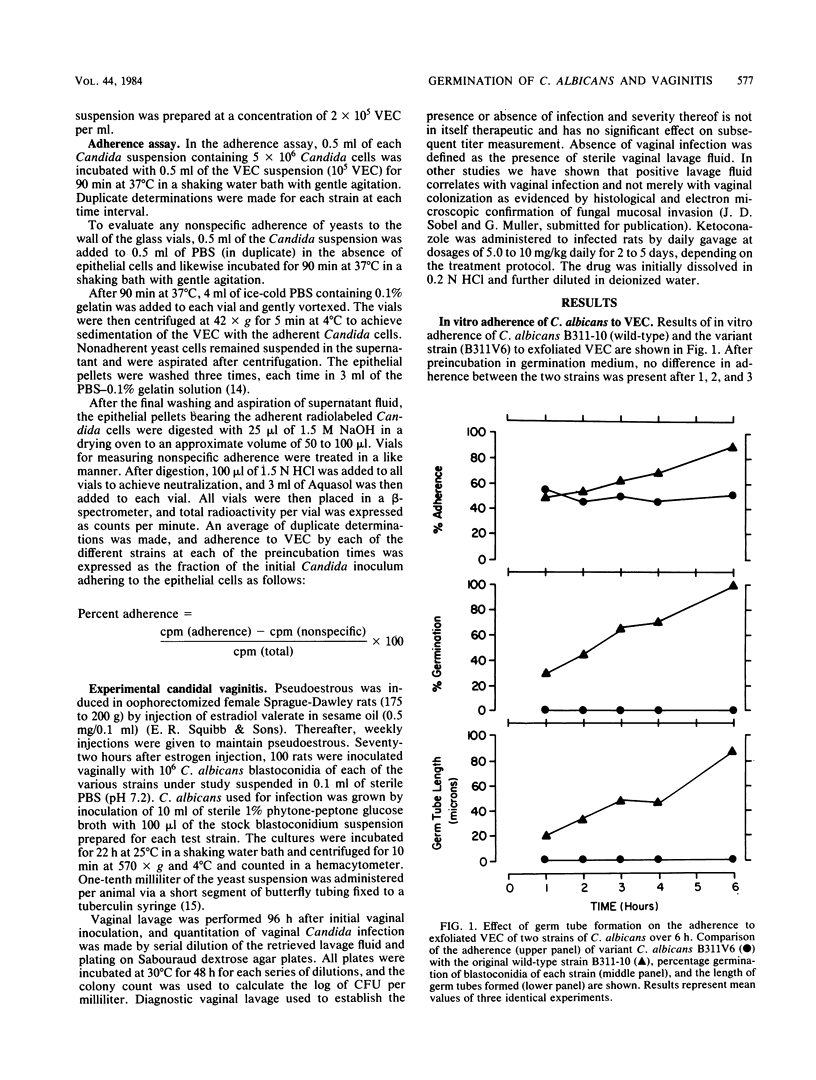
Abstract
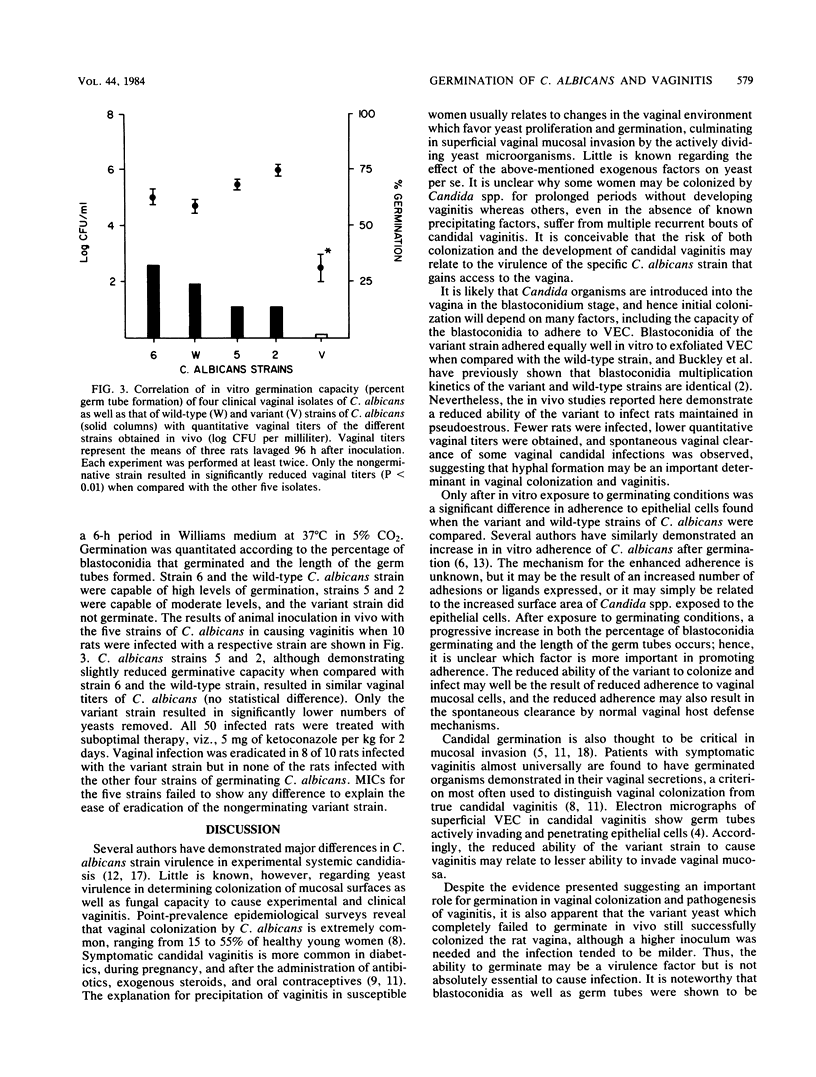
A variant strain of Candida albicans incapable of hyphal production at 37 degrees C was used to study the role of germ tube formation in the pathogenesis of experimental vaginal candidiasis in rats. No difference was observed in the in vitro adherence at 25 degrees C of blastoconidia of the variant strain to vaginal epithelial cells when compared with the parent wild-type, germ tube-producing strain and multiple clinical isolates of C. albicans. However, after exposure to conditions favoring germ tube production, the adherence of the variant strain to epithelial cells was significantly less than that of germinated strains (P less than 0.01). In vivo animal studies revealed that the variant strain was less likely to result in vaginal colonization and infection than the wild-type strain and the other clinical isolates. Furthermore, infection, when established, was milder, often transient, and with significantly lower titers of cultured vaginal microorganisms obtained by lavage. Electron microscopic studies confirmed the failure of the variant strain to produce hyphae in vivo. The capacity of C. albicans to produce hyphae appears to be an important but nonessential virulence factor in the pathogenesis of candidal vaginitis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borgers M., De Brabander M., Van Den Bossche H., Van Cutsem J. Promotion of pseudomycelium formation of Candida albicans in culture: a morphological study of the effects of miconazole and ketoconazole. Postgrad Med J. 1979 Sep;55(647):687–691. doi: 10.1136/pgmj.55.647.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley H. R., Price M. R., Daneo-Moore L. Isolation of a variant of Candida albicans. Infect Immun. 1982 Sep;37(3):1209–1217. doi: 10.1128/iai.37.3.1209-1217.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowa N., Taxer S. S., Howard D. H. Germination of Candida albicans induced by proline. Infect Immun. 1976 Mar;13(3):830–835. doi: 10.1128/iai.13.3.830-835.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEBHARDT L. P., HILL D. W. Morphological transformation of Candida albicans in tissues of mice. Proc Soc Exp Biol Med. 1956 Jul;92(3):640–644. doi: 10.3181/00379727-92-22570. [DOI] [PubMed] [Google Scholar]
- García-Tamayo J., Castillo G., Martínez A. J. Human genital candidiasis: histochemistry, scanning and transmission electron microscopy. Acta Cytol. 1982 Jan-Feb;26(1):7–14. [PubMed] [Google Scholar]
- KOZINN P. J., TASCHDJIAN C. L., BURCHALL J. J., WIENER H. Transmission of P32-labeled Candida albicans to newborn mice at birth. AMA J Dis Child. 1960 Jan;99:31–34. doi: 10.1001/archpedi.1960.02070030033006. [DOI] [PubMed] [Google Scholar]
- Kimura L. H., Pearsall N. N. Adherence of Candida albicans to human buccal epithelial cells. Infect Immun. 1978 Jul;21(1):64–68. doi: 10.1128/iai.21.1.64-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. D., Lee J. C., Morris A. L. Adherence of Candida albicans and other Candida species to mucosal epithelial cells. Infect Immun. 1980 Feb;27(2):667–674. doi: 10.1128/iai.27.2.667-674.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie D. W. Morphogenesis of Candida albicans in vivo. Sabouraudia. 1964 Jun;3(3):225–232. [PubMed] [Google Scholar]
- Mattia E., Carruba G., Angiolella L., Cassone A. Induction of germ tube formation by N-acetyl-D-glucosamine in Candida albicans: uptake of inducer and germinative response. J Bacteriol. 1982 Nov;152(2):555–562. doi: 10.1128/jb.152.2.555-562.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson M. D., Smith H. Production of germ tubes by virulent and attenuated strains of Candida albicans. J Infect Dis. 1981 Dec;144(6):565–569. doi: 10.1093/infdis/144.6.565. [DOI] [PubMed] [Google Scholar]
- Sobel J. D., Muller G. Ketoconazole in the prevention of experimental candidal vaginitis. Antimicrob Agents Chemother. 1984 Feb;25(2):281–282. doi: 10.1128/aac.25.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel J. D., Myers P. G., Kaye D., Levison M. E. Adherence of Candida albicans to human vaginal and buccal epithelial cells. J Infect Dis. 1981 Jan;143(1):76–82. doi: 10.1093/infdis/143.1.76. [DOI] [PubMed] [Google Scholar]
- Sobel J. D., Obedeanu N. Effects of subinhibitory concentrations of ketoconazole on in vitro adherence of Candida albicans to vaginal epithelial cells. Eur J Clin Microbiol. 1983 Oct;2(5):445–452. doi: 10.1007/BF02013902. [DOI] [PubMed] [Google Scholar]
- TASCHDJIAN C. L., REISS F., KOZINN P. J. Experimental vaginal candidiasis in mice; its implications for superficial candidiasis in humans. J Invest Dermatol. 1960 Feb;34:89–94. [PubMed] [Google Scholar]
- Wingard J. R., Dick J. D., Merz W. G., Sandford G. R., Saral R., Burns W. H. Differences in virulence of clinical isolates of Candida tropicalis and Candida albicans in mice. Infect Immun. 1982 Aug;37(2):833–836. doi: 10.1128/iai.37.2.833-836.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG G. The process of invasion and the persistence of Candida albicans injected intraperitoneally into mice. J Infect Dis. 1958 Mar-Apr;102(2):114–120. doi: 10.1093/infdis/102.2.114. [DOI] [PubMed] [Google Scholar]