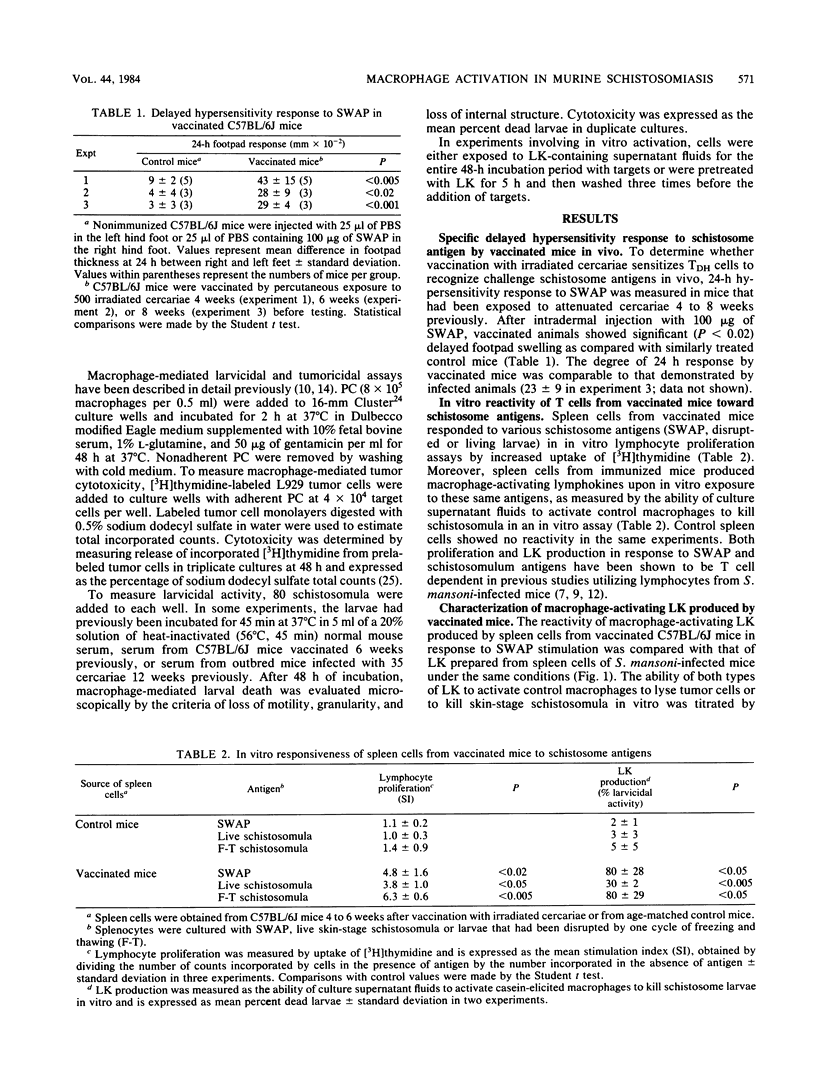
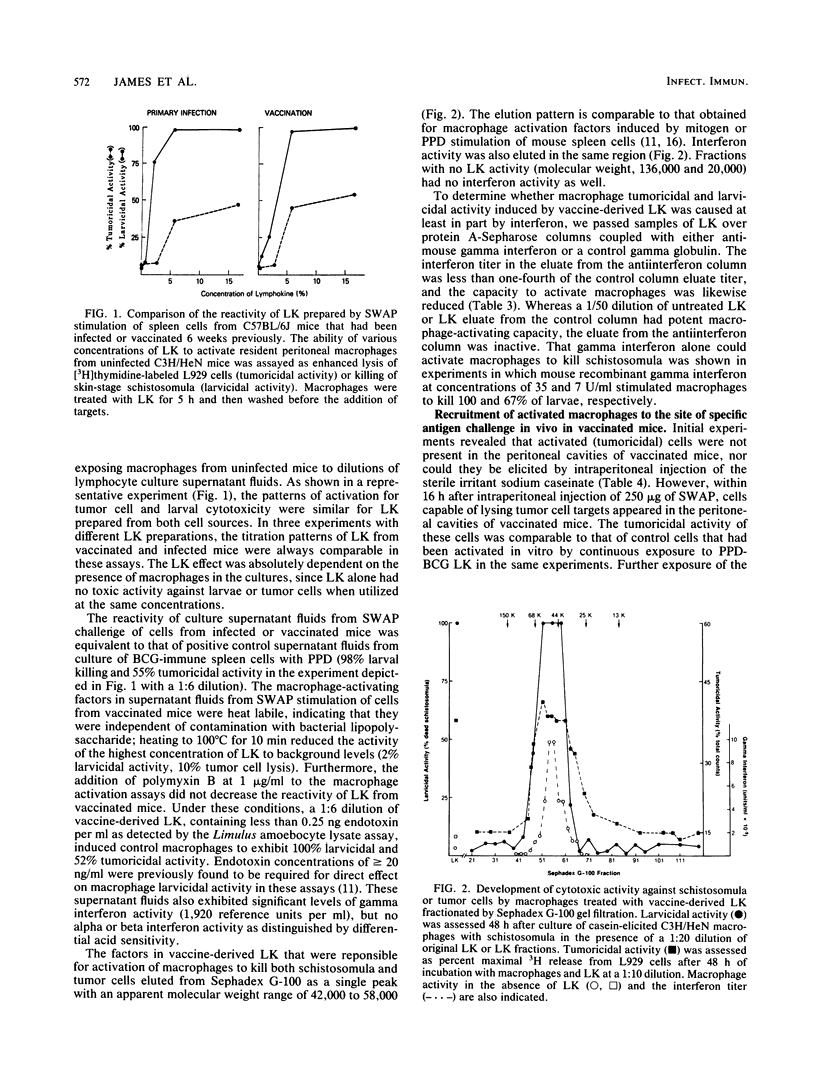
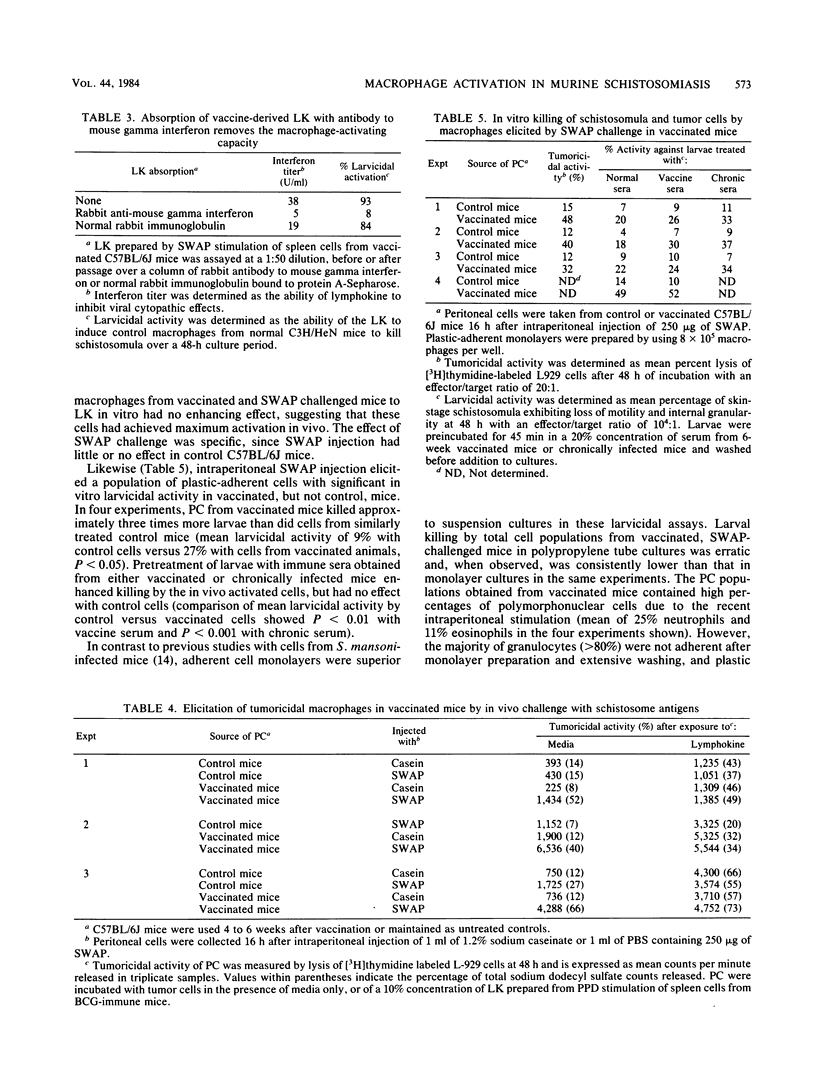
Abstract
Cell-mediated immune responses contributing to macrophage activation were compared in mice that demonstrated partial resistance to challenge Schistosoma mansoni infection as a result of vaccination with radiation-attenuated cercariae or of ongoing low-grade primary infection. Vaccinated mice developed significant delayed hypersensitivity reactions to soluble schistosome antigens in vivo. Splenocytes from vaccinated animals responded to in vitro culture with various specific antigens (soluble adult worm extract, living or disrupted schistosomula) by proliferation and production of macrophage-activating lymphokines as did lymphocytes from S. mansoni-infected animals. Macrophage-activating factors produced by spleen cells from vaccinated mice upon specific antigen stimulation eluted as a single peak on Sephadex G-100 with a molecular weight of approximately 50,000 and contained gamma interferon activity. Moreover, peritoneal macrophages with larvicidal and tumoricidal activity were recovered from vaccinated mice after intraperitoneal challenge with soluble schistosome antigens, a procedure also observed to elicit activated macrophages in S. mansoni-infected animals. These observations demonstrate that vaccination with irradiated cercariae stimulates many of the same cellular responses observed after primary S. mansoni infection, and suggest that lymphokine-activated macrophages may participate in the effector mechanism of vaccine-induced and concomitant immunity to challenge schistosome infection. This is the first demonstration of a potential immune effector mechanism in the irradiated vaccine model.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boraschi D., Meltzer M. S. Defective tumoricidal capacity of macrophages from A/J mice. II. Comparison of the macrophage cytotoxic defect of A/J mice with that of lipid A-unresponsive C3H/HeJ mice. J Immunol. 1979 Apr;122(4):1592–1597. [PubMed] [Google Scholar]
- Boraschi D., Meltzer M. S. Defective tumoricidal capacity of macrophages from P/J mice: characterization of the macrophage cytotoxic defect after in vivo and in vitro activation stimuli. J Immunol. 1980 Aug;125(2):771–776. [PubMed] [Google Scholar]
- Dean D. A., Minard P., Murrell K. D., Vannier W. E. Resistance of mice to secondary infection with Schistosoma mansoni. II. Evidence for a correlation between egg deposition and work elimination. Am J Trop Med Hyg. 1978 Sep;27(5):957–965. doi: 10.4269/ajtmh.1978.27.957. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Johnson H. M., Farrar J. J. Regulation of the production of immune interferon and cytotoxic T lymphocytes by interleukin 2. J Immunol. 1981 Mar;126(3):1120–1125. [PubMed] [Google Scholar]
- Gazzinelli G., de Oliveira C. C., Figueiredo E. A., Pereira L. H., Coelho P. M., Pellegrino J. Schistosoma mansoni: biochemical evidence for morphogenetic change from cercaria to schistosomule. Exp Parasitol. 1973 Oct;34(2):181–188. doi: 10.1016/0014-4894(73)90077-5. [DOI] [PubMed] [Google Scholar]
- James S. L. In vitro proliferative response to living schistosomula by T lymphocytes from mice infected with Schistosoma mansoni. Parasitology. 1981 Aug;83(Pt 1):147–162. doi: 10.1017/s0031182000050125. [DOI] [PubMed] [Google Scholar]
- James S. L., Labine M., Sher A. Mechanisms of protective immunity against Schistosoma mansoni infection in mice vaccinated with irradiated cercariae. I. Analysis of antibody and T-lymphocyte responses in mouse strains developing differing levels of immunity. Cell Immunol. 1981 Nov 15;65(1):75–83. doi: 10.1016/0008-8749(81)90053-8. [DOI] [PubMed] [Google Scholar]
- James S. L., Lazdins J. K., Hieny S., Natovitz P. Macrophages as effector cells of protective immunity in murine schistosomiasis. VI. T cell-dependent, lymphokine-mediated, activation of macrophages in response to Schistosoma mansoni antigens. J Immunol. 1983 Sep;131(3):1481–1486. [PubMed] [Google Scholar]
- James S. L., Lazdins J. K., Meltzer M. S., Sher A. Macrophages as effector cells of protective immunity in murine schistosomiasis. Cell Immunol. 1982 Mar 1;67(2):255–266. doi: 10.1016/0008-8749(82)90218-0. [DOI] [PubMed] [Google Scholar]
- James S. L., Leonard E. J., Meltzer M. S. Macrophages as effector cells of protective immunity in murine schistosomiasis. IV. Coincident induction of macrophage activation for extracellular killing of schistosomula and tumor cells. Cell Immunol. 1982 Nov 15;74(1):86–96. doi: 10.1016/0008-8749(82)90008-9. [DOI] [PubMed] [Google Scholar]
- James S. L., Sher A. Immune mechanisms that stimulate mouse leukocyte migration in response to schistosomula of Schistosoma mansoni. J Immunol. 1980 Apr;124(4):1837–1844. [PubMed] [Google Scholar]
- James S. L., Sher A., Lazdins J. K., Meltzer M. S. Macrophages as effector cells of protective immunity in murine schistosomiasis. II. Killing of newly transformed schistosomula in vitro by macrophages activated as a consequence of Schistosoma mansoni infection. J Immunol. 1982 Apr;128(4):1535–1540. [PubMed] [Google Scholar]
- James S. L., Sher A. Mechanisms of protective immunity against Schistosoma mansoni infection in mice vaccinated with irradiated cercariae III. Identification of a mouse strain, P/N, that fails to respond to vaccination. Parasite Immunol. 1983 Nov;5(6):567–575. doi: 10.1111/j.1365-3024.1983.tb00773.x. [DOI] [PubMed] [Google Scholar]
- James S. L., Skamene E., Meltzer M. S. Macrophages as effector cells of protective immunity in murine schistosomiasis. V. Variation in macrophage schistosomulacidal and tumoricidal activities among mouse strains and correlation with resistance to reinfection. J Immunol. 1983 Aug;131(2):948–953. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leonard E. J., Ruco L. P., Meltzer M. S. Characterization of macrophage activation factor, a lymphokine that causes macrophages to become cytotoxic for tumor cells. Cell Immunol. 1978 Dec;41(2):347–357. doi: 10.1016/0008-8749(78)90232-0. [DOI] [PubMed] [Google Scholar]
- Loveless S. E., Wellhausen S. R., Boros D. L., Heppner G. H. Tumoricidal macrophages isolated from liver granulomas of Schistosoma mansoni-infected mice. J Immunol. 1982 Jan;128(1):284–288. [PubMed] [Google Scholar]
- Minard P., Dean D. A., Jacobson R. H., Vannier W. E., Murrell K. D. Immunization of mice with cobalt-60 irradiated Schistosoma mansoni cercariae. Am J Trop Med Hyg. 1978 Jan;27(1 Pt 1):76–86. doi: 10.4269/ajtmh.1978.27.76. [DOI] [PubMed] [Google Scholar]
- Minard P., Dean D. A., Vannier W. E., Murrell K. D. Effect of immunization on migration of Schistosoma mansoni through lungs. Am J Trop Med Hyg. 1978 Jan;27(1 Pt 1):87–93. doi: 10.4269/ajtmh.1978.27.87. [DOI] [PubMed] [Google Scholar]
- Murrell K. D., Clark S., Dean D. A., Vannier W. E. Influence of mouse strain on induction of resistance with irradiated Schistosoma mansoni cercariae. J Parasitol. 1979 Oct;65(5):829–831. [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne L. C., Georgiades J. A., Johnson H. M. Classification of interferons with antibody to immune interferon. Cell Immunol. 1980 Jul 15;53(1):65–70. doi: 10.1016/0008-8749(80)90426-8. [DOI] [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Macrophage activation for tumor cytotoxicity: development of macrophage cytotoxic activity requires completion of a sequence of short-lived intermediary reactions. J Immunol. 1978 Nov;121(5):2035–2042. [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Macrophage activation for tumor cytotoxicity: induction of tumoricidal macrophages by supernatants of PPD-stimulated Bacillus Calmette-Guérin-immune spleen cell cultures. J Immunol. 1977 Sep;119(3):889–896. [PubMed] [Google Scholar]
- Schreiber R. D., Pace J. L., Russell S. W., Altman A., Katz D. H. Macrophage-activating factor produced by a T cell hybridoma: physiochemical and biosynthetic resemblance to gamma-interferon. J Immunol. 1983 Aug;131(2):826–832. [PubMed] [Google Scholar]
- Schultz R. M., Kleinschmidt W. J. Functional identity between murine gamma interferon and macrophage activating factor. Nature. 1983 Sep 15;305(5931):239–240. doi: 10.1038/305239a0. [DOI] [PubMed] [Google Scholar]
- Sher A., Hieny S., James S. L., Asofsky R. Mechanisms of protective immunity against Schistosoma mansoni infection in mice vaccinated with irradiated cercariae. II. Analysis of immunity in hosts deficient in T lymphocytes, B lymphocytes, or complement. J Immunol. 1982 Apr;128(4):1880–1884. [PubMed] [Google Scholar]
- Simon P. L., Farrar J. J., Dind P. D. Biochemical relationship between murine immune interferon and a killer cell helper factor. J Immunol. 1979 Jan;122(1):127–132. [PubMed] [Google Scholar]
- Smithers S. R., Terry R. J. The immunology of schistosomiasis. Adv Parasitol. 1969;7:41–93. doi: 10.1016/s0065-308x(08)60434-0. [DOI] [PubMed] [Google Scholar]
- Stirewalt M. A., Minnick D. R., Fregeau W. A. Definition and collection in quantity of schistosomules of Schistosoma mansoni. Trans R Soc Trop Med Hyg. 1966;60(3):352–360. doi: 10.1016/0035-9203(66)90299-9. [DOI] [PubMed] [Google Scholar]


