 23.70 | 2108 | 44 (2.09)c [1.6–2.8] | 9 (0.43)d [0.02–0.8] | 3.44 | 0.3486 |
| April 2002–August 2005 | 39.15 | 3073 | 43 (1.40)c [1.0–1.9] | 6 (0.20)d [0.1–1.9] | 4.33 | 0.3312 | |
| MDA with vector control | July 1999–March 2002 | 1.49 | 373 | 6 (1.61)c [0.7–3.5] | 2 (0.54)d [0.1–1.9] | 1.00 | 0.0080 |
| April 2002–August 2005 | 0.83 | 354 | 3 (0.85)c [0.3–2.5] | 0 (0)d [1.1] | 0 | 0 |
a 95% binomial confidence interval. b Transmission intensity index = mosquitoes caught per man hour x proportion infective x mean L3. c No significant difference among % infected (based on χ² or Fisher’s exact test). d No significant difference among % infective (based on χ² or Fisher’s exact test).
Table 2. Entomological parameters estimated from the indoor landing Culex quinquefasciatus, Tirukoilur, south India.
| Treatment in villages | Period | Mosquitoes caught per worker hour | Number dissected | Number infected (%) with 95% CIa | Number infective (%) with 95% CIa | Mean number of third-stage larvae (L3) | Mean annual biting rate | Mean annual infective biting rate | Mean annual transmission potential |
|---|---|---|---|---|---|---|---|---|---|
| Mass drug administration (MDA) alone | July 1999–March 2002 | 14.44 | 4678 | 64 (1.37)b [1.1–1.7] | 7 (0.15)c [0.1–0.3] | 4.14 | 54 309 | 159.70 | 326.98 |
| April 2002–August 2005 | 4.16 | 549 | 9 (1.64)b [0.9–3.1] | 1 (0.18)c [0.03–1.0] | 15.00 | 17 661 | 30.42 | 456.25 | |
| MDA with vector control | July 1999–March 2002 | 0.17 | 66 | 0 (0)b [5.5] | 0 (0)c [5.5] | 0 | 1833 | 0 | 0 |
| April 2002–August 2005 | 0.32 | 66 | 0 (0)b [5.5] | 0 (0)c [5.5] | 0 | 2008 | 0 | 0 |
a 95% binomial confidence interval. b No significant difference among % infected (based on χ² or Fisher’s exact test). c No significant difference among % infective (based on χ² or Fisher’s exact test).
Considerable numbers of filarial larvae (including L3 stages) were found in the mosquitoes caught in the villages receiving MDA alone. Very few (Table 1) or no (Table 2) filarial larvae were found in villages receiving MDA with vector control but very few mosquitoes were available for dissection here. The very low or zero infection and infectivity rate had a wide 95% confidence limit and did not differ significantly from those in villages receiving MDA alone.
Filarial infection status
Microfilaraemia
We observed that the prevalence and intensities of microfilaraemia decreased sharply (in the survey during 1997) in villages receiving both MDA alone and MDA with vector control (88% to 92%),10 after two MDAs using DEC + IVR (carried out in 1995 and 1996) (Table 3). In the subsequent survey in 1999 (without any MDAs in between) the microfilarial prevalence and intensities resurged in villages receiving MDA alone but did not do so in villages receiving MDA with vector control.
Table 3. Microfilaraemia status before and after two rounds of mass drug administration (diethylcarbamazine and ivermectin) alone or combined with vector control, Tirukoilur, India.
| Treatment in villages | Microfilaraemia prevalence (%) |
Microfilaraemia intensity
(geometric mean intensities; GMI) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-mass drug administration (MDA) (1994) | Post-MDA |
Pre- MDA (1994) | Post-MDA |
||||||||
| 1997a | % decreaseb | 1999c | % decreaseb | 1997a | % decreaseb | 1999c | % decreaseb | ||||
| MDA alone | 15.19 (724) | 1.81 (609) | 88.1d | 4.74 (591) | 68.8d | 0.4804 | 0.0517 | 89.2d | 0.1174 | 75.6d | |
| MDA with vector control | 15.09 (795) | 1.24 (645) | 91.8d | 2.08 (673) | 86.2d | 0.5569 | 0.0491 | 91.2d | 0.0431 | 91.9d | |
a After two rounds of MDA (in 1995 and 1996). b Decrease with respect to pre-MDA. c No MDAs during 1997 and 1998. d P< 0.05.
We found microfilaraemia in two age groups during 1999–2002 and 2003–04 (Table 4). The observed microfilaraemia prevalences continued to be lower among villages receiving MDA with vector control than among villages receiving MDA alone, but the differences were either not significant or of only borderline significance.
Table 4. Microfilaraemia prevalences in two age groups in villages that received mass drug administration alone or combined with vector control, Tirukoilur, India.
| Treatment in villages | Age group | 1999–2002 |
2003–04 |
|
|---|---|---|---|---|
| % positive (number tested) | % positive (number tested) | |||
| Mass drug administration (MDA) alone | 2–5 | 0.9a (333) | 0.85a (353) | |
| MDA with vector control | 0a (379) | 0b (327) | ||
| MDA alone | 15–25 | 4.2c (334) | 3.3c,d (608) | |
| MDA with vector control | 1.5d (395) | 1.8d (621) |
a,b,c,d Data sharing the same superscript letter do not differ statistically significantly (based on χ² or Fisher’s exact test).
Antigenaemia
We found statistically comparable estimates of AGP in 1999 for villages receiving MDA alone and those receiving MDA with vector control — 16.75% and 14.41% (χ² = 1.14; P = 0.2855), respectively. The AGP among children 2–5 years old was low (5.33%) in villages receiving MDA with vector control as compared to villages receiving MDA only (7.32%), but the difference was not statistically significant on Fisher’s exact test (P > 0.05). Similarly, the AGP in the age group 15–25 years were not significantly different between the two treatment arms during 1999.
Antigenaemia rates for 1999–2002 and 2003–04 (Table 5) were significantly less among villages receiving MDA with vector control than for villages receiving MDA alone for the age group 2–5 years. We found a definite impact of vector control on the age group 15–25 years among villages receiving MDA with vector control, whereas there was no significant impact relating to age group among villages receiving MDA only. Villages receiving MDA with vector control did show a highly significant decrease in antigenaemia rates between the periods 1999–2002 and 2003–04.
Table 5. Antigenaemia prevalences in two age groups in villages that received mass drug administration alone or combined with vector control, Tirukoilur, India.
| Treatment in villages | Age group | 1999–2002 |
2003–04 |
|
|---|---|---|---|---|
| % positive (number tested) | % positive (number tested) | |||
| Mass drug administration (MDA) alone | 2–5 | 7.2a (333) | 4.5a (200) | |
| MDA with vector control | 3.4a (366) | 2.0b (200) | ||
| MDA alone | 15–25 | 20.4c (334) | 19.5c (200) | |
| MDA with vector control | 17.7d (361) | 5.5e (200) |
a,b,c,d,e Data sharing the same superscript letter do not differ statistically significantly (based on χ² test).
Discussion
We provide strong evidence of the benefit of integrating vector control with MDA (Table 1, Table 2, Table 3 and Table 5). Culicines exhibit the phenomenon of limitation or negative density-dependence,13,14 in which the parasite yield (L3) increases when the number of ingested microfilariae is low. These vectors are capable of picking up microfilariae and developing L3 larvae after feeding on very low-level microfilaraemia carriers.15,16 In areas where limitation occurs, eradication of filariasis is hard to achieve.15 Although intensive MDA using DEC (and some vector control) has been carried out in Polynesia for more than 50 years, elimination was not attained on any of the islands where W. bancrofti was transmitted by Aedes polynesiensis.17 Prevalence decreased in a few years from 30–35% to 3–6%, and then was maintained at this level for several decades. A test of interruption of MDA in one of the islands resulted in filariasis prevalence returning to initial levels within five years. We found that it was advantageous to use polystyrene bead treatment (Table 1 and Table 2) to maximize the chances of success in decreasing vector density. The much lower mosquito densities in villages receiving MDA with vector control confirm that transmission was very low there. The zero values calculated for TII and ATP in 2002–05 in three villages are very encouraging but we cannot assert that there was absolutely no transmission in these villages.
Microfilaraemia
We did not observe a significant short-term decrease in microfilaraemia when vector control was used as an adjunct to MDA (Table 3 and Table 4). While we found evidence that on stopping MDA resurgence occurred (Table 3), we believe that this could be prevented if the vector population is suppressed by using polystyrene beads, similar to the experience reported from Zanzibar.18
Antigenaemia
Estimation of antigenaemia prevalence is becoming increasingly important to evaluate the impact of filarial control programmes, especially when microfilaraemia decreases to near zero in specific age groups, as in French Polynesia.19 Antigenaemia positivity was reported to be low in villages where the ATP was also low.20 A greater proportion of antigenaemia-positive children (91%) from villages receiving MDA with vector control were amicrofilaraemic and expected to have lower antigen levels compared to antigenaemia-positive and microfilaraemia-positive children21,22 highlighting the role of vector control in restricting worm burden.
We observed a significant benefit of vector control when used as an adjunct to MDA in decreasing antigenaemia levels among young children (age group 2–5 years). There was no apparent decrease in antigenaemia from MDA alone among young adults (age group 15–25 years), but a very significant decrease when vector control was used as adjunct to MDA (Table 5).
Role of vector control in filariasis elimination
We found that annual MDAs alone decreased the filarial infection load in the community if there were no lapses. However, residual microfilaraemia of 0.4% and antigenaemia positivity of 4.6% were observed even after 36 years of filariasis control in French Polynesia.19 MDA with “DEC drug combination” was found to be more effective than DEC alone in decreasing filarial infection variables.23,24 Vector control was found to be important during any lapse in the MDA programme.9,18 The importance of vector control methods has been emphasized, as they play a key role in the prevention of disease transmission.25 In China, the campaign against lymphatic filariasis turned successful when vector control was integrated with other intervention measures, such as DEC administration (selective and mass treatment, and as fortified salt), resulting in the interruption of filarial transmission without any resurgence.26,27 In Brazil,28 Zanzibar8,18 and India,10 the impact of MDA in combination with vector control has been extensively studied. The usefulness of polystyrene beads in decreasing the vector population in different field settings has been established.29 Furthermore, it has been reported that even a lower drug coverage can achieve the set control criteria with the inclusion of a vector control component to MDA, therefore decreasing the number of years required to attain the target of infection elimination.30 Thus, we believe that the integration of vector control with MDA can decrease the time required for elimination by complementing the benefits brought about by MDA. The maintenance of low transmission levels for a sufficiently long period to interrupt transmission is a more affordable and sustainable way to eliminate filariasis, especially when communities can be empowered to carry out simple vector control operations along with MDA. Achievement of <100 ATP and <0.5 TII, are considered as levels necessary for preventing the occurrence of new infections.31
While our data indicate that using MDA alone was initially successful (Table 3) in decreasing the prevalence of filariasis, we noted no further decrease with prolonged use of MDA alone (Table 4 and Table 5) until it was complemented by vector control approaches. Thus, we advocate the integration of vector control in the Global Programme to Eliminate Lymphatic Filariasis. ■
Acknowledgements
We wish to thank the director general of the Indian Council of Medical Research, New Delhi, India, for providing research facilities. We are grateful to the director of the Tamil Nadu Public Health Department and his staff for their cooperation in the field study. We thank the staff of the Centre for Research in Medical Entomology at Madurai and its Field Station at Tirukoilur for their excellent cooperation in carrying out this study. We also thank R Balasubramanian, Assistant Programmer, Statistics, at the Centre for Research in Medical Entomology for his help in the data analyses and the medical officers for their clinical expertise during the MDA programme. We are grateful to the panchayat presidents and the villagers in the study communities for their active cooperation in conducting the field trials. We appreciate the excellent help, particularly related to word processing, rendered by A Venkatesh of the Centre for Research in Medical Entomology, Madurai.
Footnotes
Funding: This investigation received partial financial support from WHO/TDR (grant ID No.940340) and WHO/CTD/FIL (ID No.990574), Geneva.
Competing interests: none declared.
References
- 1.Michael E, Bundy DA. Global mapping of lymphatic filariasis. Parasitol Today. 1997;13:472–6. doi: 10.1016/S0169-4758(97)01151-4. [DOI] [PubMed] [Google Scholar]
- 2.Ottesen EA, Duke BO, Karam M, Behbehani K. Strategies and tools for the control/elimination of lymphatic filariasis. Bull World Health Organ. 1997;75:491–503. [PMC free article] [PubMed] [Google Scholar]
- 3.Ottesen EA. The Global Programme to Eliminate Lymphatic Filariasis. Trop Med Int Health. 2000;5:591–4. doi: 10.1046/j.1365-3156.2000.00620.x. [DOI] [PubMed] [Google Scholar]
- 4.Bockarie MJ, Tisch DJ, Kastens W, Alexander ND, Dimber Z, Bockarie F, et al. Mass treatment to eliminate filariasis in Papua New Guinea. N Engl J Med. 2002;347:1841–8. doi: 10.1056/NEJMoa021309. [DOI] [PubMed] [Google Scholar]
- 5.Curtis CF, Feachem RG. Sanitation and Culex pipiens mosquitoes: a brief review. J Trop Med Hyg. 1981;84:17–25. [PubMed] [Google Scholar]
- 6.Jayasekera N, Kalpage KS, De Silva CS. The significance of low-density microfilaraemia in the transmission of Wuchereria bancrofti by Culex (Culex) quinquefasciatus Say in Sri Lanka. Trans R Soc Trop Med Hyg. 1991;85:250–4. doi: 10.1016/0035-9203(91)90044-Y. [DOI] [PubMed] [Google Scholar]
- 7.Burkot T, Durrheim D, Melrose W, Speare R, Ichimori K. The argument for integrating vector control with multiple drug administration campaigns to ensure elimination of lymphatic filariasis. Filaria J. 2006;5:10. doi: 10.1186/1475-2883-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maxwell CA, Curtis CF, Haji H, Kisumku S, Thalib AI, Yahya SA. Control of bancroftian filariasis by integrating therapy with vector control using polystyrene beads in wet pit latrines. Trans R Soc Trop Med Hyg. 1990;84:709–14. doi: 10.1016/0035-9203(90)90158-B. [DOI] [PubMed] [Google Scholar]
- 9.Sunish IP, Rajendran R, Mani TR, Munirathinam A, Tewari SC, Hiriyan J, et al. Resurgence in filarial transmission after withdrawal of mass drug administration and the interrelationship between antigenaemia and microfilaraemia — a longitudinal study. Trop Med Int Health. 2002;7:59–69. doi: 10.1046/j.1365-3156.2002.00828.x. [DOI] [PubMed] [Google Scholar]
- 10.Reuben R, Rajendran R, Sunish IP, Mani TR, Tewari SC, Hiriyan J, et al. Annual single-dose diethylcarbamazine plus ivermectin for control of bancroftian filariasis: comparative efficacy with and without vector control. Ann Trop Med Parasitol. 2001;95:361–78. doi: 10.1080/00034980120065796. [DOI] [PubMed] [Google Scholar]
- 11.Rao CK, Sundaram RM, Venkatanarayana M, Rao JS, Chandrasekharan A, Rao CK. Epidemiological studies on bancroftian filariasis in East Godavari district (Andhra Pradesh): entomological aspects. J Commun Dis. 1981;13:81–91. [PubMed] [Google Scholar]
- 12.Vollset SE. Confidence intervals for a binomial proportion. Stat Med. 1993;12:809–24. doi: 10.1002/sim.4780120902. [DOI] [PubMed] [Google Scholar]
- 13.Pichon G, Prod’hon J, Riviere F. A distribution law for microfilaria ingested by mosquitoes biting human carriers. Preliminary results. C R Acad Sci Hebd Seances Acad Sci D. 1975;280:717–9. [Article in French] [PubMed] [Google Scholar]
- 14.Farid HA, Hammad RE, Hassan MM, Ramzy RM, El Setouhy M, Weil GJ. Effects of combined diethylcarbamazine and albendazole treatment of bancroftian filariasis on parasite uptake and development in Culex pipiens L. Am J Trop Med Hyg. 2005;73:108–14. [PubMed] [Google Scholar]
- 15.Southgate BA, Bryan JH. Factors affecting transmission of Wuchereria bancrofti by anopheline mosquitoes. 4. Facilitation, limitation, proportionality and their epidemiological significance. Trans R Soc Trop Med Hyg. 1992;86:523–30. doi: 10.1016/0035-9203(92)90096-U. [DOI] [PubMed] [Google Scholar]
- 16.McGreevy PB, Kolstrup N, Tao J, McGreevy MM, Marshall TF. Ingestion and development of Wuchereria bancrofti in Culex quinquefasciatus, Anopheles gambiae and Aedes aegypti after feeding on humans with varying densities of microfilariae in Tanzania. Trans R Soc Trop Med Hyg. 1982;76:288–96. doi: 10.1016/0035-9203(82)90170-5. [DOI] [PubMed] [Google Scholar]
- 17.Pichon G. Limitation and facilitation in the vectors and other aspects of the dynamics of filarial transmission: the need for vector control against Anopheles-transmitted filariasis. Ann Trop Med Parasitol. 2002;96:S143–52. doi: 10.1179/000349802125002509. [DOI] [PubMed] [Google Scholar]
- 18.Maxwell CA, Mohammed K, Kisumku U, Curtis CF. Can vector control play a useful supplementary role against bancroftian filariasis? Bull World Health Organ. 1999;77:138–43. [PMC free article] [PubMed] [Google Scholar]
- 19.Esterre P, Plichart C, Sechan Y, Nguyen NL. The impact of 34 years of massive DEC chemotherapy on Wuchereria bancrofti infection and transmission: the Maupiti cohort. Trop Med Int Health. 2001;6:190–5. doi: 10.1046/j.1365-3156.2001.00682.x. [DOI] [PubMed] [Google Scholar]
- 20.Tisch DJ, Hazlett FE, Kastens W, Alpers MP, Bockarie MJ, Kazura JW. Ecologic and biologic determinants of filarial antigenemia in bancroftian filariasis in Papua New Guinea. J Infect Dis. 2001;184:898–904. doi: 10.1086/323324. [DOI] [PubMed] [Google Scholar]
- 21.Chanteau S, Glaziou P, Luquiaud P, Plichart C, Moulia-Pelat JP, Cartel JL. Og4C3 circulating antigen, anti-Brugia malayi IgG and IgG4 titers in Wuchereria bancrofti infected patients, according to their parasitological status. Trop Med Parasitol. 1994;45:255–7. [PubMed] [Google Scholar]
- 22.Nicolas L. New tools for diagnosis and monitoring of bancroftian filariasis parasitism: the Polynesian experience. Parasitol Today. 1997;13:370–5. doi: 10.1016/S0169-4758(97)01125-3. [DOI] [PubMed] [Google Scholar]
- 23.Rajendran R, Sunish IP, Mani TR, Munirathinam A, Abdullah SM, Arunachalam N, et al. Impact of two annual single-dose mass drug administrations with diethylcarbamazine alone or in combination with albendazole on Wuchereria bancrofti microfilaraemia and antigenaemia in south India. Trans R Soc Trop Med Hyg. 2004;98:174–81. doi: 10.1016/S0035-9203(03)00042-7. [DOI] [PubMed] [Google Scholar]
- 24.Tisch DJ, Michael E, Kazura JW. Mass chemotherapy options to control lymphatic filariasis: a systematic review. Lancet Infect Dis. 2005;5:514–23. doi: 10.1016/S1473-3099(05)70192-4. [DOI] [PubMed] [Google Scholar]
- 25.Townson H, Nathan MB, Zaim M, Guillet P, Manqa L, Bos R, et al. Exploiting the potential of vector control for disease prevention. Bull World Health Organ. 2005;83:942–7. [PMC free article] [PubMed] [Google Scholar]
- 26.Sun DJ, Chen PL. Filariasis surveillance at the post-control stage in China. Southeast Asian J Trop Med Public Health. 1992;23:369–76. [PubMed] [Google Scholar]
- 27.Cao W, Van der Ploeg CP, Ren Z, Habbema JD. Success against lymphatic filariasis. World Health Forum. 1997;18:17–20. [PubMed] [Google Scholar]
- 28.Regis L, Furtado AF, Oliveira CM, Bezerra CB, Silva LR, Araujo J, et al. Integrated control of the filariasis vector with community participation in an urban area of Recife, Pernambuco, Brazil. Cad Saude Publica. 1996;12:473–82. doi: 10.1590/s0102-311x1996000400005. [Article in Portugese] [DOI] [PubMed] [Google Scholar]
- 29.Curtis CF, Malecela-Lazaro M, Reuben R, Maxwell CA. Use of floating layers of polystyrene beads to control populations of the filaria vector Culex quinquefasciatus. Ann Trop Med Parasitol. 2002;96:S97–104. doi: 10.1179/000349802125002446. [DOI] [PubMed] [Google Scholar]
- 30.Michael E, Malecela-Lazaro MN, Kabali C, Snow LC, Kazura JW. Mathematical models and lymphatic filariasis control: endpoints and optimal interventions. Trends Parasitol. 2006;22:226–33. doi: 10.1016/j.pt.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Ramaiah KD, Das PK, Dhanda V. Estimation of permissible levels of transmission of bancroftian filariasis based on same entomological parasitological results of a 5-year vector control programme. Acta Trop. 1994;56:89–96. doi: 10.1016/0001-706X(94)90043-4. [DOI] [PubMed] [Google Scholar]