Abstract
Objective
To assess the availability and affordability of medicines used to treat cardiovascular disease, diabetes, chronic respiratory disease and glaucoma and to provide palliative cancer care in six low- and middle-income countries.
Methods
A survey of the availability and price of 32 medicines was conducted in a representative sample of public and private medicine outlets in four geographically defined areas in Bangladesh, Brazil, Malawi, Nepal, Pakistan and Sri Lanka. We analysed the percentage of these medicines available, the median price versus the international reference price (expressed as the median price ratio) and affordability in terms of the number of days’ wages it would cost the lowest-paid government worker to purchase one month of treatment.
Findings
In all countries ≤ 7.5% of these 32 medicines were available in the public sector, except in Brazil, where 30% were available, and Sri Lanka, where 28% were available. Median price ratios varied substantially, from 0.09 for losartan in Sri Lanka to 30.44 for aspirin in Brazil. In the private sector in Malawi and Sri Lanka, the cost of innovator products (the pharmaceutical product first given marketing authorization) was three times more than generic medicines. One month of combination treatment for coronary heart disease cost 18.4 days’ wages in Malawi, 6.1 days’ wages in Nepal, 5.4 in Pakistan and 5.1 in Brazil; in Bangladesh the cost was 1.6 days’ wages and in Sri Lanka it was 1.5. The cost of one month of combination treatment for asthma ranged from 1.3 days’ wages in Bangladesh to 9.2 days’ wages in Malawi. The cost of a one-month course of intermediate-acting insulin ranged from 2.8 days’ wages in Brazil to 19.6 in Malawi.
Conclusion
Context-specific policies are required to improve access to essential medicines. Generic products should be promoted by educating professionals and consumers, by implementing appropriate policies and incentives, and by introducing market competition and/or price regulation. Improving governance and management efficiency, and assessing local supply options, may improve availability. Prices could be reduced by improving purchasing efficiency, eliminating taxes and regulating mark-ups.
Résumé
Objectif
Évaluer la disponibilité et l’accessibilité économique dans six pays à revenu faible ou moyen de médicaments utilisés pour le traitement de maladies cardiovasculaires, des diabètes, d’affections respiratoires chroniques et du glaucome, ainsi que pour les soins palliatifs dispensés aux cancéreux.
Méthodes
Une enquête sur la disponibilité et le prix de 32 médicaments a été menée sur un échantillon représentatif d’officines pharmaceutiques publiques et privées, situées dans quatre régions géographiquement définies du Bengladesh, du Brésil, du Malawi, du Népal, du Pakistan et du Sri Lanka. Nous avons analysé le pourcentage de ces médicaments disponible, leur prix médian par rapport au prix international de référence (exprimé sous forme de ratio de prix médian) et leur accessibilité économique, évaluée d’après le nombre de jours de salaire que devrait débourser le fonctionnaire le moins payé pour acheter un mois de traitement.
Résultats
Dans tous les pays étudiés, 7,5% ou moins de ces 32 médicaments étaient disponibles dans le secteur public, sauf au Brésil, où la proportion de médicaments disponibles était de 30%, et au Sri Lanka, où elle était de 28%. Les ratios de prix médians étaient très variables, allant de 0,09 pour le losartan au Sri Lanka à 30,44 pour l’aspirine au Brésil. Dans le secteur pharmaceutique privé du Malawi et du Sri Lanka, le coût des produits innovants était trois fois plus élevé que celui des génériques. Un mois de traitement associé pour une maladie coronarienne coûtait 18,4 jours de salaire au Malawi, 6,1 au Népal, 5,4 au Pakistan, 5,1 au Brésil, 1,6 au Bangladesh et 1,5 au Sri Lanka. Le coût d’un mois de traitement associé contre l’asthme allait de 1,3 jour de salaire au Bangladesh à 9,2 jours de salaire au Malawi. Celui d’une cure d’un mois d’insuline à action intermédiaire variait de 2,8 jours de salaire au Brésil à 19,6 jours de salaire au Malawi.
Conclusion
Des stratégies adaptées au contexte sont nécessaires pour améliorer l’accès aux médicaments essentiels. Il convient de promouvoir l’usage des médicaments génériques à travers l’éducation des professionnels et des consommateurs, la mise en œuvre de politiques et d’incitations appropriées et la mise en place d’une concurrence commerciale et/ou d’une réglementation des prix. Une administration et une gestion plus efficaces, ainsi qu’une évaluation des options d’approvisionnement locales, pourraient permettre une plus grande disponibilité des médicaments. Une baisse des prix serait possible en améliorant l’efficacité des services d’achat, en supprimant les taxes et en réglementant les marges bénéficiaires.
Resumen
Objetivo
Evaluar la disponibilidad y asequibilidad de diversos medicamentos utilizados para tratar las enfermedades cardiovasculares, la diabetes, las enfermedades respiratorias crónicas y el glaucoma y para proporcionar atención paliativa contra el cáncer en seis países de ingresos bajos y medios.
Métodos
Se llevó a cabo una encuesta sobre la disponibilidad y el precio de 32 medicamentos en una muestra representativa de puntos de venta públicos y privados en cuatro zonas geográficas concretas de Bangladesh, el Brasil, Malawi, Nepal, el Pakistán y Sri Lanka. Analizamos el porcentaje disponible de esos medicamentos, el precio mediano en comparación con el precio de referencia internacional (expresado como proporción del precio mediano) y la asequibilidad expresada en función del número de días de sueldo que le costaría al funcionario público peor pagado adquirir el tratamiento necesario para un mes.
Resultados
En el conjunto de todos los países, se podía disponer en el sector público de un porcentaje inferior o igual al 7,5% de esos 32 medicamentos, exceptuando los casos del Brasil, donde se tenía acceso al 30%, y de Sri Lanka, donde se podía acceder al 28%. Las proporciones del precio mediano diferían muy ampliamente, desde el 0,09 para el losartan en Sri Lanka hasta el 30,44 para la aspirina en el Brasil. En el sector privado de Malawi y Sri Lanka, el costo de los productos innovadores era tres veces superior al de los genéricos. Un mes de tratamiento combinado para la cardiopatía coronaria costaba 18,4 días de sueldo en Malawi, 6,1 días en Nepal, 5,4 en el Pakistán y 5,1 en el Brasil; en Bangladesh el costo era de 1,6 días de sueldo, y en Sri Lanka de 1,5. El costo de un mes de tratamiento combinado para el asma se situaba entre 1,3 días de sueldo en Bangladesh y 9,2 días en Malawi. Y el costo de un régimen de un mes de insulina de acción intermedia oscilaba entre 2,8 días de sueldo en el Brasil y 19,6 en Malawi.
Conclusión
Se requieren políticas adaptadas al contexto para conseguir mejorar el acceso a los medicamentos esenciales. Es necesario promover los productos genéricos educando a los profesionales y a los consumidores, implementando políticas e incentivos apropiados, e introduciendo medidas de regulación de los precios y/o de la competencia en el mercado. La mejora de la gobernanza y la eficiencia de la gestión, así como la evaluación de las opciones de suministro locales, pueden contribuir a mejorar la disponibilidad. Y es posible reducir los precios mejorando la eficiencia de las compras, eliminando impuestos y regulando los márgenes de beneficio.
ملخص
الەدف
تقيـيم توافر الأدوية المستخدمة في معالجة الأمراض القلبية الوعائية والسكري والأمراض التنفسية المزمنة والزَرَق، والقدرة على شرائەا، وعلى تقديم الرعاية الملطفة للسرطان في ستة من البلدان المنخفضة والمتوسطة الدخل.
الطريقة
أجرينا مسحاً حول توافر وأسعار 32 دواءً، ضمن عينة ممثِّلة لمنافذ توزيع الأدوية في القطاع الخاص والقطاع العام، في أربع مناطق جغرافية محددة في كل من بنغلاديش والبرازيل وملاوي ونيبال وباكستان وسيريلانكا. وأجرينا تحليلاً للنسبة المئوية لتوافر ەذە الأدوية، ولمتوسط أسعارەا مقابل السعر الدولي المرجعي (معبرين عن ذلك بالنسبة الوسطية للسعر)، وتحليلاً للقدرة على دفع النفقات، ممثلاً بأجور عدد أيام العمل التي تمكِّن أقل العاملين الحكوميين من شراء أدوية المعالجة اللازمة لمدة شەر.
الموجودات
توافر 7.5% أو أقل من ەذە الأدوية التي يبلغ عددەا 32 دواء في جميع البلدان، باستثناء البرازيل التي توافر فيەا 30% من الأدوية، وسيريلانكا التي توافر فيەا 28% من الأدوية. أما نسب أسعار الأدوية فكانت تختلف بشكل واضح بين 0.09 للوسارتان في سيريلانكا إلى 30.44 للأسبرين في البرازيل. أما في القطاع الخاص في كل من مالاوي وسيريلانكا، فإن تكاليف الأدوية المبتكرة (وەي المستحضرات التي منحت التـرخيص لأول مرة في السوق) يزيد على الأدوية الجنيسة بثلاثة أضعاف. إن توليفة معالجة شەرية لمرض القلب التاجي تكلف 18.4 أجر يوم عمل في ملاوي، و61 في نيبال، و5.4 في باكستان و5.1 في البرازيل. أما في بنغلاديش فقد كانت التكاليف 1.6 أجر يوم عمل، وفي سريلانكا 1.5 أجر يوم عمل. وكانت تكلفة توليفة أدوية معالجة الربو لمدة شەر تـتراوح بين 1.3 أجر يوم عمل في بنغلاديش و9.2 أجر يوم عمل في ملاوي. أما تكاليف المعالجة الشەرية بالأنسولين المتوسط الأمد فتـتـراوح بين 2.8 من أجور الأيام في البرازيل و19.6 أجر يوم عمل في ملاوي.
الاستنتاج
تمس الحاجة إلى سياسات خاصة بكل سياق على حدة لتحسين إتاحة الأدوية الأساسية. ويجب تعزيز الأدوية الجنيسة بتثقيف أرباب المەن الطبية والمستەلكين، وبتنفيذ السياسات الملائمة وتقديم الحوافز، وبإدخال المنافسة في السوق وبتنظيم الأسعار. وقد يحسن تطوير الحَوْكَمة والإدارة الفعَّالة، وتقيـيم اختيارات الإمداد المحلي من توافر الأدوية. ويمكن خفض الأسعار بتحسين الكفاءات عند الشراء وتخفيض الضرائب وتنظيم عملية رفع السعر.
Introduction
Globally, approximately 35 million deaths (60% of all deaths) are attributable to chronic diseases each year, with more than 30 million deaths (52% of all deaths) due to cardiovascular disease (accounting for 30% of all deaths), cancer (13% of all deaths), chronic respiratory disease (7% of all deaths) and diabetes (2% of all deaths).1 The global burden of disease resulting from all noncommunicable conditions,1 which includes premature death and disability, is 49%; 80% of these deaths occur in low- and middle-income countries.1,2 Medicines represent a substantial proportion of the economic costs of treating chronic diseases in these countries. For example, in Latin America and the Caribbean it is estimated that medicine costs account for 44% of the direct medical costs of diabetes.3 Further, in low- and middle-income countries 50–90% of the population have to pay for medicines themselves,4 rendering treatment unaffordable for many.
A significant proportion of chronic disease morbidity and mortality can be prevented if medications are made accessible and affordable. In patients with a high risk of cardiovascular disease, aspirin, beta-blockers, angiotensin-converting enzyme (ACE) inhibitors and lipid-lowering medicines reduce the risk of future vascular events by about a quarter each. When used together, these medicines have the potential to reduce the relative risk by 75% and substantially reduce the recurrence of cardiovascular events.5 Similarly, making cost-effective treatments available to patients with asthma and diabetes may lead to substantial reductions in morbidity and mortality.6–9
Several studies have examined the availability, price and affordability of essential medicines, however none have focused specifically on medicines used to treat chronic diseases.10–13 Little data exist on whether patients have access to affordable medicines for chronic diseases in low- and middle-income countries. A health-care facility-based study of practice patterns used for the secondary prevention of cardiovascular disease in 10 low- and middle-income countries looked at the proportion of patients with coronary heart disease and patients with cerebrovascular disease receiving medicine.14 The proportion of coronary heart disease patients receiving aspirin was 81.2%; for beta-blockers it was 48.1%; for ACE inhibitors it was 39.8%; and for statins it was 29.8%. The proportions for patients with cerebrovascular disease were: 70.6% for aspirin; 22.8% for beta-blockers; 37.8% for ACE inhibitors; and 14.1% for statins.
There are many reasons why medicines are not used more often. These include poor availability, a lack of affordability, poor prescribing practices and a lack of patient adherence.14
Thus, despite the availability of cost-effective interventions, gaps in the treatment of chronic diseases persist. WHO is therefore initiating a global initiative to improve the care of chronic diseases in low- and middle-income countries.15 As part of the initiative’s preliminary activities, a survey of selected medicines used to treat chronic diseases was undertaken in selected countries to determine:
whether medicines were available, affordable and how much they cost;
whether there were variations in availability, price or affordability between the public and private sectors and between innovator brands (that is, the product that receives marketing authorization first, which is usually the patented version of the medicine) and generic equivalents; and
the contribution that mark-ups, taxes and other costs add to the final price of medicine.
Our general hypothesis was that the essential medicines used to treat chronic diseases in low- and middle-income countries have limited availability and may not be affordable.
Methods
The survey was conducted in three low–middle income countries (Brazil, only in Rio Grande do Sul state; Pakistan and Sri Lanka) and three low-income countries (Bangladesh, Malawi and Nepal). These countries were selected based on the logistic feasibility of the study and commitment of the investigating teams to implement policies and programmes to improve the affordability and availability of medicines. Standardized methods developed and tested by WHO and Health Action International were used. With these methods, data is collected on the availability and price of medicines found in public and private medicine outlets; a country’s pharmaceutical policies; the cost of procuring medicine in the public sector and whether it is available; and any additional charges applied to medicines throughout the distribution chain.16 Availability was defined differently in these surveys to the method described in the WHO–Health Action International manual. If any dose or form of the products being surveyed was available in a country then the product was classified as being available. In the WHO–Health Action International method only a specific dose and form is surveyed for price and availability.
Selection of outlets
Facilities were selected using a multistage random sampling method. In each country, a main urban centre and three administrative regions within one-day’s drive of the urban centre were randomly selected as survey areas. For each area, a list of public medicine outlets (i.e. medicine outlets administered by the state, usually the Ministry of Health) was compiled and a minimum of four outlets were randomly selected. The main public hospital, and at least four randomly selected public medicine outlets (e.g. other hospitals, dispensaries) constituted the public sector sample. Private, for-profit medicine outlets (such as pharmacies or shops where people can purchase medicine) closest to each of these public outlets were selected for the private sector sample. In most areas, there were many more private outlets than public outlets. Altogether 20 public sector and 20 private sector outlets were sampled in Bangladesh and Brazil. In Malawi, 20 public sector and 16 private sector outlets were sampled. In Nepal, 41 public sector and 44 private sector outlets were surveyed. In Pakistan 20 public sector and 60 private sector outlets were surveyed. In Sri Lanka 50 public sector and 49 private sector outlets were surveyed. The sample size was the same as or larger than that recommended in the WHO–Health Action International manual.
Data collection
The survey collected information on 32 medicines used to treat cardiovascular disease, diabetes, chronic respiratory disease (particularly asthma), glaucoma and to provide palliative cancer care (see Box 1, available at http://www.who.int/bulletin). These conditions were selected because together they account for 52% of deaths worldwide and 20% of the global burden of disease. Medicines were selected based on their potential impact on the burden of disease and their availability in standard formulations; the majority of these medicines are included in the WHO’s list of essential medicines.17 In keeping with the WHO–Health Action International methods, only registered products were included in the survey. For each medicine, data were collected on the price and availability of the innovator brand, the country’s “most-sold generic equivalent” of all the 32 medicines (sales volume) and the “lowest priced generic” found at each outlet. The most-sold generic equivalents were identified using publicly available official figures or by using data from the government body responsible for the pharmaceutical sector. Where such data were inadequate, information was gathered by telephone from 10 large, geographically dispersed pharmacies in the area. All surveys were conducted during an 8–12 week period in 2005. Price data on public sector procurement were obtained by using a recent order from each country’s centralized medicine procurement agency. Data on mark-ups, taxes and other costs that contributed to the final price of a medicine were collected by tracking the prices of selected medicines backwards from medicine outlets to central sources in the country.
Box 1. List of 32 medicines surveyeda.
| Medicines by disease | Dose | Category |
|---|---|---|
| Cardiovascular disease | ||
| Aspirin | 100mg | Antiplatelet |
| Atenolol | 50 mg | Antihypertensive |
| Benzathine benzylpenicillin | 2.4M IUs | Antibiotic |
| Captopril | 25 mg | Antihypertensive |
| Enalaprila | 10 mg | Antihypertensive |
| Erythromycin | 250 mg | Antibacterial |
| Furosemide | 40 mg | Diuretic |
| Hydrochlorothiazide | 25 mg | Antihypertensive |
| Isosorbide dinitrate | 5 mg | Anti-angina |
| Losartanb | 50 mg | Antihypertensive |
| Lovastatinb | 20 mg | Lipid lowering |
| Methyldopab | 250 mg, 500 mg | Antihypertensive |
| Nifedipine (sustained release) | 20 mg | Antihypertensive |
| Phenoxymethyl penicillin | 250 mg | Antibiotic |
| Propranolol hydrochloride | 40 mg | Antihypertensive |
| Spironolactone | 25 mg | Diuretic |
| Streptokinaseb | 1 500 000 IU | Anti-thrombotic |
| Chronic respiratory disease | ||
| Aminophylline | 100 mg | Anti-asthmatic |
| Beclometasone inhaler | 0.05 mg/dose | Anti-asthmatic |
| Ipratropium bromidec | 20 µg/dose | Anti-asthmatic |
| Prednisoloneb | 5 mg 25 mg | Anti-inflammatory |
| Salbutamol inhaler | 0.1 mg/dose | Anti-asthmatic |
| Diabetes | ||
| Glibenclamide | 5 mg | Antidiabetic |
| Insulin isophane | 100 IU/ml | Antidiabetic |
| Insulin soluble | 40 IU/ml | Antidiabetic |
| Insulin soluble | 100 IU/ml | Antidiabetic |
| Insulin zinc suspensionb | 40 IU/ml | Antidiabetic |
| Insulin zinc suspension | 100 IU/ml | Antidiabetic |
| Metformin | 500 mg | Antidiabetic |
| Palliative cancer care | ||
| Codeine | 30 mg | Opioid analgesic |
| Morphine sulfatec | 30 mg | Opioid analgesic |
| Glaucoma | ||
| Timolol eye drops | 0.25% | Antiglaucoma |
a For calculation of median price ratios for the above medicines, the international reference price was obtained from the 2005 version of Management Sciences for Health’s International drug price indicator guide.18 The supplier price was used unless otherwise indicated.
b 2005 Management Sciences for Health’s agency price used.
c No 2005 Management Sciences for Health reference price available.
Training and standardization
The following steps were taken to ensure that data were standardized and of high quality. A standardized operations manual and data collection forms were used. All medicine outlets in a survey area were identified before sampling. Training workshops were held for the data collection teams; these included a data collection pilot test. Data were collected by pairs of survey personnel. A supervisor validated 10% of all data collected from medicine outlets. Data were entered in triplicate and verified; and missing or erroneous entries were rectified. Data entry was standardized and checked by two operators.
Data analysis
As part of our analysis we determined the percentage of the 32 medicines available (i.e. the percentage of outlets in which any dose or form of the medicine was found) and the median price ratio. This ratio compares a medicine’s median price (a minimum of four prices are required) to its international reference price (the procurement prices for multisource generic medicines offered to low- and middle-income countries by non-profit-making suppliers or agencies, if the supplier price is not available) obtained from Management Sciences for Health’s 2005 International drug price indicator guide.18 For the availability analysis, different strengths of the same medicine were combined to yield a measure of overall availability. For patients’ prices, median price ratios of ≤ 1.5 were considered reasonable for generic medicines; ratios for innovators are usually higher.19 Affordability was estimated using median medicine prices and the average salary of the lowest-paid government worker and calculating the number of days’ wages required to purchase a one-month course of treatment.
Findings
Results refer to the availability and price of medicines on the day of data collection; they are not comparable between countries. The exchange rate of local currency to US dollars is the commercial “buy” rate on the first day of data collection.
Overall results
Availability in public and private sectors
Compared with the private sector, the total availability of medicines in the public sector was considerably lower in all countries (Table 1). Availability in the private sector was substantially higher, particularly in Brazil and Sri Lanka; the median percentage of the lowest-price generic medicine available in Brazil was 70% and in Sri Lanka it was 79%. In most countries, generic medicines were more widely available than innovator brands in both sectors. The availability of innovators was high in the private sector in Brazil and Pakistan; in Pakistan innovators were more widely available than generic medicines.
Table 1. Median percentage of 32 essential medicines available in both the private and public sectors on day of data collection in selected low- and middle-income countriesa.
| Country | Public sectorb |
Private sector |
||||||
|---|---|---|---|---|---|---|---|---|
| Innovatorc brand | Most-sold generic | Lowest price generic | Innovator brand | Most-sold generic | Lowest price generic | |||
| Bangladesh | 0.0 (0.0–7.5) | 0.0 (0.0–7.5) | 5.0 (0.0–10.0) | 10.0 (0.0–80.0) | 25.0 (5.0–72.5) | 30.0 (6.3–85.0) | ||
| Brazil | 0.0 (0.0–0.0) | 0.0 (0.0–5.0) | 30.0 (0.0–70.0) | 65.0 (25.0–80.0) | 40.0 (10.0–55.0) | 70.0 (20.0–90.0) | ||
| Malawi | 0.0 (0.0–0.0) | 0.0 (0.0–17.5) | 5.0 (0.0–40.0) | 0.0 (0.0–0.0) | 12.5 (0.0–34.4) | 37.5 (0.0–87.5) | ||
| Nepal | 0.0 (0.0–0.0) | 5.0 (2.5–7.5) | 7.5 (2.5–12.5) | 0.0 (0.0–4.5) | 23.9 (2.8–50.0) | 28.4 (9.7–65.9) | ||
| Pakistan | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 5.0 (0.0–30.0) | 51.7 (1.7–93.3) | 6.7 (1.7–65.0) | 31.7 (5.0–76.7) | ||
| Sri Lanka | 4.0 (0.0–12.5) | 14.0 (0.0–34.5) | 28.0 (8.0–75.0) | 4.1 (0.0–15.3) | 13.3 (0.0–69.4) | 78.6 (2.0–96.4) | ||
a Values are the overall availability expressed as the median percentage available (i.e. the median percentage of medicine outlets where the medicine was found). Values include all doses combined. b Values are percentages (25th–75th percentile). c See text for description of innovator brands, most-sold generics and lowest price generics.
Prices in the public sector
In the public sector in Bangladesh, Brazil, Malawi, Pakistan and state hospitals in Sri Lanka, medicines are generally provided free of charge. In Sri Lanka, patients must pay at public pharmacies. In Malawi, patients are required to pay for some medicines that are not on the national essential medicine list and are therefore not supplied through the Central Medical Store, however these are rarely available. In Nepal’s public sector, government-supplied medicines are free, but because they are often not available public facilities obtain medicines from other sources (e.g. community drug treatment programmes) and then sell them to patients.20
Generic medicines were the predominant type of product available in the public sector, with most priced between 0.5 times and 1.5 times their international reference price. Where the price of innovator brands was reported, these cost about 2–3 times their international reference price.
Prices in the private sector
The median value of the median price ratio of all available medicines is presented in Table 2. In Nepal and Pakistan there was little variation in prices among private outlets; in Malawi, prices varied substantially among private outlets and were generally higher than in other countries surveyed.
Table 2. Median price ratios by product type for 32 essential medicines in selected low- and middle-income countriesa.
| Country | Median value of median price ratiob |
||
|---|---|---|---|
| Innovator brand | Most-sold generic | Lowest price generic | |
| Bangladesh | 1.31 (n = 14)c | 1.30 (n = 15) | 1.14 (n = 19) |
| Brazil | 16.15 (n = 21) | 11.04 (n = 19) | 6.34 (n = 20) |
| Malawi | 6.28 (n = 3) | 4.58 (n = 12) | 4.51 (n = 17) |
| Nepal | 3.53 (n = 6) | 2.24 (n = 17) | 2.05 (n = 20) |
| Pakistan | 1.72 (n = 20) | 4.16 (n = 11) | 1.64 (n = 16) |
| Sri Lanka | 2.23 (n = 13) | 0.97 (n = 14) | 1.05 (n = 18) |
a Only medicines found in > 4 outlets are included in the analysis. Data presented are not comparable across countries or product types. b See text for description of median price ratio, innovator brands, most-sold generics and lowest price generics. c n is the total number of medicines included in the analysis.
To compare prices between innovator brands and generic equivalents, the median of the median price ratios were calculated only for those medicines for which both product types were found (Table 3). Innovator products were priced 34% higher than the lowest price generics in Bangladesh, 40% higher in Nepal, 90% higher in Pakistan, 135% higher in Brazil, 175% higher in Sri Lanka and 257% higher in Malawi. Sometimes the cost of innovator brands was 10 times higher than their generic equivalent.
Table 3. Price comparison across product types for 32 essential medicines in selected low- and middle-income countriesa.
| Country | Median value of median price ratiob |
||
|---|---|---|---|
| Innovator brand vs most-sold generic | Innovator brand vs lowest price generic | Most-sold generic vs lowest price generic | |
| Bangladesh | 1.76 vs 1.76 (n = 9) | 1.59 vs 1.18 (n = 12) | 1.30 vs 1.14 (n = 15) |
| Brazil | 16.15 vs 11.04 (n = 19) | 14.93 vs 6.34 (n = 21) | 11.04 vs 6.44 (n = 19) |
| Malawi | 9.40 vs 2.02 (n = 1) | 9.40 vs 2.63 (n = 1) | 4.58 vs 4.82 (n = 12) |
| Nepal | 7.84 vs 6.08 (n = 2) | 4.77 vs 3.41 (n = 4) | 2.24 vs 2.24 (n = 17) |
| Pakistan | 6.34 vs 4.16 (n = 9) | 3.55 vs 1.87 (n = 13) | 4.16 vs 3.82 (n = 11) |
| Sri Lanka | 3.69 vs 1.10 (n = 8) | 3.11 vs 1.13 (n = 11) | 0.97 vs 0.91 (n = 14) |
a Only medicines for which prices were found for both product types being compared are included in the analysis. Ipratropium bromide and morphine sulfate are not included in the analysis because international reference prices are not available. b See text for description of median price ratio, innovator brands, most-sold generics and lowest price generics.
Prices paid by patients in public and private sectors
In Nepal, private sector prices were 66.3% higher than patients’ prices in the public sector. At public pharmacies in Sri Lanka, prices were almost the same as those in the private sector. In other countries medicines are generally free to patients being treated in the public sector.
Treatment of specific chronic diseases
Availability
The availability of certain key medicines (in any dose) used for chronic diseases was poor in several countries (Table 4). Hydrochlorothiazide had poor availability in the public sector in Bangladesh, and in both the public and private sectors in Nepal and Pakistan. Both ACE inhibitors and statins have been shown to prevent recurrent attacks in patients with coronary heart disease and are important for secondary prevention. The availability of ACE inhibitors was poor in the pubic sector in Bangladesh and Malawi and in the both sectors in Nepal. The availability of statins was poor in the public sector of all countries and in the private sector in Bangladesh and Nepal. Innovator brand statins were more readily available than generic medicines in Bangladesh, Malawi and Pakistan. Benzathine benzylpenicillin, used for rheumatic fever, had poor availability in Bangladesh, Nepal and Sri Lanka. Streptokinase, for thrombolytic therapy, which has been shown to significantly reduce mortality in patients with myocardial infarction (due to coronary artery disease), was rarely available in all countries.
Table 4. Medicines used to treat chronic diseases with poor availability (described as percentage of outlets in which at least one innovator or one generic is found) in selected low- and middle-income countriesa.
| Medicines by disease (formulation) | % availableb |
|||||
|---|---|---|---|---|---|---|
| Bangladeshc | Brazild | Malawie | Nepalf | Pakistang | Sri Lankah | |
| Cardiovascular disease | ||||||
| Hydrochlorothiazide (capsules and tablets) | 5, 85 | 85, 100 | 70, 88 | 10, 21 | 0, 7 | 100, 98 |
| Captopril (capsules and tablets) | 10, 75 | 85, 100 | 5, 63 | 0, 5 | 65, 100 | 58, 98 |
| Enalapril (capsules and tablets) | 10, 65 | 40, 100 | 0, 81 | 10, 46 | 30, 98 | 20, 76 |
| Lovastatini (capsules and tablets) | 0, 10 | 0, 75 | 0, 56 | 8, 0 | 0, 70 | 20, 96 |
| Streptokinase (vial for injection) | 0, 10 | 0, 5 | 0, 0 | 5, 11 | 5, 15 | 18, 0 |
| Benzathine benzylpenicillin (injection) | 0, 0 | 80, 85 | 100, 50 | 18, 66 | 10, 52 | 52, 10 |
| Diabetes | ||||||
| Insulin soluble (injection) | 5, 60 | 40, 10 | 25, 6 | 8, 23 | 0, 53 | 40, 8 |
| Insulin isophane (injection) | 5, 20 | 50, 35 | 0, 0 | 3, 18 | 0, 55 | 48, 12 |
| Insulin zinc suspension (injection) | 0, 0 | 0, 0 | 30, 25 | 3, 9 | NA, NA | NA, NA |
| Asthma | ||||||
| Beclometasone inhaler | 5, 55 | NA, NA | 0, 38 | 0, 16 | 0, 82 | 34, 98 |
| Palliative cancer care | ||||||
| Codeine (capsules and tablets) | 0, 0 | 0, 0 | 5, 6 | 13, 68 | NA, NA | 0, 0 |
| Morphine sulphate (capsules and tablets) | 0, 0 | 0, 10 | 30, 0 | 0, 0 | 0, 0 | 12, 4 |
NA, not available. a Medicines included in this analysis are those with poor availability in more than one country surveyed. Analysis includes all doses and forms combined. b Values are percentage available in public sector, percentage available in private sector. c Data for Bangladesh from 20 public outlets and 20 private outlets. d Data for Brazil from 20 public outlets and 20 private outlets. e Data for Malawi from 20 public outlets and 16 private outlets. f Data for Nepal from 41 public outlets and 44 private outlets. g Data for Pakistan from 20 public outlets and 60 private outlets. h Data for Sri Lanka from 50 public outlets and 49 private outlets. i Atorvastatin (as lovastatin) is not commercially available in Malawi.
In the case of diabetes, the availability of insulin preparations was poor in all countries, with generic preparations rarely available except in Brazil. Beclometasone inhalers, required for daily management of persistent bronchial asthma, were rarely available or not found in the public sector in all countries except Sri Lanka; they had poor availability in the private sector in Bangladesh, Malawi and Nepal.
For palliative cancer care, the availability of opiates was poor in all countries except Nepal (where it was available in the private sector), possibly due to the regulation of this class of drug.
Affordability
The affordability of treatment was estimated as the number of days’ wages the lowest-paid government worker would be required to pay to purchase from the private sector a one-month course of medicine at the standard or common dose.
Patients with established coronary heart disease require aspirin, a statin, a beta-blocker and an ACE inhibitor to prevent a recurrence of vascular events.5 A one-month’s course of this combination therapy using the lowest priced generic medicines cost 1.5 days’ wages in Sri Lanka; more than 5 days’ wages in Brazil, Nepal and Pakistan; and more than 18 days’ wages in Malawi (Fig. 1). In Malawi, treatment using the more widely available innovator statin increased the monthly cost to 48.8 days’ wages.
Fig. 1.
Affordability of standard treatment for coronary heart disease in the private sector in selected low- and middle-income countriesa
a Affordability expressed as the number of days’ wages that it would cost the lowest-paid government worker to purchase one month of treatment with generic equivalents for a daily combination of 100 mg aspirin, 100 mg atenolol, 10 mg ACE inhibitor and 20 mg statin.
b Daily wage of lowest-paid government worker used for calculation (in US$): Bangladesh 1.85; Rio Grande do Sul State, Brazil 5.50; Malawi 1.36; Nepal 1.42; Pakistan 2.02; Sri Lanka 2.63. Information collected in local currency was converted into US$ using the exchange rate (commercial “buy” rate) on the first day of the survey.
c Data for asprin 75 mg tablet and innovator statin (from two medicine outlets) were used for calculation, because of the low availability of aspirin 100 mg and generic statin.
d Data from public sector.
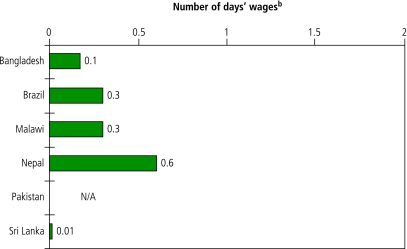
In all six countries one month of monotherapy with hydrochlorothiazide was affordable (Fig. 2). Monotherapy with any of the other antihypertensives included in the survey (atenolol, captopril, enalapril, sustained-release nifedipine, methyldopa, losartan and propranolol hydrochloride,) costs more than 1 day’s wage in at least two countries.
Fig. 2.
Affordability of standard treatment for hypertension in the private sector in selected low- and middle-income countriesa
N/A, not available.
a Affordability expressed as the number of days’ wages that it would cost the lowest-paid government worker to purchase one month of daily treatment with the generic equivalent of 25 mg hydrochlorothiazide.
b Daily wage of lowest-paid government worker used for calculation (in US$): Bangladesh 1.85; Rio Grande do Sul State, Brazil 5.50; Malawi 1.36; Nepal 1.42; Pakistan 2.02; Sri Lanka 2.63. Information collected in local currency was converted into US$ using the exchange rate (commercial “buy” rate) on the first day of the survey.
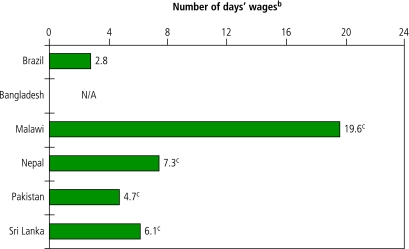
In all countries, patients with diabetes could purchase at least one of the two oral antidiabetics included in the survey (glibenclamide and metformin) with less than 1 days’ wage. Conversely, one month of treatment with intermediate-acting insulin preparations often cost more than several days’ wages (Fig. 3).
Fig. 3.
Affordability of standard treatment for diabetes in the private sector in selected low- and middle-income countriesa
NA, not available.
a Affordability expressed as the number of days’ wages that it would cost the lowest-paid government worker to purchase one month of daily treatment with generic equivalent of 40 IU intermediate-acting insulin.
b Daily wage of lowest-paid government worker used for calculation (in US$): Bangladesh 1.85; Rio Grande do Sul State, Brazil 5.50; Malawi 1.36; Nepal 1.42; Pakistan 2.02; Sri Lanka 2.63. Information collected in local currency was converted into US$ using the exchange rate (commercial “buy” rate) on the first day of the survey.
c Data for innovator brand product was used because of the low availability of generic products.
Patients with persistent asthma require a steroid inhaler for ongoing management and a beta-stimulant inhaler for relief of acute attacks. Monthly treatment with this combination therapy costs more than 9 days’ wages in Malawi and several days’ wages in countries where both medicines were found (Fig. 4).
Fig. 4.
Affordability of standard treatment for bronchial asthma in the private sector in selected low- and middle-income countriesa
N/A, not available.
a Affordability expressed as the number of days’ wages that it would cost the lowest-paid government worker to purchase one month of treatment with generic equivalents of a combination of 400 µg beclometasone/day and ⩽ 2 puffs of salbutamol inhaler 3 times/day.
b Daily wage of lowest-paid government worker used for calculation (in US$): Bangladesh 1.85; Rio Grande do Sul State, Brazil 5.50; Malawi 1.36; Nepal 1.42; Pakistan 2.02; Sri Lanka 2.63. Information collected in local currency was converted into US$ using the exchange rate (commercial “buy” rate) on the first day of the survey.
c Data for innovator beclometasone was used because of the low availability of the generic product.
Final price and international reference price
In the private sector, generic aspirin costs more than 10 times its international reference price in Nepal (median price ratio of 12) and in Sri Lanka (median price ratio of 10), as did generic hydrochlorothiazide in Nepal (median price ratio of 11). In Brazil, aspirin costs 30 times it international reference price and hydrochlorothiazide costs 25 times it reference price. Propranolol products with the best availability cost more than 8 times the international price in Nepal (median price ratio of 9), Brazil (median price ratio of 16) and Pakistan (median price ratio of 8).
Components of price
As medicines travelled through the private-sector distribution chain, price increases resulted primarily from wholesale and retail mark-ups (Table 5). The total add-on costs applied to the manufacturer’s price ranged from 18% in Pakistan to more than 90% in countries where there were no regulatory policies, such as Malawi (data not shown). (Complete information on price components could be obtained from only two countries and for Pakistan this information was available only for a local product.)
Table 5. Price components and cumulative mark-up for one imported essential medicine and one locally produced essential medicine in the private sector in three selected countriesa.
| Chargeb | Malawi |
Nepal |
Pakistan |
||||
|---|---|---|---|---|---|---|---|
| Imported product | Local product | Imported product | Local product | Local product | |||
| Import tariff | US$ 8.70 (1200 kwacha) | NA | 5% | NA | NA | ||
| Port charges | 0.5% | NA | Up to 3% | NA | 1% | ||
| Clearance and freight | Air: 1%; Road or surface: 0.75% | NA | Up to 3% | NA | 1% | ||
| Pre-shipment inspection | NA | NA | 0.125–1% | NA | Quality control testing: US$ 49.00–81.00 (3000–5000 rupees) | ||
| Pharmacy board fee | US$ 250/year | US$ 10.90/year (1500 kwacha/year) | NA | NA | US$ 19.00 (1200 rupees) | ||
| Importers’ margins | Varies | NA | 5% | NA | NA | ||
| Value-added tax | Exempt | Exempt | Exempt | Exempt | Exempt | ||
| Central government tax | Exempt | Exempt | Exempt | Exempt | NA | ||
| State government tax | Exempt | Exempt | Exempt | Exempt | NA | ||
| Wholesale mark-up | 20–30% | 20–30% | 5–10% | 10% | 5–10% | ||
| Retail mark-up | Not regulated: 35–65% observed | Not regulated | 16% | 16% | 10–15% | ||
NA, not applicable (the charge does not exist). a Complete information on price components and cumulative mark-up could be obtained from only two countries and for Pakistan information was available only for a local product. The imported essential medicine and the locally produced essential medicine in the private sector were selected by the investigators based on the availability of reliable information. b Currency exchange rates used in these calculations were: US$ 1.00 = 136.6 Malawian kwacha, 72.1 Nepalese rupees, and 60.5 Pakistani rupees.
The efficiency of the public supply chain was assessed by comparing procurement prices with final prices. Government procurement prices were comparable to international reference prices. Median price ratios for procurement (that is, the median of the ratios of the procurement price to the international reference price) for the lowest priced generic medicines ranged from 0.47 in Sri Lanka to 0.85 in Pakistan and 0.91 for innovator brands in Malawi (data not shown), indicating that these countries have efficient public sector procurement systems. This means that the basket of medicines in Sri Lanka costs 47% of the international reference price for the same medicines. Final prices in the public sector were comparable to procurement prices, with mark-ups of 0% for the lowest priced generic medicines in Sri Lanka (in state hospitals), 8.6% in Malawi and 15.4% in Pakistan (data not shown), suggesting that these countries have financially efficient distribution systems.
Discussion
The results of this preliminary analysis support our hypothesis and highlight the need to improve the availability of medicine for chronic diseases, particularly in the public sector, and to ensure that the medicines used in treatment regimens are affordable.
Although many medicines for chronic diseases are theoretically provided free or at low cost in the public sector, their availability was poor. These results are particularly striking given that different doses were used to determine availability, a deviation from the WHO–Health Action International methods that specify the dose to be included in the survey. Stock-outs due to poor estimates of consumption and cash-flow constraints are possible explanations, however the specific causes of poor availability require further investigation.
Because the availability of these medicines in the public sector is poor, the majority of patients must purchase medicine from the private sector or forego treatment if they cannot afford it. The availability of medicines in the private sector, though better than in the public sector, was still poor in most countries. In Malawi and Sri Lanka, the availability of locally manufactured medicines was generally better than medicines that were not produced domestically, indicating the potentially important role local manufacturing may have in increasing the supply of medicines.
In the private sector in all countries except Bangladesh, innovator brands were more costly than their generic equivalents. High premiums on branded medicines become an issue when generic equivalents are not stocked, when innovator brands are prescribed or dispensed preferentially, and when generic substitution is prohibited. Wide variation in median price ratios was also observed for different medicines in the private sector, suggesting that there are inconsistencies in procurement efficiency or mark-ups, or both. In Nepal and Pakistan, prices showed little variation among private outlets. This may reflect price regulations in Pakistan; in Nepal, where prices are not regulated, market competition may be a key factor. In Malawi, prices varied substantially among private outlets and were generally higher than those in other countries surveyed, possibly owing to a lack of price regulation or price information.
For patients with chronic diseases who require multiple medications for adequate management, monthly treatment costs may be equivalent to several days’ wages of the lowest-paid government worker; they are, therefore, unaffordable. Further, many people in low- and middle-income countries earn less than the wages of the lowest-paid government worker, which were used to calculate affordability in this study. Given that 8% of the population in Brazil lives below the international poverty line of US$ 1.00 per day, even treatments that seem to be affordable are out-of-reach of a large number of people. The percentage of people in Sri Lanka living below the poverty line is also 8%; in Pakistan the proportion is 13%; in Bangladesh it is 36%; in Nepal it is 39%; and in Malawi it is 42%.21
Hydrochlorothiazide is an inexpensive first-line antihypertensive,22 yet it had poor availability (0–10%) in several countries and had a high median price ratio when compared with the other antihypertensive medicines surveyed. Similarly, although aspirin is recommended for most patients with established coronary heart disease,5 it had the highest median price ratio of all medicines in both the public and private sectors in Nepal and Sri Lanka, and in the private sector in Brazil and Pakistan. These results suggest that a review of guidelines for medicine selection at the supplier level may be warranted, especially in the public sector. Further, practice guidelines and lists of essential medicines should be regularly reviewed and revised to reflect current evidence on the rational use of medicines, and physicians, pharmacists and patients should be made aware of best practices.
Caution is required when interpreting median price ratios since a high international reference price may lead to a low median price ratio and vice versa. Although these ratios may provide insight into the efficiency of procurement and the magnitude of mark-ups applied at the country level, they do not reveal any information about affordability. Medicines with low median price ratios may still be unaffordable for the general population. Further, the affordability of a medicine may vary significantly across countries. In this respect, the influence of the daily wage of the lowest-paid government worker must also be considered since higher wages will lead to improvements in affordability.
A mix of policies that specifically address a country’s circumstances is required to improve the availability and affordability of treatments for chronic diseases. Improvements in governance and management efficiency, and a realistic assessment of local supply options could increase availability. Strategies that can be used to lower prices include ensuring there is adequate and sustainable financing through national and international sources, pooling procurement and other arrangements to improve purchasing efficiency, eliminating taxes and tariffs, regulating mark-up charges and regularly monitoring the distribution chain. In Malawi and Nepal, for example, medicines are exempt from value added tax and central government tax, while in Pakistan prices are regulated through the enforcement of maximum mark-ups and maximum selling prices.
Patients purchasing innovator brands from private outlets usually pay substantially more than they would for generic equivalents. The availability and use of generic preparations should be facilitated through the implementation of several strategies, including educating health professionals to use standard treatment guidelines that link price and affordability and encourage generic prescribing; increasing consumers’ awareness of the availability, affordability and acceptability of generics; implementing policies that make generic substitution compulsory or allowing higher mark-ups for generic products; developing a remuneration policy for dispensing fees and implementing fixed margins, rather than allowing unregulated mark-ups, in order to favour generic products; promoting market competition; and, where market forces fail, regulating prices.
This survey revealed the substantial part donations play in the supply of drugs to the public sector in countries such as Bangladesh and Malawi. Although donations temporarily increase availability, they are not a sustainable solution. However, most international drug companies do not appear to be concerned about developing markets in poor countries; these account for less than one quarter of annual global pharmaceutical sales.23
This study has provided some insight into issues related to the price, availability and affordability of key medicines used to treat chronic diseases. The results are limited by the fact that medicine outlets surveyed were within one-day’s travel from a major urban centre; data were subject to outside influences, such as market fluctuations and delivery schedules; some data were unavailable or missing, or both; and international reference prices were sometimes based on a single agency’s or supplier’s price, rather than on a median price. Further, the study failed to capture information on medicines obtained through informal channels, such as street vendors. Notwithstanding these shortcomings, the results highlight priority areas for action by ministries of health and other stakeholders. Broad debate and dialogue are needed to identify the best way for different players to contribute to improving access to affordable medicines for treating chronic diseases.
Conclusion
Patients with chronic diseases, such as cardiovascular disease, diabetes and asthma, require a reliable supply of affordable medicines. In the absence of such a supply, avoidable mortality and morbidity will occur. A range of policy options and technical options exist to enable governments to ensure that medicines for chronic diseases are consistently available and affordable. A commitment by governments to meet the needs of their citizens who suffer from chronic diseases is urgently required. ■
Acknowledgements
The advice and guidance of the following are acknowledged: C Le Galès-Camus, R Beaglehole, M Mostafa Zaman, CN Mwikisa, K Wagner, K Bile Mohamud, K Tun, S N Khaltaev, G Roglic. The country project teams that participated in this collaborative study are thanked and include: in Bangladesh, CM Hasan, MA Rashid; in Malawi, R Pendame, E Soliman; in Nepal, NM Shrestha, BB Thapa, B Subba Rao, GM Khan; in Pakistan, S Nishtar, A Badar; and in Sri Lanka AR Wickremasinghe.
Footnotes
Competing interests: None declared.
References
- 1.Preventing chronic diseases: a vital investment. Geneva: WHO; 2005.
- 2.The world health report 2003: shaping the future. Geneva: WHO; 2003.
- 3.Barcelo A, Aedo C, Rajpathak S, Robles S. The cost of diabetes in Latin America and the Caribbean. Bull World Health Organ. 2003;81:19–27. [PMC free article] [PubMed] [Google Scholar]
- 4.Quick JD, Hogerzeil HV, Velasquez G, Rago L. Twenty-five years of essential medicines. Bull World Health Organ. 2002;80:913–4. [PMC free article] [PubMed] [Google Scholar]
- 5.Prevention of recurrent heart attacks and strokes in low and middle income populations: evidence-based recommendations for policy-makers and health professionals. Geneva: WHO; 2003.
- 6.Mitchell EA, Didsbury PB, Kruithof N, Robinson E, Milmine M, Barry M, et al. A randomized controlled trial of an asthma clinical pathway for children in general practice. Acta Paediatr. 2005;94:226–33. doi: 10.1080/08035250410020235. [DOI] [PubMed] [Google Scholar]
- 7.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- 8.Saenz A, Fernandez-Esteban I, Mataix A, Ausejo M, Roque M, Moher D. Metformin monotherapy for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;3:CD002966. doi: 10.1002/14651858.CD002966.pub3. [DOI] [PubMed] [Google Scholar]
- 9.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 10.Kishuna A. Drug pricing survey in KwaZulu-Natal. Essential Drugs Monitor [Online journal] 2003;32:4-7. Available from: http://mednet2.who.int/edmonitor/32/32_2.pdf
- 11.Madden J, Kotwani A. Availability of essential medicines: an example from Rajasthan, India. Essential Drugs Monitor [Online journal] 2003;33:17. Available from: http://mednet2.who.int/edmonitor/33/EDM33_17_Availability_e.pdf
- 12.Madden J, Balasubramaniam K, Kibwage I. Components of patient prices: examples from Sri Lanka and Kenya. Essential Drugs Monitor [Online journal] 2003;33:18. Available from: http://mednet2.who.int/edmonitor/33/EDM33_18_Components_e.pdf
- 13.Madden J. Comparing pilot survey results from different countries. Essential Drugs Monitor [Online journal] 2003;33:19-20. Available from: http://mednet2.who.int/edmonitor/33/EDM33_19-20_Comparing_e.pdf
- 14.Mendis S, Abegunde D, Yusuf S, Ebrahim S, Shaper G, Ghannem H, et al. WHO study on Prevention of REcurrences of Myocardial Infarction and StrokE (WHO-PREMISE). Bull World Health Organ. 2005;83:820–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Wise J. Polypill holds promise for people with chronic disease. Bull World Health Organ. 2005;83:885–7. [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization, Health Action International. Medicine prices: a new approach to measurement. Working draft for field testing and revision, (WHO/EDM/PAR/2003.2), 2003. Available from: http://www.haiweb.org/medicineprices/manual/manuals/MedicinePrices.pdf
- 17.World Health Organization. Essential medicines: WHO model list, 14th edition, 2005. Available from: http://whqlibdoc.who.int/hq/2005/a87017_eng.pdf
- 18.Management Sciences for Health. International drug price indicator guide, 2005. Available from: http://erc.msh.org/mainpage.cfm?file=1.0.htm&module=DMP&language=English
- 19.Gelders S, Ewen M, Noguchi N, Laing R. Price, availability and affordability: an international comparison of chronic disease medicines, (WHO-EM/EDB/068/E), 2006. Available from: http://mednet3.who.int/medprices/CHRONIC.pdf
- 20.International Bank for Reconstruction and Development, World Bank. His Majesty`s Government, Ministry of Health, and the World Health Organization (HMG/WHO) Nepal (1995), Report on Evaluation of Community Drug Supply Scheme (WHO supported). Kathmandu: Planning and Foreign Aid Division, Dept Health Services, Ministry of Health, His Majesty`s Govt of Nepal; 1995.
- 21.The World Bank. World development indicators2005. Available from: http://devdata.worldbank.org/wdi2005/index2.htm
- 22.World Health Organization. International Society of Hypertension. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–92. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Vaknin S. Global differential pricing, 2002. Available from: http://www.globaltreatmentaccess.org/content/press_releases/a02/060602_UPI_HGAP_phrma.html