Abstract
Objective
To determine the prevalence and intensity of Schistosoma japonicum infection and associated morbidity, and to estimate the infected human and buffalo populations in the Dongting Lake region, Hunan province, China.
Methods
We used data from the third national schistosomiasis periodic epidemiological survey (PES) of 2004. These included 47 144 human serological and 7205 stool examinations, 3893 clinical examinations and questionnaire surveys, and 874 buffalo stool examinations, carried out in 47 villages in Hunan province. Serological examinations were performed using the enzyme linked immunosorbent assay technique and human stool samples were examined by the Kato-Katz method. Stools from buffaloes and other domestic animals were examined for schistosome infection by the miracidial hatching test.
Findings
Sero-prevalence was 11.9% (range: 1.3–34.9% at the village level), and the rate of egg-positive stools was estimated at 1.9% (0–10.9%) for the same population. The prevalence of infection among buffaloes was 9.5% (0–66.7%). Extrapolating to the entire population of the Dongting Lake region, an estimated 73 225 people and 13 973 buffaloes were infected. Most frequently reported symptoms were abdominal pain (6.2%) and bloody stools (2.7%). More than half of the clinically examined people reported having had at least one prior antischistosomal treatment.
Conclusion
There was a significant reduction in the number of humans infected with S. japonicum since the previous national PES carried out in 1995, partially explained by large-scale chemotherapy campaigns. However, a near-stable number of buffalo infections suggest continuing human re-infection, which may lead to future increases in human prevalence.
Résumé
Objectif
Déterminer la prévalence et l’intensité de l’infection à Schistosoma Japonicum et la morbidité associée. Estimer l’importance des populations d’êtres humains et de buffles contaminés dans la région du lac de Dongting, dans la province du Hunan, en Chine.
Méthodes
Nous avons utilisé des données provenant de la troisième enquête épidémiologique périodique sur la schistosomiase (PES) de 2004. Ces données étaient les résultats de 47 144 examens sérologiques, de 7205 examens de selles et de 3893 examens cliniques et enquêtes par questionnaire pratiqués chez des êtres humains, ainsi que de 874 examens d’excréments de buffles, effectués dans 47 villages du Hunan. Les examens sérologiques ont été réalisés par la méthode immuno-enzymatique en phase solide et les examens de selles humaines par la technique de Kato-Katz. On a recherché la présence d’une infection à schistosomes sur des selles de buffles et d’autres animaux domestiques par le test d’éclosion des miracidia.
Résultats
La séroprévalence était de 11,9% (plage : 1,3 – 34,9% au niveau du village) et la proportion de selles positives pour le test d’éclosion a été estimée à 1,9% (0 – 10,9%) dans la même population. La prévalence de l’infection parmi les buffles était de 9,5% (0 – 66,7%). En extrapolant ces résultat à l’ensemble de la population de la région du Lac Dongting, on a estimé que 73 225 personnes et 13 973 buffles étaient contaminés. Les symptômes les plus fréquemment rapportés étaient des douleurs abdominales (6,2%) et des selles sanglantes (2,7%). Plus de la moitié des participants ayant subi un examen clinique ont signalé au moins un traitement antischistosomique antérieur.
Conclusion
L’étude a permis de constater une réduction notable du nombre d’êtres humains contaminés par S. japonicum depuis la précédente enquête PES de 1995, réduction partiellement explicable par les campagnes de chimiothérapie à grande échelle. Cependant, le nombre relativement stable de buffles contaminés laisse prévoir une recontamination permanente des humains, susceptible de conduire à une recrudescence de la prévalence de la schistosomiase chez l’homme.
Resumen
Objetivo
Determinar la prevalencia e intensidad de la infección por Schistosoma japonicum y la morbilidad a ella asociada, y estimar las poblaciones humanas y de búfalos infectadas en la región del lago Dongting, en la provincia china de Hunan.
Métodos
Usamos datos del tercer estudio epidemiológico nacional periódico sobre la esquistosomiasis de 2004. Como parte del mismo se hicieron 47 144 pruebas serológicas y 7205 análisis de heces en personas, 3893 exploraciones clínicas y encuestas a base de cuestionarios, y 874 análisis de heces en búfalos, en un total de 47 aldeas de la provincia de Hunan. Los análisis serológicos se realizaron mediante una técnica de inmunosorción enzimática, y las muestras de heces humanas fueron examinadas mediante el método de Kato-Katz. La presencia de infección por esquistosoma en las heces de búfalos y otros animales domésticos se analizó mediante la prueba de incubación de miracidia.
Resultados
La seroprevalencia fue del 11,9% (intervalo: 1,3%-34,9% a nivel de aldea), y la tasa de resultados positivos para la presencia de huevos en las heces fue del 1,9% (0%-10,9%) para la misma población. La prevalencia de infección entre los búfalos fue del 9,5% (0%-66,7%). Extrapolando a la totalidad de la población de la región del lago Dongting, se estimó que estaban infectadas unas 73 225 personas y 13 973 búfalos. Los síntomas notificados con más frecuencia fueron el dolor abdominal (6,2%) y las heces sanguinolentas (2,7%). Más de la mitad de los participantes explorados clínicamente declararon haber sido sometidos antes a tratamiento antiesquistosómico por lo menos una vez.
Conclusión
Se observó una reducción importante del número de personas infectadas por S. japonicum desde el estudio nacional realizado en 1995, lo que puede atribuirse en parte a las campañas de antibioticoterapia emprendidas a gran escala. Sin embargo, el número prácticamente inalterado de infecciones detectadas en la población de búfalos lleva a pensar que persisten las reinfecciones humanas, y ello podría traducirse en un futuro aumento de la prevalencia en el hombre.
ملخص
الەدف
معرفة معدل انتشار وشدة العدوى بالبلەارسيات اليابانية والمراضة المصاحبة لەا، وتقدير مجموعات السكان والجواميس المصابة بالعدوى في منطقة بحيرة دونغتنغ في ولاية ەونان في الصين.
الطريقة
استخدمنا المعطيات المستفادة من المسح الوبائي الدوري الوطني لداء البلەارسيات في عام 2004. وقد اشتمل على 144 47 اختباراً سيرولوجياً، و7250 اختباراً للبراز، و3893 فحصاً سريرياً ومسحاً بالاستبيانات، و874 اختباراً لبراز الجواميس، نفذت في 47 قرية في ولاية ەونان. وقد أجريت الاختبارات السيرولوجية باستخدام أسلوب إليزا (مقايسة الممتز المناعي المرتبط بالإنزيم)، وطريقة كاتو-كاتز لفحص البراز، كما فحصت عينات براز الجواميس والحيوانات المنزلية الأخرى بحثاً عن العدوى بالبلەارسيات باختبار تفقيس الطُفَيْلات.
الموجودات
بلغ معدل الانتشار السيرولوجي 11.9% ( وتراوح بين 1.3 و34.9 على مستوى القرية)، وبلغ معدل إيجابية البيوض في البراز 1.9% (وتراوح بين 0 و10.9%) بالنسبة لنفس المجموعات السكانية. أما معدل انتشار العدوى بين الجواميس فكان 9.5% (وتراوح بين (0 و66.7%) وباستقراء ەذە النتائج على مجمل السكان في منطقة بحيرة دونغتنغ، يقدر أن 225 73 من السكان و973 13 من الجواميس مصابون بالعدوى. وأكثر الأعراض التي يُبَلَّغ عنەا ەي الألم البطني (6.2%) والبراز المدمَّى (2.7%). وأبلغ ما يزيد على نصف المشاركين في الفحص السريري عن معالجة سابقة لمرة واحدة على الأقل بالأدوية المضادة للبلەارسيات.
الاستنتاج
كان ەناك نقص واضح في عدد المصابين بعدوى البلەارسيات اليابانية منذ إجراء المسح الوبائي الدوري الوطني لداء البلەارسيات عام 1995، وقد عُلِّل ذلك، ولو بشكل جزئي، بالحملات الواسعة النطاق للمعالجة الكيميائية. ومع ذلك فإن عدداً يكاد يكون ثابتاً من العدوى لدى الجواميس يشير إلى استمرار السراية بين البشر، وەو ما قد يؤدي إلى زيادة مستقبلية في معدل الانتشار بين الناس.
Introduction
Schistosomiasis japonica remains endemic in seven provinces in China.1,2 Human infection is acquired during the course of domestic or occupational activities, in particular fishing and farming.3,4 Acute infection may result in fever, weakness, diarrhoea, abdominal pain and hepatomegaly. Chronic disease involves granuloma formation, tissue inflammation, liver lesions and fibrosis, which may persist after infection has been cleared.5,6 Schistosoma japonicum is also known to infect 45 species of animals, of which water buffaloes are especially important for transmission.7 The zoonotic nature of schistosomiasis japonica renders control particularly challenging. Despite 50 years of intensive control in China, the disease remains of considerable public health concern, with an estimated 843 000 people and 100 250 bovines infected in 2003.1 Major endemic foci occur in the marsh and lake areas of southern China, particularly the Dongting Lake region bordering Hubei and Hunan provinces, and the Poyang Lake region in Jiangxi province.2
In the 1990s, praziquantel-based morbidity control became the central feature of China’s national schistosomiasis control programme, supported through a 10-year World Bank Loan Project (WBLP).8,9 The estimated number of human infections was reduced by over 50%, from 1 471 000 in 1989 to 695 000 in 2000.9 Recent data indicate that schistosomiasis might be spreading, and re-emerging in settings where the disease had previously been controlled.10,11 The causes are multifactorial, including severe flooding,12 water-resource developments such as the construction of the Three Gorges Dam and the resulting ecological transformations,13 climate change,14,15 market and health sector reforms,16 increased population density and migration, as well as the termination of the WBLP.9,17
Concerns about the re-emergence of schistosomiasis, particularly in the densely populated lake regions, call for a re-estimation of the number of current infections in humans and domestic animals. Here we have determined the level and extent of schistosomiasis in the Dongting Lake region, including estimates of disease-associated morbidity, based on data from the third national schistosomiasis periodic epidemiological survey (PES), carried out in 2004. The results are compared to the previous PES done in 1995 and the observed changes discussed.
Materials and methods
Study area and population
A nationwide cross-sectional epidemiological survey, using a cluster-randomized design, was carried out by the Chinese Ministry of Health in October/November 2004, with a total of 250 987 people examined for schistosomiasis in 239 villages.18 In Hunan province, the survey was conducted in 47 of the 2832 villages from the Dongting Lake region where schistosomiasis is endemic. The region covers an area of approximately 15 000 km² and accounts for 7.2% of the total area and 6.1% (i.e. 4 133 137) of the total population of Hunan province. There were an estimated 124 265 buffaloes in the region at the time of the study.18
Sampling procedures
Sampling followed the standard protocols of the national schistosomiasis PES. Briefly, in China there are eight distinct schistosome-endemic settings within three topological areas, namely (i) lake areas, (ii) water course network areas, and (iii) hilly areas.9 The Dongting Lake region comprises five of the eight endemic settings, i.e. (i) lake fork, (ii) grassy lake beach, (iii) lake embankment, (iv) inside embankment (lake areas), and (v) hills (hilly areas). Within each endemic setting, villages were grouped according to an estimated prevalence of S. japonicum among local residents as follows: (i) high (≥11%), (ii) upper moderate (6–10.9%), (iii) lower moderate (1–5.9%), and (iv) low (< 1%). Usually, one out of 100 villages from every endemic setting/prevalence group combination was selected at random; if there were less than 100 villages in any of the 20 combinations, one village was selected at random. All inhabitants of the selected villages aged between 5 and 65 years were invited to participate, but there were a few younger or older participants included in the final data set.
Within each sampled village, 100 domestic animals (comprising buffaloes, cattle, pigs and goats) were selected at random for faecal examination. If there were less than 100 animals in a village, all were included in the examination.
In addition to the standard study design of the first (1989) and second (1995) national schistosomiasis PES, the third PES (2004) consisted of a supplementary morbidity component, administered to one randomly re-selected village from each prevalence group within the lake-embankment endemic setting.
Serological, parasitological and clinical examinations
A two-pronged diagnostic approach, which has been widely and effectively applied in China over the past decades,19,20 was used to investigate S. japonicum infections among human participants. First, serum was extracted from 2 ml venous blood taken from each subject and examined by indirect enzyme linked immunosorbent assay (ELISA) for the occurrence of anti-soluble egg antigen (SEA) IgG antibodies.19 Second, SEA-ELISA-positive individuals were asked to provide a stool specimen from which three Kato-Katz thick smears were prepared and examined under a light microscope by experienced laboratory technicians who counted S. japonicum eggs per slide. A random sample of SEA-ELISA-negative individuals (< 5%) was also subjected to the Kato-Katz technique. Infection intensity was expressed as the number of eggs per gram of faeces (epg).21 Individuals with watery stools were not included in the examination due to the dilutive effect of watery stools on schistosome eggs. For quality control, 10% of slides were randomly selected and re-examined by a senior microscopist.
The miracidial hatching test5,7 was undertaken on single stool specimens taken from domestic animals as a marker of S. japonicum infection.
Human clinical examinations, involving liver and spleen palpations, were performed by a community nurse, examining liver tenderness. Then, a portable ultrasound (Sonolayer-L SAL-33B; Toshiba) was used for assessment of size, texture, fibrosis and other abnormalities of the liver, portal vein diameter, interior portal vein diameter, spleen size and biliary duct abnormalities. Standard positions, views, measurements and classification protocols were followed.12,22 Clinically examined subjects were also interviewed for the presence of symptoms (e.g. headache, diarrhoea and blood in stool) with a recall period of 2 weeks and whether they had previous antischistosomal treatment history.
Consent and anthelmintic treatment
Written informed consent was obtained from each individual or, for those below the age of 15 years, from their parents/legal guardians; verbal informed consent was obtained from the domestic animal owners. ELISA-positive individuals, apart from pregnant women, were treated with praziquantel (single oral dose of 40 mg/kg). All S. japonicum-positive animals were also treated with single oral doses of praziquantel (buffaloes at 25 mg/kg, cattle at 30 mg/kg, goats at 20 mg/kg and pigs at 60mg/kg).
Data management and statistical analyses
Data were double-entered into FoxPro (version 6.0), cross checked and subsequently analysed with SPSS version 13.0 (Chicago, USA). Separate analyses were carried out for the SEA-ELISA results and the combination of the SEA-ELISA plus the Kato-Katz thick smear examinations. Infection intensities were categorised as light (1–100 epg), moderate (101–400 epg) or heavy (> 400 epg). All variables including sex, age group and endemic setting were explored individually by χ² statistics. Infection intensity was explored by Student’s t-test and the Kruskal–Wallis test.
Estimates of the number of infected people and buffaloes were made using Microsoft Excel 2002. Population structure and numbers were obtained from the 2003 data. Data were stratified according to sampling procedure as follows: five schistosome-endemic settings, four endemic groups, sex and four age groups, giving a total of 160 separate estimations. The standard error (SE) of each estimate was converted to a variance; all variances were summed to provide an overall variance, SE and 95% confidence interval (CI). Buffalo numbers were obtained from the Department of Animal Husbandry, and were stratified according to endemic setting/group only.
Logistic and negative binomial regression models were fitted for S. japonicum infection status and intensity, respectively, to assess for significant associations with morbidity indicators.
Results
Study compliance and operational results
The five schistosome-endemic settings of the Dongting Lake region were represented in the sample by a ratio of 1:1:5:2:1 for the lake fork, grassy lake beach, lake embankment, inside embankment and hills, respectively. There were a total of 47 villages; hence each village accounted for ~2% of the overall sampled population, which totalled 83 604 individuals. Among them, 47 144 (56.4%) had complete data sets, with infection status for S. japonicum based on the SEA-ELISA examination and, among those SEA-ELISA-positive, three Kato-Katz thick smears from a single stool specimen. There were 24 640 (52.3%) males and 22 504 (47.7%) females. Age ranged from 1 month to 99 years, although 98.3% of the sampled population was between 5 and 65 years. The median age of males and of females was 36 years, and the standard deviation (SD) was 17.7 and 17.0 years, respectively: 3893 individuals (2083 or 53.5% males) were also examined clinically.
Infection with S. japonicum
Table 1 displays a summary of the infection prevalence and intensity found among human participants. The SEA-ELISA examination revealed the presence of antibodies in 5625 (11.9%) individuals. Among them, 874 (15.5%) were found S. japonicum egg-positive by the Kato-Katz method. Of the 41 519 individuals with negative SEA-ELISA results, 1580 (3.8%) were selected for subsequent stool examination, all of whom were S. japonicum egg-free. Thus, the overall prevalence of S. japonicum, considering all negative SEA-ELISA results as ‘true’ negative Kato-Katz results, was 1.9% (874/47 144).
Table 1. Prevalence, as indicated by SEA-ELISA examination alone and in combination with Kato-Katz thick smears, and intensity of infection with Schistosoma japonicum in the sample population, according to sex, age category and endemic setting, shown for a sample of 47 144 individuals in the 2004 periodic epidemiological survey (PES) from the Dongting Lake region of Hunan province, Chinaa.
| Variable |
S. japonicum infection status |
S. japonicum infection intensity (epg) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Infected: SEA-ELISA N (%) | Infected: SEA-ELISAb plus Kato-Katz N (%) | Geometric mean intensity epg (SD) | Light (1–100) N (%) | Moderate (101–400) N (%) | Heavy (>400) N (%) | ||||
| Year | 2004 | 1995c | 2004 | 1995c | 2004 | 2004 | 2004 | 2004 | |
| Sex | |||||||||
| Male | 3758 (15.3) | 2383 (9.9) | 673 (2.7) | 17.6 (n.a.) | 28.6 (10.3) | 581 (86.3) | 80 (11.9) | 12 (1.8) | |
| Female | 1867 (8.3) | 1105 (5.4) | 201 (0.9) | 17.4 (n.a.) | 24.7 (23.3) | 181 (90.1) | 17 (8.5) | 3 (1.5) | |
| Age category (years) | |||||||||
| 0–19 | 708 (5.6) | 383 (3.1) | 59 (0.5) | 12.6 (n.a.) | 21.9 (3.4) | 55 (93.2) | 4 (6.8) | 0 | |
| 20–39 | 1813 (12.5) | 1675 (8.1) | 267 (1.8) | 17.6 (n.a.) | 27.4 (24.2) | 236 (88.4) | 24 (9.0) | 7 (2.6) | |
| 40–59 | 2550 (15.7) | 1430 (12.4) | 460 (2.8) | 18.8 (n.a.) | 27.7 (19.2) | 396 (86.1) | 57 (12.4) | 7 (1.5) | |
| ≥60 | 554 (15.1) | – | 88 (2.4) | – | 32.8 (17.6) | 75 (85.2) | 12 (13.6) | 1 (1.1) | |
| Endemic setting | |||||||||
| Lake fork | 698 (13.2) | 582 (11.2) | 187 (3.5) | 8.3 (n.a.) | 51.7 (35.1) | 134 (71.6) | 43 (23.0) | 10 (5.4) | |
| Lake embankment | 3038 (13.5) | 1493 (9.2) | 576 (2.6) | 23.9 (n.a.) | 21.8 (9.6) | 541 (93.9) | 33 (5.7) | 2 (0.4) | |
| Inside embankment | 1157 (10.6) | 473 (2.5) | 63 (0.6) | 34.7 (n.a.) | 25.6 (9.1) | 33 (71.7) | 11 (23.9) | 2 (4.4) | |
| Lakebeach | 500 (9.5) | 940 (22.5) | 46 (0.9) | 11.4 (n.a.) | 43.6 (34.3) | 53 (84.1) | 9 (14.3) | 1 (1.6) | |
| Hills | 232 (7.4) | 0 | 2 (0.1) | 0 | 91.9 (3.3) | 1 (50) | 1 (50) | 0 | |
| Total | 5625 (11.9) | 3488 (7.8) | 874 (1.9) | 17.6 (n.a.) | 27.6 (18.3) | 762 (87.2) | 97 (11.1) | 15 (1.7) | |
epg, eggs per gram; SD, standard deviation; SEA-ELISA, soluble egg antigen - indirect enzyme linked immunosorbent assay. a Data from the 1995 PES are shown for comparison. b In the second national schistosomiasis PES, in 1995, the miracidial hatching test was performed on stool samples from all individuals, followed by Kato-Katz thick smear examination of positive individuals, while in 2004 the initial screening of the participants was done by an SEA-ELISA examination.24 c Data obtained from the Hunan Provincial Office of the Leading Group for Schistosomiasis and Endemic Diseases Control.
Male study participants were more likely to be infected with S. japonicum for both SEA-ELISA examination alone (χ² = 541.5, degree of freedom (d.f.) = 1, P < 0.001), and for SEA-ELISA combined with Kato-Katz (χ² = 218.4, d.f. = 1, P < 0.001). Likewise, older age-groups were significantly more likely to be infected with S. japonicum than their younger counterparts, for SEA-ELISA alone (χ² = 902.8, d.f. = 13, P < 0.001) and the combined results (χ² = 270.7, d.f. = 13, P < 0.001).
The geometric mean infection intensity was 27.6 epg. Males had significantly heavier infections than females (t-test statistic = 6.92, d.f. = 47 142, P < 0.001). Infection intensity was also significantly associated with age group (Kruskal–Wallis H = 270.9, d.f. = 13, P < 0.001). Of the 874 egg-positive people, 762 (87.2%) had a light infection, whereas moderate and heavy infections were found in 97 (11.1%) and 15 (1.7%) people, respectively.
S. japonicum infections were found in 83 (9.5%) out of 874 water buffaloes, 13 (6.1%) out of 215 cattle and 9 (4.9%) out of 184 goats. No infections were found in 116 pigs examined.
The prevalence of human schistosomiasis had decreased by 75.6% when compared to the second PES in 1995, yet the geometric mean infection intensity increased by 10 epg (Table 1).
Differences in infection status and intensity
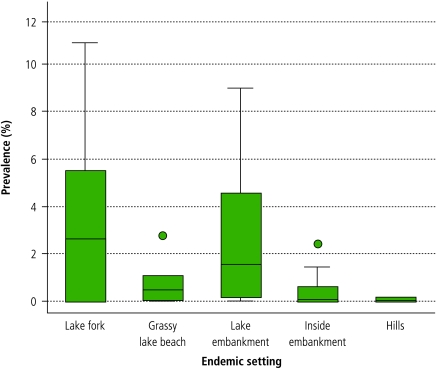
There was a significant difference in the schistosome prevalence for each endemic setting based on the SEA-ELISA (χ² = 170.7, d.f. = 4, P < 0.001) and the combined SEA-ELISA plus Kato-Katz results (χ² = 325.0, d.f. = 4, P < 0.001) (Fig. 1).
Fig. 1.
Human prevalence of Schistosoma japonicum, shown for 47 sampled villages within five endemic settings, in the Dongting Lake region of Hunan province
A horizontal line within the box indicates mean prevalence; outliers are represented by a dark circle.
Infection intensity was also significantly associated with endemic setting (Kruskal–Wallis H = 326.1, d.f. = 4, P < 0.001). At the village level, the geometric mean intensity of infection varied from 8.0 epg to 214.9 epg.
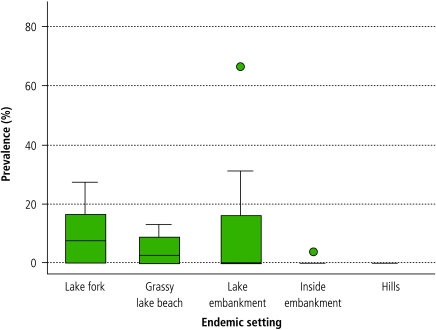
Fig. 2 shows that the buffalo infection prevalence also varied significantly according to endemic setting and village (range: 0–66.7%).
Fig. 2.
Buffalo prevalence of Schistosoma japonicum, shown for 47 sampled villages within five endemic settings, in the Dongting Lake region of Hunan province
A horizontal line within the box indicates mean prevalence; outliers are represented by a dark circle.
Estimates of people and buffaloes infected with S. japonicum
The estimated number of S. japonicum-infected people residing in the Dongting Lake region at the time of the study was 73 225 (95% CI: 61 679–84 772); more than three-quarters were male (78.0%). An estimated 55 238 (75.4%) lived in the lake embankment, 11 236 (15.3%) in the inside embankment, 5051 (6.9%) in the lake fork, 1496 (2.0%) in the grassy lake beach, and the remaining 204 (0.3%) in the hills.
The number of buffaloes infected was estimated at 13 973 (95% CI: 13 666–14 281). Of these, 12 889 (92.2%) were situated in the lake embankment, 457 (3.3%) in the lake fork, 419 (3.0%) in the inside embankment, and 208 (1.5%) in the grassy lake-beach; no infected buffaloes were present in the hills.
Schistosome-associated symptoms and morbidity, and treatment history
Table 2 summarizes the results from three bivariate regression models fitted on S. japonicum infection status (for SEA-ELISA alone and combined SEA-ELISA plus Kato-Katz) and intensity. Infected individuals were significantly more likely to report the presence of blood in stool and abdominal pain within 2 weeks preceding interview. Infection status determined by SEA-ELISA alone also showed a significant positive association with an increased portal vein diameter.
Table 2. Bivariate models showing the relationship of Schistosoma japonicum infection status (logistic regression), and infection intensity (negative binomial regression), with demographic descriptors, self-reported morbidity indicators and clinical disease among 3893 study participants from the 2004 PES in four villages in the lake embankment endemic setting, Dongting Lake region.
|
S. japonicum infection status |
S. japonicum infection intensity (epg) |
|||||||
|---|---|---|---|---|---|---|---|---|
| SEA-ELISA |
Combined SEA-ELISA plus Kato-Katz |
|||||||
| Variable | N | Adjusted OR (95% CI) | P-value* | Adjusted OR (95% CI) | P-value* | Adjusted IRR (95% CI) | P-value* | |
| Sex | < 0.001 | < 0.001 | 0.003 | |||||
| Female | 1810 | 1.00 | 1.00 | 1.00 | ||||
| Male | 2083 | 2.59 (2.10–3.20) | 3.04 (1.88–4.90) | 3.87 (1.70–8.85) | ||||
| Age category (in years) | < 0.001 | < 0.001 | < 0.001 | |||||
| 0–19 | 1056 | 1.00 | 1.00 | 1.00 | ||||
| 20–39 | 1205 | 3.94 (2.71–5.72) | 2.67 (1.30–5.49) | 1.42 (0.98–3.30) | ||||
| 40–59 | 1369 | 6.08 (2.24–8.72) | 3.64 (1.83–7.24) | 1.99 (0.56–2.86) | ||||
| ≥60 | 263 | 8.38 (5.39–13.00) | 4.57 (1.92-10.87) | 1.79 (0.68–2.99) | ||||
| Village | < 0.001 | 0.002 | < 0.001 | |||||
| Gaoqi | 962 | 1.00 | 1.00 | 1.00 | ||||
| Lianhe | 984 | 3.94 (2.95–5.26) | 3.72 (1.79–5.75) | 3.64 (2.08–5.23) | ||||
| Qixin | 930 | 0.03 (0.01–0.12) | 3.94 (1.95–5.94) | 2.56 (1.05–3.67) | ||||
| Zensheng | 1017 | 3.11 (2.32–4.17) | 4.21 (1.73–6.25) | 5.10 (4.86–7.01) | ||||
| Prior treatment history | 2058 | 6.04 (4.67–7.82) | < 0.001 | 10.32 (4.99–21.34) | < 0.001 | n.s. | ||
| Abdominal pain | 243 | 1.35 (1.22–1.56) | < 0.001 | 0.20 (0.03–1.45) | 0.034 | 0.03 (0.01–1.15) | 0.001 | |
| Blood in stool | 124 | 3.36 (0.84–6.14) | < 0.001 | 2.48 (1.57–3.81) | 0.002 | 3.17 (2.88–3.98) | < 0.001 | |
| Liver tenderness | 153 | n.s. | n.s. | 6.67 (4.08–9.02) | < 0.001 | |||
| Portal vein diameter (cm) | < 0.001 | n.s. | < 0.001 | |||||
| ≤8 | 194 | 1.00 | 1.00 | |||||
| 9–10 | 916 | 4.07 (1.26–7.14) | 4.12 (1.23–6.80) | |||||
| 11–12 | 2131 | 5.11 (2.89–8.72) | 3.21 (2.85–4.48) | |||||
| 13 | 452 | 5.61 (4.86–9.98) | 5.57 (4.02–7.33) | |||||
| 14 | 180 | 7.75 (5.60–9.83) | 6.37 (4.13–8.68) | |||||
| ≥15 | 20 | 7.00 (5.47–8.87) | 7.75 (5.69–9.13) | |||||
| Interior portal vein diameter (cm) | n.s. | n.s. | < 0.001 | |||||
| 6–8 | 1051 | 1.00 | ||||||
| 9–11 | 2429 | 4.77 (2.22–6.51) | ||||||
| ≥12 | 413 | 6.07 (4.01–8.88) | ||||||
CI, confidence interval; epg, eggs per gram; IRR, incidence risk ratio; OR, odds ratio; SEA-ELISA, soluble egg antigen - indirect enzyme linked immunosorbent assay. n.s. signifies variables not significant in the respective bivariate model. P-values based on the likelihood ratio test.
Similarly, infection intensity showed a significant positive association with abdominal pain, blood in stool, liver tenderness, increased portal vein diameter and increased interior portal vein diameter.
Of the 3893 individuals examined clinically, 2058 (52.9%) reported receiving praziquantel (one prior treatment: 26.3%, two prior treatments: 9.8%, at least three prior treatments: 16.8%). Prevalence, but not intensity, of infection was significantly associated with treatment history. The self-reported year of the most recent treatment ranged from 1958 to 2003, with more than half (53.7%) of the treatments having occurred in 2003.
Discussion
The third national schistosomiasis PES in 2004 revealed an overall S. japonicum prevalence in humans of 1.9% in the Dongting Lake region, or an estimated 73 225 infected people at the time of the survey. This is a 75.6% reduction since the second PES conducted in 1995. It is noteworthy that over the same timeframe the geometric mean infection intensity increased. We found a S. japonicum prevalence in buffaloes of 9.5%, translating to an estimated 13 973 infected buffaloes in the region, a slight increase (+1.1%) since 1995.23 Abdominal pain and blood in stool were the most common self-reported symptoms, while liver tenderness, increased portal vein diameter and increased interior portal vein diameter, revealed by clinical examination and ultrasonography, showed positive associations with human infection. Prior treatment histories with antischistosomal drugs were common.
There are three factors that may have led to underestimations in the number of infections. First, an important epidemiological feature of schistosomiasis is its focal distribution; sampling only 1% of villages may have resulted in some highly-endemic villages being missed.24 Second, we found a significantly higher prevalence and intensity of infection in males, particularly those aged 25–30 years. Many of these were absent during the cross-sectional epidemiological survey (data not shown), possibly working in the fields, and hence at an elevated risk of infection. Third, day-to-day and intra-specimen variations in egg output can underestimate prevalence in the case of limited stool sampling and diagnostic effort.25 Although we examined three Kato-Katz thick smears, all were derived from a single stool specimen, producing a test-sensitivity ranging from 20–70%.26
In previous PESs the miracidial hatching test was used to examine all individuals, while a Kato-Katz thick smear examination – three smears derived from a single stool specimen – provided intensity data on individuals who tested positive by miradicial hatching. While the study design remained the same for all PESs, in 2004 the diagnostic methods of the previous surveys were replaced by initial screening with an SEA-ELISA examination followed by the Kato-Katz technique of SEA-ELISA positive individuals. Intra-survey comparisons are possible, however, due to the similar sensitivities and specificities of the methodologies used.27 In all three PESs, animal examinations were based on the miracidial hatching test undertaken on a single stool specimen. A recent study has investigated the use of a Bayesian modelling approach to further improve the accuracy of estimations based on the combined SEA-ELISA plus Kato-Katz results, taking into account the lack of sensitivity of either diagnostic test.26
The reduced number of estimated human infections compared to the results from previous PESs is certainly a result of mass drug administration targeting villages with infection prevalences above 5% one year before our study. Overall, this may have reduced infection prevalence by up to 50%.28 Behavioural changes brought on by recent improvements in health awareness may also have reduced the prevalence, particularly in females.29 Approximately 90% of houses in the region have an adequate water supply, reducing the exposure of women to schistosome-contaminated lake and marshland areas.30 However, fishing and farming, the two most common male occupations in the region, require frequent lake-water exposure and close interaction with animal hosts.31 It is noteworthy that a study focusing on school children in the Dongting Lake region found a significantly higher S. japonicum prevalence among girls than boys.32 Since 1998 there had been a marked reduction in the frequency of floods along the central parts of the Yangtze River, due in part to the construction of the Three Gorges Dam, further reducing the prevalence of infection. However, long-term consequences of the dam may negatively impact on the transmission of schistosomiasis.33
The PES included the examination of animal populations because of the zoonotic nature of schistosomiasis japonica. We found a buffalo infection prevalence of 9.5%, ranging from 0% to 66.7% at the village level. In accordance with another recent study carried out in Anhui province, we found S. japonicum infections in cattle (6.1%) and goats (4.9%).7 These data reflect the actual scenario, i.e. the estimated number of infected buffaloes remained remarkably stable since 1995, signifying continual schistosomiasis transmission in the Dongting Lake region. Animal infection intensities were not recorded, yet egg output from all hosts is a significant component in S. japonicum transmission dynamics.34 Furthermore, our prediction, based on mathematical modelling, is that buffaloes can be responsible for up to 75% of human transmission in the lake and marshland areas.35 These results call for rigorous monitoring not only in the human population, but also in domestic animals, and perhaps in oncomelanid intermediate host snails.
With regard to associated morbidity, a total of 3893 individuals were interviewed with a standardized questionnaire and examined by ultrasonography for schistosome-associated morbidity. Self-reported blood in stool and abdominal pain were associated with S. japonicum infection status and intensity, while liver tenderness, an increased portal vein diameter and an increased interior portal vein diameter were associated with infection intensity only, a result of high egg burdens.36 Where control of schistosomiasis has been successful, the likelihood of a loss of immunity to infection necessitates, careful monitoring and surveillance of acute cases in relation to post-transmission control.37 Over 50% of those clinically examined had been treated with praziquantel at least once in their lifetime, with the majority of treatments administered within one year before the current survey. Importantly, our results showed a significant positive association between treatment history and infection, suggesting that treatments had been targeted to the at-risk population. This finding is confirmatory that people’s knowledge on prior antischistosomal treatment history can be used as a marker for rapid identification of individuals and/or communities at highest risk of S. japonicum.38
In summary, the approach taken by the national schistosomiasis control programme, i.e. screening all participants by serology followed by stool examination of sero-positives, provided an effective means of analysing a large number of people, although the prevalence of infection was underestimated.26,34 This might be a result of a low sensitivity of the ELISA examination used as the first step in screening patients, especially in areas of low prevalence. However, mass drug administration, increased health awareness and reduced flooding have resulted in a lower number of human infections in the Dongting Lake region.39 The slight increase in buffalo prevalence suggests that schistosomiasis may re-emerge, particularly in foci within the lake-embankment endemic setting, where the majority of buffalo infections occur.35 It is clear that buffaloes must be targeted in all future control efforts if further progress is to be made in the control and possible elimination of schistosomiasis in China. Control of schistosomiasis, and other neglected tropical diseases, is a crucial step towards achieving several of the Millennium Development Goals (MDGs),40,41 and hence our results are of considerable public health relevance.42 We recommend the large-scale treatment of domestic livestock, particularly buffaloes, and, perhaps, buffalo vaccination,43 in addition to smaller scale, selective human treatment, in concert with health education and environmental management interventions that are readily adapted to the local eco-epidemiological settings. ■
Acknowledgments
We thank all study participants, staff from Hunan province and colleagues from the Chinese Ministry of Health who were involved in the third national schistosomiasis PES in 2004.
Footnotes
Funding: We acknowledge financial support from the NHMRC (Australia)/Wellcome Trust (UK) (ICRG Award) (DPM, GMW and YSL) and the Swiss National Science Foundation through project nos. PBBSB–109011 (GR) and PPOOB–102883 (JU). JB is the holder of a Northcote Graduate Scholarship.
Competing interests: None declared.
References
- 1.Utzinger J, Zhou XN, Chen MG, Bergquist R. Conquering schistosomiasis in China: the long march. Acta Trop. 2005;96:69–96. doi: 10.1016/j.actatropica.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Zhou XN, Wang LY, Chen MG, Wu XH, Jiang QW, Chen XY, et al. The public health significance control of schistosomiasis in China – then and now. Acta Trop. 2005;96:97–105. doi: 10.1016/j.actatropica.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Booth M, Guyatt HL, Li YS, Tanner M. The morbidity attributable to Schistosoma japonicum infection in 3 villages in Dongting Lake region, Hunan province, PR China. Trop Med Int Health. 1996;1:646–54. doi: 10.1046/j.1365-3156.1996.d01-93.x. [DOI] [PubMed] [Google Scholar]
- 4.Ross AGP, Li YS, Sleigh AC, Williams GM, Hartel GF, Forsyth SJ, et al. Measuring exposure to S. japonicum in China. I. Activity diaries to assess water contact comparison to other measures. Acta Trop. 1998;71:213–28. doi: 10.1016/S0001-706X(98)00063-1. b. [DOI] [PubMed] [Google Scholar]
- 5.Ross AGP, Bartley PM, Sleigh AC, Olds GR, Li YS, Williams GM, et al. Schistosomiasis. N Engl J Med. 2002;346:1212–20. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- 6.Fenwick A, Keiser J, Utzinger J. Epidemiology, burden and control of schistosomiasis with particular consideration to past and current treatment trends. Drugs Future. 2006;31:413–25. doi: 10.1358/dof.2006.031.05.984953. [DOI] [Google Scholar]
- 7.Wang TP, Vang Johansen M, Zhang SQ, Wang FF, Wu WD, Zhang GH, et al. Transmission of Schistosoma japonicum by humans and domestic animals in the Yangtze River valley, Anhui province, China. Acta Trop. 2005;96:198–204. doi: 10.1016/j.actatropica.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Yuan HC, Guo JG, Bergquist R, Tanner M, Chen XY, Wang HZ. The 1992-1999 World Bank schistosomiasis research initiative in China: outcome and perspectives. Parasitol Int. 2000;49:195–207. doi: 10.1016/S1383-5769(00)00045-3. [DOI] [PubMed] [Google Scholar]
- 9.Chen XY, Wu LY, Cai JM, Zhou XN, Zheng J, Guo JG, et al. Schistosomiasis control in China: the impact of a 10-year World Bank Loan Project (1992–2000). Bull World Health Organ. 2005;83:43–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Li KL, Yang GH, Duan SS, Xia GH. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2002;20:235–7. [Re-emergence of schistosomiasis in Dali City after the criteria of transmission control were met] [PubMed] [Google Scholar]
- 11.Liang S, Yang C, Zhong B, Qiu D. Re-emerging schistosomiasis in hilly mountainous areas of Sichuan, China. Bull World Health Organ. 2006;84:139–44. doi: 10.2471/BLT.05.025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou XN, Acosta L.Willingham AL3rd, Leonardo LR, Chen MG, Aligui G, et alRegional Network for Research, Surveillance and Control of Asian Schistosomiasis (RNAS). Acta Trop 200282305–11. 10.1016/S0001-706X(02)00024-4 [DOI] [PubMed] [Google Scholar]
- 13.Xu XJ, Wei FH, Yang XX, Dai YH, Yu GY, Chen LY, et al. Possible effects of the Three Gorges Dam on the transmission of Schistosoma japonicum on the Jiang Han plain, China. Ann Trop Med Parasitol. 2000;94:333–41. doi: 10.1080/00034983.2000.11813548. [DOI] [PubMed] [Google Scholar]
- 14.Zhou XN, Yang K, Hong QB, Sun LP, Yang GJ, Liang YS, et al. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2004;22:262–5. [Prediction of the impact of climate warming on transmission of schistosomiasis in China] [PubMed] [Google Scholar]
- 15.Yang GJ, Vounatsou P, Zhou XN, Tanner M, Utzinger J. A potential impact of climate change and water resource development on the transmission of Schistosoma japonicum in China. Parassitologia. 2005;47:127–34. doi: 10.1016/S1383-5769(98)80282-1. [DOI] [PubMed] [Google Scholar]
- 16.Bian Y, Sun Q, Zhao Z, Blas E. Market reform: a challenge to public health – the case of schistosomiasis control in China. Int J Health Plann Manage. 2004;19:S79–94. doi: 10.1002/hpm.771. [DOI] [PubMed] [Google Scholar]
- 17.Dongbao Y, Ross AG, Musheng X, Li YS, Yan C. Highlights on the World Bank Loan Schistosomiasis Control Program in China (1991-1998): a special focus on Hunan province. Southeast Asian J Trop Med Public Health. 1999;30:657–63. [PubMed] [Google Scholar]
- 18.Zhou XN, Chen JX, Chen MG, Bergquist R. The National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention: a new administrative structure for schistosomiasis control. Acta Trop. 2005;96:296–302. doi: 10.1016/j.actatropica.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Zhu YC. Immunodiagnosis and its role in schistosomiasis control in China: a review. Acta Trop. 2005;96:130–6. doi: 10.1016/j.actatropica.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Wu GL. A historical perspective on the immunodiagnosis of schistosomiasis in China. Acta Trop. 2002;82:193–8. doi: 10.1016/S0001-706X(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 21.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 22.Hatz CFR. The use of ultrasound in schistosomiasis. Adv Parasitol. 2001;48:225–84. doi: 10.1016/s0065-308x(01)48007-9. [DOI] [PubMed] [Google Scholar]
- 23.Li YS, Zhao ZY, Ellis M, McManus DP. Applications outcomes of periodic epidemiological surveys for schistosomiasis related economic evaluation in the People’s Republic of China. Acta Trop. 2005;96:266–75. doi: 10.1016/j.actatropica.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 24.Spear RC, Seto E, Liang S, Birkner M, Hubbard A, Qiu D, et al. Factors influencing the transmission of Schistosoma japonicum in the mountains of Sichuan province of China. Am J Trop Med Hyg. 2004;70:48–56. [PubMed] [Google Scholar]
- 25.Yu JM, de Vlas SJ, Yuan HC, Gryseels B. Variations in fecal Schistosoma japonicum egg counts. Am J Trop Med Hyg. 1998;59:370–5. doi: 10.4269/ajtmh.1998.59.370. [DOI] [PubMed] [Google Scholar]
- 26.Wang XH, Wu XH, Zhou XN. Bayesian estimation of community prevalences of Schistosoma japonicum infection in China. Int J Parasitol. 2006;36:895–902. doi: 10.1016/j.ijpara.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Ross AGP, Sleigh AC, Lin YS, Davis GM, Williams GM, Zheng J, et al. Schistosomiasis in the People’s Republic of China: prospects and challenges for the 21st century. Clin Microbiol Rev. 2001;14:270–95. doi: 10.1128/CMR.14.2.270-295.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams GM, Sleigh AC, Li YS, Feng Z, Davis GM, Chen H, et al. Mathematical modelling of schistosomiasis japonica: comparison of control strategies in the People’s Republic of China. Acta Trop. 2002;82:253–62. doi: 10.1016/S0001-706X(02)00004-9. [DOI] [PubMed] [Google Scholar]
- 29.Guo JG, Cao CL, Hu GH, Lin H, Li D, Zhu R, et al. The role of ‘passive chemotherapy’ plus health education for schistosomiasis control in China during maintenance consolidation phase. Acta Trop. 2005;96:177–83. doi: 10.1016/j.actatropica.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Tang M, Fan Y, Wang G. Zhonghua Yu Fang Yi Xue Za Zhi. 1996;30:23–5. [Comprehensive cost-benefit evaluation for the improvement of rural water supply in Hunan province] [PubMed] [Google Scholar]
- 31.Ross AGP, Li YS, Sleigh AS, Yi L, Williams GM, Wu WZ, et al. Epidemiologic features of Schistosoma japonicum among fishermen other occupational groups in the Dongting Lake region (Hunan province) of China. Am J Trop Med Hyg. 1997;57:302–8. doi: 10.4269/ajtmh.1997.57.302. [DOI] [PubMed] [Google Scholar]
- 32.Zhou H, Ohtsuka R, He Y, Yuan L, Yamauchi T, Sleigh AC. Impact of parasitic infections dietary intake on child growth in the schistosomiasis-endemic Dongting Lake region, China. Am J Trop Med Hyg. 2005;72:534–9. [PubMed] [Google Scholar]
- 33.Ross AG, Li YS, Williams GM, Jiang Z, McManus DP. Dam worms. Biologist (London) 2001;48:121–4. [PubMed] [Google Scholar]
- 34.Fang JC, Wu ZW, Liu XS, Yi MY, Luo SY, Zeng LS, et al. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2005;23:300–3. [Study on the strategy of interrupting schistosomiasis transmission in a hilly new endemic area of Taoyuan County] [PubMed] [Google Scholar]
- 35.Guo JG, Li YS, Gray D, Ning A, Hu G, Chen H, et al. A drug-based intervention study on the importance of buffaloes for human Schistosoma japonicum infection around Poyang Lake, People’s Republic of China. Am J Trop Med Hyg. 2006;74:335–41. [PubMed] [Google Scholar]
- 36.Warren KS, Su DL, Xu ZY, Yuan HC, Peters PA, Cook JA, et al. Morbidity in schistosomiasis japonica in relation to intensity of infection. A study of two rural brigades in Anhui province, China. N Engl J Med. 1983;309:1533–9. doi: 10.1056/NEJM198312223092501. [DOI] [PubMed] [Google Scholar]
- 37.Hirayama K. Immunogenetic analysis of post-schistosomal liver fibrosis. Parasitol Int. 2004;53:193–6. doi: 10.1016/j.parint.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 38.Zhou H, Ross AGP, Hartel GF, Sleigh AC, Williams GM, McManus DP, et al. Diagnosis of schistosomiasis japonica in Chinese schoolchildren by administration of a questionnaire. Trans R Soc Trop Med Hyg. 1998;92:245–50. doi: 10.1016/S0035-9203(98)90997-X. [DOI] [PubMed] [Google Scholar]
- 39.Zhao GM, Zhao Q, Jiang QW, Chen XY, Wang LY, Yuan HC. Surveillance for schistosomiasis japonica in China from 2000 to 2003. Acta Trop. 2005;96:288–95. doi: 10.1016/j.actatropica.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 40.Fenwick A, Molyneux DH, Nantulya V. Achieving the Millennium Development Goals. Lancet. 2005;365:1029–30. doi: 10.1016/S0140-6736(05)71134-X. [DOI] [PubMed] [Google Scholar]
- 41.Hotez PJ, Molyneux DH, Fenwick A, Ottesen E, Ehrlich Sachs S, Sachs JD. Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and malaria. PLoS Med. 2006;3:e102. doi: 10.1371/journal.pmed.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fenwick A. Waterborne infectious diseases – could they be consigned to history? Science. 2006;313:1077–81. doi: 10.1126/science.1127184. [DOI] [PubMed] [Google Scholar]
- 43.McManus DP. Prospects for development of a transmission blocking vaccine against Schistosoma japonicum. Parasite Immunol. 2005;27:297–308. doi: 10.1111/j.1365-3024.2005.00784.x. [DOI] [PubMed] [Google Scholar]