Abstract
We recently reported development of an experimental model for the study of nitric oxide (NO·) toxicology in vivo. SJL mice were injected with superantigen-bearing RcsX (pre-B-cell lymphoma) cells, which migrated to the spleen and lymph nodes, where their rapid growth induced activation of macrophages to produce large amounts of NO· over a period of several weeks. In the experiments described here, we used this model to investigate mutagenesis in splenocytes exposed to NO· during RcsX cell growth. Transgenic mice were produced by crossbreeding animals of the pUR288 transgenic C57BL/6 and SJL strains. RcsX cells were injected into F1 mice and NO· production was confirmed by quantification of urinary nitrate, the ultimate metabolite of NO·. Mutant frequency in the lacZ gene of the pUR288 plasmid was determined in DNA isolated from spleen (target) and kidney (nontarget) tissues. A significant elevation in mutant frequency was found in the spleen, but not in the kidney, of tumor-bearing mice. Furthermore, increases in mutant frequency in the spleen as well as NO· production were abrogated by administration of N-methylarginine, a NO· inhibitor, to mice following injection of RcsX cells. These results indicate that NO· had mutagenic activity in RcsX tumor-bearing mice and thus support a possible role for its involvement in the carcinogenic process.
Keywords: mutation, transgenic mice, pUR288
Elevation of risk for certain cancers by persistent inflammation has been suggested by extensive observational data (1, 2), but a causal relationship has yet to be established for any cancer. Central to proposed mechanisms through which inflammation may contribute to carcinogenesis is the migration of inflammatory cells to sites of infection or injury accompanied by release of reactive free radicals. The radicals, in turn, are hypothesized to deregulate cellular processes, increase cell turnover, and cause mutations that together facilitate the carcinogenic process (3, 4). Recent data have shown that the free radical nitric oxide (NO·) is present in inflammatory conditions associated with malignancies (5, 6), while studies in several in vitro experimental systems demonstrated that it is capable of inducing cellular damage and mutations (reviewed in refs. 2 and 7–9).
To assess in vivo toxicological responses to excess NO· production in intact animals, an experimental model utilizing the SJL mouse was developed (10, 11). In this model, mice were injected with superantigen-bearing RcsX (pre-B-cell lymphoma) cells. The superantigen is known to stimulate host Vβ16+ T cells (12) to secrete cytokines which, in turn, support the growth of RcsX tumor cells (13). In the course of this immune response, inducible NO synthase (iNOS) expression was induced in macrophages located in spleen and lymph nodes, resulting in a 50-fold increase in NO· production 14 days after tumor cell injection (11).
The experiments described here were designed to investigate mutagenesis in splenocytes exposed to NO· produced by splenic macrophages in response to RcsX cell growth. The target gene used was the lacZ gene of the pUR288 plasmid (14, 15), which was introduced by breeding C57BL/6#60 (pUR288 transgenic mice) with SJL mice. The spleen and lymph nodes of RcsX lymphoma-bearing C57BL/6 × SJL F1 (designated SJL#60) mice comprised an environment in which splenocytes were subjected to macrophage-derived NO· over a prolonged period of time. A significant increase in mutant frequency was observed in the lacZ transgene recovered from DNA of spleen, but not kidneys, of tumor-bearing SJL#60 mice. The increase was prevented by administration of the NO· synthase inhibitor, NG-methyl l-arginine acetate (NMA). This report demonstrates mutagenicity associated with endogenously produced NO· and suggests a potential mechanism for its involvement in the carcinogenic process.
MATERIALS AND METHODS
Animals.
Ten male SJL mice (The Jackson Laboratory) were bred with 10 female C57BL/6#60 transgenic mice homozygous for the pUR288 plasmid (kindly provided by J. Vijg, Beth Israel Hospital, Boston). Southern blot analyses were performed on DNA from male F1 offsprings to confirm that the transgene was uniformly inherited. Twelve- to 14-week-old female C57BL/6#60 × SJL F1 mice were fed a low nitrate control diet (AYN-76A; Bio-Serve, Frenchtown, NJ) for 3 weeks to minimize the background rate of nitrate excretion. Animals were then weighed and placed in pairs in metabolic cages, and their drinking water was replaced with either a 30 mM solution of the NO· synthase inhibitor NMA (Chem Biochem Research, Salt Lake City) or 30 mM ammonium acetate (Sigma). Two days later, six mice receiving NMA and six mice receiving ammonium acetate were each injected intravenously with 0.2 ml PBS containing 107 cells of the RcsX line (kindly provided by N. Ponzio, State University of New Jersey Medical Center, Newark) isolated from lymph nodes of SJL mice bearing actively growing tumors. Urine was collected on alternate days into tubes containing 0.5 ml 0.5 M NaOH to inhibit bacterial growth. As a measure of endogenous production of NO·, total urinary nitrate concentration was determined as described (16) and normalized to body weight. Animals were killed by CO2 asphyxiation 20 days after injection of RcsX cells; spleen, liver, and kidneys were removed from each animal and immediately frozen in liquid nitrogen.
DNA Isolation.
Tissues were homogenized by Polytron sonication in lysis buffer (10 mM Tris/150 mM NaCl/10 mM EDTA/1% SDS/100 μg/ml ribonuclease A). After homogenization, proteinase K was added to a final concentration of 0.5 mg/ml, and the samples were incubated overnight at 37°C. Samples were extracted once with phenol/chloroform/isoamyl alcohol (10:10:1, vol/vol), and twice with chloroform. DNA was precipitated in cold absolute ethanol, spooled onto a glass rod and washed with 5 ml cold ethanol. Purified DNA was air dried for 10 min and solubilized in 0.5 ml TE buffer (10 mM Tris·HCl, pH 7.5/1 mM EDTA).
Plasmid Rescue and Mutation Assay.
The mutation assay was performed essentially as described by Dolle et al. (15), based on the following general strategy. The pUR288 transgenic plasmid contains the Escherichia coli pBR322 origin of replication together with lacZ and ampicillin resistance genes. The plasmid is integrated into the genome of C57BL/6#60 transgenic mice in tandem arrays containing 40 copies of the lacZ gene. In these experiments, the plasmid was isolated by digestion of tissue DNA with HindIII, followed by incubation with magnetic beads coated with lacI protein, which captured plasmid by binding to the lacZ promoter. After washing, isopropyl β-d-thiogalactoside was added to the beads to release the plasmid. The isolated plasmid was circularized by ligation and transformed by electroporation into the indicator bacterial strain E. coli C (Δlac, galE). Plating efficiency was determined by plating 0.1% of the transformed bacteria on medium containing ampicillin and 5-bromo-4-chloro-3-indolyl β-d-galactoside; mutants were identified and enumerated by plating the remainder of the bacteria on medium containing ampicillin and phenyl β-d-galactopyranoside. In the presence of active β-galactosidase, phenyl β-d-galactopyranoside is converted to galactose. The first two genes of the galactose operon convert galactose to UDP-galactose. Mutational inactivation of galE, the third gene in the operon, blocks conversion of UDP-galactose to UDP-glucose resulting in accumulation of the toxic intermediate UDP-galactose in bacteria expressing functional lacZ (14, 17). Thus, bacteria harboring wild-type lacZ plasmids grow in the presence of ampicillin alone (plating efficiency), but fail to grow in the presence of phenyl β-d-galactopyranoside. In contrast, bacteria containing mutant lacZ plasmids grow under both conditions. Mutant frequency is therefore expressed as the ratio of phenyl β-d-galactopyranoside resistant colonies to ampicillin-resistant colonies.
Isolation and Characterization of Mutant Plasmids.
Total numbers of mutants isolated from specific tissue DNA samples varied from 7 to 139 (see Table 1). Whenever possible (the majority of instances), 24 independent mutant bacterial clones were selected from each mouse for characterization; otherwise, all mutants isolated were characterized. Mutant colonies were transferred from selective medium into 2 ml tubes containing 500 μl TB medium (12 g/liter Bacto-tryptone/24 g/liter Bacto-yeast extract/0.4% glycerol/17 mM KH2PO4/72 mM K2HPO4/25 μg/ml kanamycin/75 μg/ml ampicillin) and incubated overnight at 37°C.
Table 1.
LacZ mutant frequencies in spleens of RcsX tumor-bearing and control mice
| Treatment | Animal code | No. of colonies (×105) | No. of mutants | Mutant frequency (×10−5) |
|---|---|---|---|---|
| Control | 303 | 5.60 | 67 | 12.0 |
| 305 | 1.28 | 7 | 5.5 | |
| 306 | 1.68 | 6 | 3.6 | |
| 307 | 6.00 | 54 | 9.0 | |
| 301 | 5.12 | 50 | 9.8 | |
| 304 | 2.62 | 20 | 7.6 | |
| 309 | 2.16 | 16 | 7.4 | |
| 310 | 9.80 | 70 | 7.1 | |
| 7.7 ± 2.6 | ||||
| RcsX | 103 | 8.80 | 139 | 15.8 |
| 105 | 4.04 | 65 | 16.1 | |
| 106 | 2.72 | 25 | 9.2 | |
| 111 | 1.92 | 25 | 13.0 | |
| 104 | 3.56 | 43 | 12.1 | |
| 109 | 3.68 | 69 | 18.8 | |
| 101 | 4.86 | 123 | 25.3 | |
| 112 | 3.56 | 60 | 16.9 | |
| 15.9 ± 4.9 | ||||
| RcsX + NMA | 203 | 8.52 | 99 | 11.6 |
| 207 | 10.56 | 106 | 10.0 | |
| 206 | 2.48 | 18 | 7.3 | |
| 204 | 12.08 | 84 | 6.9 | |
| 201 | 9.40 | 100 | 10.6 | |
| 208 | 6.48 | 82 | 12.7 | |
| 205 | 3.60 | 52 | 14.4 | |
| 210 | 6.72 | 66 | 9.8 | |
| 10.4 ± 2.5 | ||||
| Control + NMA | 406 | 7.96 | 64 | 8.0 |
| 402 | 9.52 | 74 | 7.8 | |
| 401 | 2.40 | 13 | 5.4 | |
| 404 | 5.32 | 37 | 6.9 | |
| 403 | 4.08 | 36 | 8.8 | |
| 405 | 3.24 | 25 | 7.7 | |
| 407 | 2.16 | 24 | 11.1 | |
| 409 | 1.70 | 25 | 14.8 | |
| 8.8 ± 2.9 |
To identify lacZ mutants containing large deletions or insertions, crude DNA preparations were isolated from bacteria in 400 μl of each culture and subjected to electrophoresis on a 1% agarose gel according to the method of Liu and Mishra (18). Bacteria containing DNA with gel mobility of the wild-type plasmid were cultured in 6 ml TB medium overnight at 37°C, after which plasmid DNA was isolated and purified using the Perfect Prep kit (5 Prime → 3 Prime, Inc.). Sequences of the lacZ gene in mutant plasmids were then determined by automated cycle sequencing (Molecular Genetics Facility, University of Georgia, Athens) using the following primers: LacZ170, 5′-ttgtgtggaatt gtgagagcgg-3′; LacZ781, 5′-tgctgcgttg gagtgacggc-3′; LacZ1382, 5′-ctgttcgcatt atccgaacc-3′; LacZ1984, 5′-gcggtgattt tggcgatacg-3′; LacZ2581, 5′-cgctggataa cgacattggc-3′. Mutations were characterized by comparison of wild-type and mutant sequences with the sequencher program (Gene Codes, Ann Arbor, MI).
Statistics.
χ2 analysis revealed no excess variability among experiments performed on different days with respect to observed mutation frequency. Similarly, no excess variability was evident with respect to mutation frequency in DNA from kidneys of individual mice. Therefore, statistical analysis of mutation frequencies in kidney DNA was carried out on unpaired samples by the one-tailed Student’s t test. Conversely, in the case of spleen DNA, excess mouse-to-mouse variability and unequal variances were found to exist in mutation frequencies in different treatment groups; therefore, mutant frequencies of spleen DNA samples were compared using the two-sample t test for independent samples with unequal variances (Satterwaite’s method) as suggested by Piegorsch et al. (19) and described by Rosner (20).
RESULTS
Tumor Cell Growth and NO· Production.
The objective of this study was to adapt the pUR288 transgenic mutant detection system to the RcsX tumor-bearing mouse model to assess the mutagenicity of endogenously produced NO·. We first attempted to generate a pUR288 transgenic mouse in the SJL background by the conventional plasmid microinjection method, but were unsuccessful due to poor reproductive performance of SJL mice. Therefore, it was necessary to produce transgenic animals by crossbreeding animals of the C57BL/6#60 strain with SJL mice (F1 offspring designated as SJL#60). The two strains were known to be antigenically compatible, and C57BL/6#60 animals had previously been shown to support the growth of SJL-derived RcsX cells (N. Ponzio, personal communication). Preliminary experiments in our laboratory confirmed the growth of RcsX cells, and also showed that iNOS was expressed in the spleen of RcsX tumor-bearing SJL#60 mice (data not shown).
Four groups, each containing eight transgenic animals, were subjected to the following treatments in this study: (i) mice bearing the RcsX tumor, (ii) mice bearing the RcsX tumor and also treated with the NO· inhibitor NMA in their drinking water, (iii) untreated control mice, and (iv) control animals also administered NMA. Mice were each injected intravenously with 107 RcsX lymphoma cells on the first day of the experiment. Urinary nitrate, the final metabolite of NO· in vivo, was quantified to confirm NO· production. Urinary nitrate excretion showed that NO· production was strongly elevated in RcsX tumor-bearing mice (P < 0.05 by day 6), and that the increase was abolished by administration of NMA in the drinking water (Fig. 1).
Figure 1.
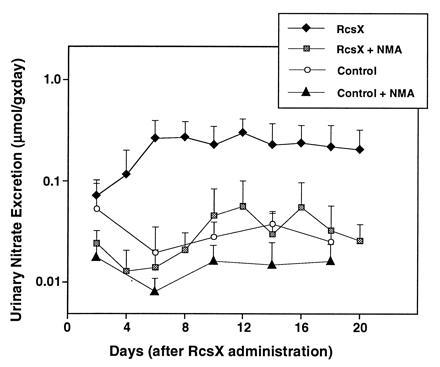
Nitrate excretion by RcsX bearing SJL#60 mice and controls. Twelve SJL mice were injected i.v. on day 1 with 107 RcsX cells. Eight RcsX-bearing and eight control mice received 30 mM NMA in drinking water starting on day 2. All mice were killed on day 20.
Administration of NMA alone did not affect the body weight of the animals, and no differences were observed in the weights or gross appearance of organs from mice receiving NMA and those from mice receiving ammonium acetate in the drinking water. Spleens from RcsX-injected mice receiving ammonium acetate weighed 0.34 ± 0.09 g and those from treated mice receiving NMA weighed 0.31 ± 0.07 g; peripheral lymph nodes from animals receiving the same treatments weighed 0.083 ± 0.30 and 0.078 ± 0.19 g, respectively.
Mutant Frequencies.
Mutant frequency was determined in eight spleens (target organ) and four kidneys (nontarget organ) isolated from RcsX tumor-bearing and control mice, and results are summarized in Tables 1 and 2. In spleens (Fig. 2), a highly significant difference was observed between mutant frequencies in RcsX tumor-bearing mice and those of all other treatment groups (compared with control mice, t = 4.18, df = 10, P < 0.005; compared with RcsX plus NMA mice, t = 2.814, df = 10, P < 0.010; and compared with control plus NMA mice, t = 3.539, df = 11, P < 0.005). A significant difference was also observed between the RcsX plus NMA and control groups (t = 2.097, df = 13, P < 0.050), but not between RcsX plus NMA and control plus NMA groups (t = 1.187, df = 13, P < 0.250). No differences in mutant frequencies were observed in kidneys of animals of any of the treatment groups. We therefore conclude that growth of the RcsX tumor in SJL#60 mice was mutagenic in host splenocytes and propose that NO· and/or other reactive species produced in response to tumor growth were responsible for the observed mutagenicity.
Table 2.
LacZ mutant frequencies in kidneys of RcsX tumor-bearing and control mice
| Treatment | Animal code | No. of colonies (×105) | No. of mutants | Mutant frequency (×10−5) |
|---|---|---|---|---|
| Control | 307 | 2.90 | 30 | 10.3 |
| 310 | 4.60 | 30 | 6.5 | |
| 303 | 2.04 | 21 | 10.3 | |
| 304 | 1.72 | 16 | 9.3 | |
| 9.1 ± 1.8 | ||||
| RcsX | 101 | 3.70 | 36 | 9.7 |
| 109 | 4.12 | 39 | 9.5 | |
| 104 | 2.96 | 24 | 8.1 | |
| 111 | 1.28 | 16 | 1.2 | |
| 8.7 ± 1.6 | ||||
| RcsX + NMA | 208 | 3.80 | 56 | 14.7 |
| 206 | 8.64 | 59 | 6.8 | |
| 201 | 1.68 | 12 | 7.1 | |
| 207 | 3.32 | 20 | 6.0 | |
| 8.7 ± 4.1 | ||||
| Control + NMA | 401 | 5.52 | 41 | 7.4 |
| 407 | 3.00 | 30 | 10.0 | |
| 405 | 1.64 | 17 | 10.4 | |
| 403 | 2.52 | 18 | 7.1 | |
| 8.7 ± 1.7 |
Figure 2.
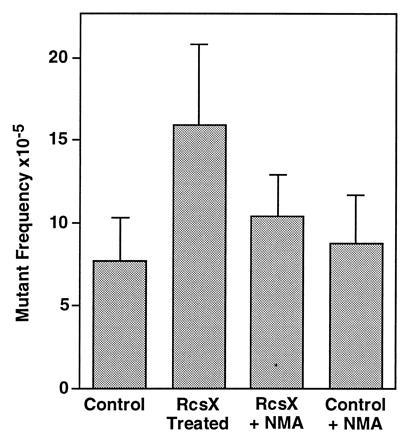
LacZ mutant frequency in the spleen of RcsX tumor-bearing SJL#60 mice.
Characterization of Spontaneous and Induced Mutants.
Activated macrophage-derived NO· has previously been reported to induce DNA strand breaks (7, 21). We hypothesized that misrepaired strand breaks may lead to large deletions in the lacZ transgene in RcsX tumor-bearing mice. To test this hypothesis, mutant plasmids isolated from control and RcsX tumor-bearing mice were sized by migration through agarose gel. An increase in the fraction of mutants with a change in the apparent molecular weight was observed [control, 67/132 (51%); RcsX treated, 94/161 (58%)] but the increase was not statistically significant.
To further characterize the mutations induced by NO·, plasmids that migrated through the agarose gel with the same apparent molecular size as wild-type plasmids were sequenced. Twenty-eight spontaneous and 24 RcsX-induced independent mutants were identified. The results are reported in Tables 3 and 4. No difference in the patterns of mutations was observed between the two treatment groups (Table 5), although the ratio of G to A transition to G to T transversion was higher in the RcsX-induced group than controls (8/4 and 7/6, respectively).
Table 3.
Spontaneous mutants in SJL#60 spleen
| Mouse ID | Position | Mutation | Sequence | Amino acid change |
|---|---|---|---|---|
| 309 | 266 | G:C to A:T | ACTGGGAAA | Trp to stop |
| 309 | 285 | C:G to T:A | TACCCAACT | Gln to stop |
| 303 | 318–340 | Multiple deletion | Frameshift | |
| 303 | 409 | A:T insertion | TTTC CGGC | Frameshift |
| 309 | 417 | G:C to T:A | ACCAGAAGC | Glu to stop |
| 305 | 441 | G:C to T:A | GCTGGAGTG | Glu to stop |
| 309 | 630 | C:G to T:A | AGGCCAGAC | Gln to stop |
| 310 | 642 | A:T to C:G | AATTATTTT | Ile to Leu |
| 310 | 686 | G:C to T:A | ACGGGCGCT | Gly silent |
| 306 | 886 | G:C to A:T | ATCAGCGAT | Ser to Asn |
| 305 | 1128 | G:C to T:A | CGCCGAAAT | Glu to stop |
| 310 | 1230 | G:C to T:A | GATTGAAAA | Glu to Lys |
| 307 | 1340 | G:C to C:G | TGCAGGATA | Gln to His |
| 301 | 1705 | C:G to T:A | GCCACCGAT | Thr to Ile |
| 307 | 1732 | 2-bp deletion | GCGCGCGTGG | Frameshift |
| 301 | 1749 | C:G to T:A | CCAGCCCTT | Pro to Ser |
| 305 | 1810 | G:C to C:G | ACGCGCCCG | Arg to Pro |
| 303 | 1849 | A:T to T:A | GGTAACAGT | Asn to Ile |
| 307 | 1895 | 1-bp deletion | AGTATCCCC | Frameshift |
| 305 | 1900+ | Multiple deletion | Frameshift | |
| 309 | 1905 | A:T to C:G | TTACAGGGC | Gln to Pro |
| 306 | 1940 | G:C to C:G | AGTCGCTGA | Ser silent |
| 310 | 1951 | A:T to T:A | AAATATGAT | Tyr to Phe |
| 307 | 1966 | G:C to T:A | AACGGCAAC | Gly to Val |
| 310 | 2020 | T:A to C:G | CAGTTCTGT | Phe to Ser |
| 310 | 2087 | G:C to C:G | ACCAGCAGC | Gln to His |
| 303 | 2110 | T:A to G:C | CGTTTATCC | Leu to stop |
| 305 | 2166 | G:C to A:T | TAACGAGCT | Glu to Lys |
Table 4.
RcsX induced mutants in SJL#60 spleen
| Mouse ID | Position | Mutation | Sequence |
|---|---|---|---|
| 106 | 250 | T:A to G:C | GTTTTACAC |
| 101 | 265 | G:C to A:T | GACTGGGAA |
| 106 | 509 | G:C to A:T | ATGCGCCCA |
| 103 | 512 | 2-bp deletion | GCGCGCCCAT |
| 106 | 676 | G:C G:C to A:T A:T | CTGTGGTGCA |
| 111 | 1145 | T:A to G:C | TCTATCGTG |
| 106 | 1158 | G:C to T:A | GGTTGAACT |
| 111 | 1194 | G:C to T:A | AGCAGAAGC |
| 111 | 1214 | C:G to T:A | GTTTCCGCG |
| 112 | 1272 | C:G to T:A | GATTCGAGG |
| 106 | 1371 | A:T to G:C | CTTTAACGC |
| 111 | 1387 | C:G to T:A | TGTTCGCAT |
| 106 | 1411 | G:C to A:T | CTGTGGTAC |
| 105 | 1524 | A:T to T:A | GGCGATGAG |
| 105 | 1570 | 1-bp deletion | CACCCGAGT |
| 103 | 1784 | 1-bp deletion | AAAAAATGGC |
| 111 | 1784 | 1-bp deletion | AAAAAATGGC |
| 111 | 1828 | A:T to T:A | TGCGAATAC |
| 106 | 1893 | T:A to A:T | TCAGTATCC |
| 103 | 1919 | G:C to T:A | CTTCGTCTG |
| 101 | 2071 | 1-bp insertion | CTGA CGGA |
| 101 | 2118 | C:G to T:A | CGGGCAAAC |
| 101 | 2130 | G:C to T:A | CGAAGTGAC |
| 112 | 2482 | G:C to A:T | AAGCGTTGG |
Table 5.
Types of mutations observed in RcsX and control treatment groups
| Mutation | Spontaneous | RcsX |
|---|---|---|
| G:C to A:T | 7 | 8 |
| Other transitions | 2 | 2 |
| G:C to T:A | 6 | 4 |
| Other transversions | 8 | 5 |
| Frameshift | 5 | 5 |
| Total | 28 | 24 |
DISCUSSION
When injected into immunologically compatible mice, RcsX cells localize to the spleen and lymphatic tissues of the host (22–24). In these target tissues, the superantigen presented on the surface of the RcsX cells induces a potent inflammatory response by stimulating Vβ16+ T cells (12). We have previously shown by Western blot analysis that this immune response resulted in iNOS expression in cells of the spleen and lymph nodes of host animals. Expression of iNOS was limited to a small number of cells in these lymphatic tissues, and iNOS-expressing cells were identified as macrophages by immunohistochemistry (11). We subsequently used RcsX tumor-bearing SJL mice to assess the role of NO· in inflammation-induced cellular damage by characterizing the pattern of apoptosis and the presence of nitrotyrosine in cells of the spleen and lymph nodes. Both the number of apoptotic nuclei and the extent of nitrotyrosine staining were elevated in these tissues, and both were diminished by administration of NMA. Furthermore, immunohistochemical evidence demonstrated that nitrotyrosine was localized in cells lying in close proximity to macrophages expressing iNOS. The rate of apoptosis and extent of nitrotyrosine staining were unaffected in the kidneys of tumor-bearing animals (unpublished data).
On the basis of this evidence, we hypothesized that exposure to NO· and other reactive species produced by macrophages in the course of tumor growth would constitute a mutagenic exposure for splenocytes of the host animal. To test this hypothesis, we used the pUR288 transgenic mouse, in which the lacZ gene serves as a mutational target. An experimental system based on a transgenic (as opposed to endogenous) target gene was chosen because of the wide range of tissues that can be studied and the technical facility involved in their use. The pUR288 system was specifically selected because it permits detection of deletions, which were predicted to increase in response to NO· exposure.
Growth of RcsX superantigen-bearing lymphoma cells led to a 10-fold increase in nitrate excretion in SJL#60 mice. Thus, NO· flux within spleen and lymph nodes (tissues in which NO·-producing macrophages were localized) was estimated to be substantially higher than in other tissues, since these account for <5% of the body mass of the animal. A statistically significant increase in mutant frequency was observed in spleen of tumor-bearing mice (15.9 × 10−5) compared with control mice (7.7 × 10−5). No increase was found in kidney (nontarget tissue) of tumor-bearing animals. Furthermore, the increase in spleen was largely blocked by administration of NMA, a NO· synthase inhibitor. Collectively, these observations support the conclusion that the increased mutation frequency was induced principally by NO·. With respect to mutation frequencies observed in spleen, overdispersion and substantial differences among variances of different treatment groups existed. Similar findings have been reported in data from other transgenic mouse models (19, 25, 26) and were not surprising, considering the complex nature of this model.
It is of interest to compare the 2-fold increase in mutant frequency observed in our experiment with previously reported responses of transgenic mice to well-characterized chemical mutagens. Increased mutation frequency induced in the livers of pUR288 transgenic C57BL/6 mice by administration of ethylnitrosourea at a dose of 250 mg/kg amounted to 11.7-fold; benzo[a]pyrene at 50 mg/kg, 5.9-fold; and x-rays at 50 rads divided over 5 days, 4-fold (27). It should be noted that these dose levels were employed to induce maximal responses. By comparison, the increase observed in our experiments was substantial in magnitude, and we propose was attributable to NO· and/or other reactive chemical species. Several factors may have been significant determinants of the magnitude of the response as well as the difference in potency in comparison with other mutagens studied to date. Within spleen (the target tissue), the kinetics of NO· production exposed splenocytes chronically over a prolonged period in contrast to the short-term, massive doses used in studies of other mutagens. NO· was therefore less likely to have overwhelmed cellular defenses and repair mechanisms. Further, delivery of NO· was localized and coordinated as part of a repertoire of radicals and signaling molecules produced by macrophages (28–30). Thus, relatively few potential target cells may have been exposed to a NO· flux large enough to have induced mutations. Patterns of nitrotyrosine staining and apoptosis previously observed in this model support this hypothesis.
On the other hand, the increase in mutant frequency observed in these experiments was relatively large compared with reported age-dependent rates of mutation accumulation. The 2-fold increase in mutant fraction observed by us was approximately equivalent to the annual increases in splenic mutant frequency in the lacI phage system [3.5 × 10−5 in newborn to 15.3 × 10−5 in 2-year-old mice (31)]; the lacZ phage system [3.2 × 10−5 in newborn to 8.3 × 10−5 in 1-year-old mice (32)]; and the pUR288 plasmid system [7 × 10−5 in 6-week-old mice to 12 × 10−5 in 18-month-old mice (27)]. The causes of spontaneous age-dependent increases in mutant frequency are unknown, but have been suggested to be attributable to endogenous production of reactive oxygen species (3). Results presented here are consistent with the postulate that endogenously produced NO· may also be a contributing factor.
The increased rate of cell replication associated with the rapid growth of RcsX cells in spleen may also have contributed to the observed increase in mutant frequency in that tissue. The importance of this factor cannot be fully evaluated on the basis of current evidence but the antimutagenic effect of NMA suggests that it was less significant than NO· as a determinant of mutation frequency. Furthermore, if cell replication was indeed involved in the process, a high fraction of sibling mutants would have been expected when mutants were sequenced. This was not the case, because in a total of 34 mutants sequenced, only 6 were siblings.
The spontaneous mutant frequencies observed in these experiments (kidney, 9.1 ± 1.8 × 10−5; spleen, 7.7 ± 2.6 × 10−5) were somewhat higher than those previously reported by Dolle et al. (15) (kidney, 5.9 × 10−5 ± 0.3; spleen, 5.4 × 10−5 ± 0.5). The basis for this difference is unknown, but may be related to characteristics specific to strains of mice used (SJL#60 vs C57BL/6). The types of spontaneous mutants identified were in agreement with previous observations by Martus et al. (27) and Dolle et al. (15) that ≈50% of spontaneous mutants observed in pUR288 transgenic mice involved size changes rather than base substitutions. Base substitutions and frameshifts comprised the remaining mutations observed in our experiments, as follows: G:C to A:T transitions, 7/28 (25%); G:C to T:A transitions, 6/28 (21%); and frameshifts, 5/28 (18%). Very few base substitutions have previously been identified in spontaneous mutants of pUR288 transgenic animals; 3/14 (21%) of these were G:C to A:T transitions (M. E. T. I. Boerrigter, personal communication). A larger data base has been accumulated regarding the spontaneous mutant pattern in mice bearing lacI and lacZ transgenes. Zhang et al. (33) report that ≈80% of spontaneous mutants contained single or multiple base substitutions, and the remainder contained frameshifts. Base pair substitutions in the liver of lacZ phage transgenic mice occurred most often in CpG islands, with G:C to A:T transitions being most common (12/27, 45%) (34). The spontaneous mutation pattern in spleen of lacI phage transgenic mice has been studied by two groups, both of whom found that base pair substitutions accounted for 80% of the mutants. In one study, the most common were G:C to T:A transversions (6/22, 27%) (33), and in the other G:C to A:T transitions (7/16, 45%) (35).
NO· has been shown to be mutagenic in a variety of experimental systems. NO· gas was mutagenic in TK6 cells (36) and caused predominantly A:T to G:C transversions in plasmids (37) and C to T transitions in a bacterial system (38). NO· donor drugs have been shown to cause predominantly G:C to A:T transversions in plasmids replicated in bacteria (39), while peroxynitrite caused strand breaks, G:T to T:A and G:C to C:G transversions (40) in the same system. Therefore, on the basis of available evidence, no pattern of mutations that can be specifically attributed to NO· has yet been characterized.
The experimental system used in our studies will be useful in extending studies of mechanisms through which inflammation contributes to carcinogenesis. Transgenic C57BL/6 × SJL F1 mice were found to respond to growth of RcsX tumor cells by production of excess amounts of NO· in spleen and lymph nodes. This response was similar to that of the SJL parental strain. In SJL mice, this syndrome develops spontaneously, and we are currently introducing the pUR288 transgene into SJL animals by backcrossing with transgenic C57BL/6 mice to take advantage of that characteristic. With the SJL transgenic animals, the possible role of NO·-induced mutagenesis in the spontaneous development of lymphoma can be assessed. Specifically, mutagenesis in the mesenteric lymph nodes, where the lymphoma is believed to arise, can be evaluated at early stages and protective effects of NO· synthase inhibitors can be assessed. The pUR288 transgenic SJL mouse could also be crossed to superoxide dismutase overexpressing or deficient mice to address the interaction of NO· and O2−. These and other related developments are currently in progress.
Acknowledgments
We thank Dr. J. Vijg for making transgenic C57BL/6 mice available to us, and Dr. N. Ponzio for providing RcsX cells as well as valuable advice on their use. We thank Dr. M. E. Dolle and M. E. T. I. Boerrigter for their technical assistance and to M. E. T. I. Boerrigter for assistance in statistical analysis of the data. Financial support was provided by Grants 2P01 CA2673 from the National Cancer Institute and 5P01 ES05622 from the National Institute for Environmental Health Sciences.
Footnotes
Abbreviations: RcsX, pre-B-cell lymphoma cells; iNOS, inducible nitric oxide synthase; NMA, NG-methyl l-arginine acetate; SJL#60, C57BL/6 × SJL F1.
References
- 1.Gordon L I, Weitzman S A. Cancer J. 1993;6:257–261. [Google Scholar]
- 2.Ohshima H, Bartsch H. Mutat Res. 1994;305:253–264. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 3.Ames B N, Shigenaga M K, Hagen T M. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trush M A, Kensler T W. Free Radicals Biol Med. 1991;10:201–209. doi: 10.1016/0891-5849(91)90077-g. [DOI] [PubMed] [Google Scholar]
- 5.Haswell-Elkins M R, Satarug S, Tsuda M, Mairiang E, Esumi H, Sithithaworn P, Mairiang P, Saaitoh M, Yongvanit P, Elkins D B. Mutat Res. 1994;305:241–252. doi: 10.1016/0027-5107(94)90244-5. [DOI] [PubMed] [Google Scholar]
- 6.Mannick E E, Bravo L E, Zarama G, Realpe J L, Zhang X J, Ruiz B, Fontham E T H, Mera R, Miller M J S, Correa P. Cancer Res. 1996;56:3238–3243. [PubMed] [Google Scholar]
- 7.Tamir S, Burney S, Tannenbaum S R. Chem Res Toxicol. 1996;9:821–827. doi: 10.1021/tx9600311. [DOI] [PubMed] [Google Scholar]
- 8.Tannenbaum, S. R., Tamir, S. & Walker, T. (1994) in DNA Damage and Cytotoxicity by Nitric Oxide, ACS Symposium Series, eds. Loeppky, R. N. & Michejda, C. J. (Am. Chem. So., Washington, DC), Vol. 553, pp. 120–135.
- 9.Brune B, Messmer U K, Sandau K. Toxicol Lett. 1995;82–83:233–237. doi: 10.1016/0378-4274(95)03481-1. [DOI] [PubMed] [Google Scholar]
- 10.Tamir S, Walker T D, Gal A, Weller A H, Li X, Fox J G, Wogan G N, Tannenbaum S R. Cancer Res. 1995;55:4391–4397. [PubMed] [Google Scholar]
- 11.Gal A, Tamir S, Tannenbaum S R, Wogan G N. Proc Natl Acad Sci USA. 1996;93:11499–11503. doi: 10.1073/pnas.93.21.11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsiagbe V, Yoshimoto T, Asakawa J, Cho S, Meruelo D, Thorbecke G. EMBO J. 1993;12:2313–2320. doi: 10.1002/j.1460-2075.1993.tb05885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lasky J, Ponzio N, Thorbecke G. J Immunol. 1988;14:679–687. [PubMed] [Google Scholar]
- 14.Gossen J A, Vijg J. BioTechniques. 1993;14:326–330. [PubMed] [Google Scholar]
- 15.Dolle M E T, Martus H J, Gossen J A, Boerrigter M E T I, Vijg J. Mutagenesis. 1996;11:111–118. doi: 10.1093/mutage/11.1.111. [DOI] [PubMed] [Google Scholar]
- 16.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 17.Gossen J A, Molijn A C, Douglas G R, Vijg J. Nucleic Acids Res. 1992;20:3254. doi: 10.1093/nar/20.12.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z, Mishra N C. BioTechniques. 1995;18:214–217. [PubMed] [Google Scholar]
- 19.Piegorsch W W, Margolin B H, Shelby M D, Johnson A, French J E, Tennant R W, Tindall K R. Environ Mol Mutagen. 1995;25:231–245. doi: 10.1002/em.2850250310. [DOI] [PubMed] [Google Scholar]
- 20.Rosner B. Fundamentals of Biostatistics. Boston: BWS–Kent; 1989. [Google Scholar]
- 21.Zingarelli B, O’Connor M, Wong H, Salzman A L, Szabo C. J Immunol. 1996;156:350–358. [PubMed] [Google Scholar]
- 22.Lerman S P, Carswell E A, Chapman J, Thorbecke G J. Cell Immunol. 1976;23:53–67. doi: 10.1016/0008-8749(76)90171-4. [DOI] [PubMed] [Google Scholar]
- 23.Ponzio N M, Powell P H, Brown H, Thorbecke G J. Int Rev Immunol. 1986;1:273–301. doi: 10.3109/08830188609056610. [DOI] [PubMed] [Google Scholar]
- 24.Stavnezer J, Laskey J, Ponzio N, Scheid M, Thorbecke G. Eur J Immunol. 1989;19:1063–1069. doi: 10.1002/eji.1830190616. [DOI] [PubMed] [Google Scholar]
- 25.Piegorsch W W, Lockhart A C, Margolin B H, Tindall K R, Gorelick N J, Gerelick J M, Short J M, Carr G J, Thompson E D, Shelby M D. Environ Mol Mutagen. 1994;23:17–31. doi: 10.1002/em.2850230105. [DOI] [PubMed] [Google Scholar]
- 26.Tinwell H, Liegibel U, Krebs O, Schmezer P, Favor J, Ashby J. Mutat Res. 1995;335:185–190. doi: 10.1016/0165-1161(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 27.Martus H J, Dolle M E, Gossen J A, Boerrigter M E, Vijg J. Mutat Res. 1995;338:203–213. doi: 10.1016/0921-8734(95)00025-2. [DOI] [PubMed] [Google Scholar]
- 28.Nathan C, Nogueira N, Juangbhanich C, Ellis J, Cohn Z. J Exp Med. 1979;149:1056–1068. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szefler S J, Norton C E, Ball B, Gross J M, Aida Y, Pabst M J. J Immunol. 1989;142:3985–3992. [PubMed] [Google Scholar]
- 30.Baron P, Constantin G, D’Andrea A, Ponzin D, Scarpini E, Scarlato G, Trinchieri G, Rossi F, Cassatella M A. Proc Natl Acad Sci USA. 1993;90:4414–4418. doi: 10.1073/pnas.90.10.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee A T, DeSimons C, Cerani A, Bucala R. FASEB J. 1994;8:545–550. doi: 10.1096/fasebj.8.8.8181674. [DOI] [PubMed] [Google Scholar]
- 32.Ono T, Miyamura Y, Ikehata H, Yamanaka H, Kurishita A, Yamamoto K, Suzuki T, Nohmi T, Hayashi M, Sofuni T. Mutat Res. 1995;338:183–188. doi: 10.1016/0921-8734(95)00023-y. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X B, Urlando C, Tao K S, Heddle J A. Mutat Res. 1995;338:189–201. doi: 10.1016/0921-8734(95)00024-z. [DOI] [PubMed] [Google Scholar]
- 34.Douglas G R, Gingerich J D, Gossen J A, Bartlett S A. Mutagenesis. 1994;9:451–458. doi: 10.1093/mutage/9.5.451. [DOI] [PubMed] [Google Scholar]
- 35.Kohler S W, Provost G S, Fieck A, Kretz P L, Bullock W O, Sorge J A, Putma D L, Short J M. Proc Natl Acad Sci USA. 1991;88:7958–7962. doi: 10.1073/pnas.88.18.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen T, Brunson D, Crespi C L, Penman B W, Wishnok J S, Tannenbaum S R. Proc Natl Acad Sci USA. 1992;89:3030–3034. doi: 10.1073/pnas.89.7.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Routledge M N, Wink D A, Keefer L K, Dipple A. Carcinogenesis. 1993;14:1251–1254. doi: 10.1093/carcin/14.7.1251. [DOI] [PubMed] [Google Scholar]
- 38.Wink D A, Kasprzak K S, Maragos C M, Elespuru R K, Misra M, Dunams T M, Celuba T A, Koch W H, Andrews A W, Allan A S, Keefer L K. Science. 1991;254:1001–1003. doi: 10.1126/science.1948068. [DOI] [PubMed] [Google Scholar]
- 39.Routledge M N, Wink D A, Keefer L K, Dipple A. Chem Res Toxicol. 1994;7:628–632. doi: 10.1021/tx00041a007. [DOI] [PubMed] [Google Scholar]
- 40.Juedes M J, Wogan G N. Mutat Res. 1996;349:51–61. doi: 10.1016/0027-5107(95)00152-2. [DOI] [PubMed] [Google Scholar]


