Abstract
Objective
To provide the international community with an estimate of the amount of financial resources needed to scale up malaria control to reach international goals, including allocations by country, year and intervention as well as an indication of the current funding gap.
Methods
A costing model was used to estimate the total costs of scaling up a set of widely recommended interventions, supporting services and programme strengthening activities in each of the 81 most heavily affected malaria-endemic countries. Two scenarios were evaluated, using different assumptions about the effect of interventions on the needs for diagnosis and treatment. Current health expenditures and funding for malaria control were compared to estimated needs.
Findings
A total of US$ 38 to 45 billion will be required from 2006 to 2015. The average cost during this period is US$ 3.8 to 4.5 billion per year. The average costs for Africa are US$ 1.7 billion and US$ 2.2 billion per year in the optimistic and pessimistic scenarios, respectively; outside Africa, the corresponding costs are US$ 2.1 billion and US$ 2.4 billion.
Conclusion
While these estimates should not be used as a template for country-level planning, they provide an indication of the scale and scope of resources required and can help donors to collaborate towards meeting a global benchmark and targeting funding to countries in greatest need. The analysis highlights the need for much greater resources to achieve the goals and targets for malaria control set by the international community.
Résumé
Objectif
Fournir à la communauté internationale une estimation des ressources financières nécessaires au développement de la lutte antipaludique en vue d’atteindre les objectifs internationaux fixés à cette lutte, et notamment de l’affectation de ces ressources par pays, par année et par intervention, ainsi qu’une indication des lacunes actuelles en matière de financement.
Méthodes
Un modèle d’évaluation des coûts a servi à estimer les coûts totaux de mise à l’échelle d’une série d’interventions largement recommandées, de services d’appui et d’activités de renforcement des programmes pour chacun des 81 pays les plus fortement touchés par le paludisme à l’état endémique. Les ressources financières nécessaires ont été évaluées pour deux scénarios élaborés à partir d’hypothèses différentes concernant l’effet des interventions sur les besoins en diagnostic et en traitement. Les dépenses de santé et les fonds actuels pour lutter contre le paludisme ont ensuite été comparés aux besoins estimés.
Résultats
Pour la période allant de 2006 à 2015, il faudra disposer au total de 38 à 45 milliards de dollars des Etats-Unis. Le coût moyen par an de la lutte antipaludique pour cette période se situera entre 3,8 et 4,5 milliards de dollars des Etats-Unis. Pour l’Afrique, les coûts moyens pour les scénarios optimiste et pessimiste seront respectivement de 1,7 et de 2,2 milliards de dollars des Etats-Unis. Hors Afrique, ces coûts seront respectivement de 2,1 et de 2,4 milliards de dollars des Etats-Unis.
Conclusion
Même s’il ne faut pas tabler sur ces estimations pour planifier le financement national de la lutte antipaludique, elles fournissent une indication de l’ordre de grandeur et de l’ampleur des ressources nécessaires et peuvent faciliter pour les donateurs l’atteinte d’une norme mondiale et le ciblage des pays ayant les plus grands besoins en matière de financement. Cette analyse fait apparaître des besoins bien supérieurs aux ressources disponibles pour réaliser les buts et les objectifs fixés par la communauté internationale pour la lutte antipaludique.
Resumen
Objetivo
Proporcionar a la comunidad internacional una estimación de la cantidad de recursos financieros necesarios para expandir la lucha antimalárica con miras a alcanzar los objetivos internacionales en ese terreno, incluidas las sumas asignadas por país, año e intervención, así como una indicación del actual déficit de financiación.
Métodos
Se empleó un modelo de cálculo de costos para estimar los costos totales de la extensión masiva de un conjunto de intervenciones ampliamente recomendadas, servicios de apoyo y actividades de fortalecimiento de programas en cada uno de los 81 países más afectados endémicamente por la malaria. Se evaluaron dos escenarios, partiendo de distintas premisas sobre el efecto de las intervenciones en las necesidades de diagnóstico y tratamiento. El gasto sanitario y la financiación actuales de la lucha contra la malaria se compararon con las necesidades estimadas.
Resultados
De 2006 a 2015 se requerirán en total entre US$ 38 000 y US$ 45 000 millones. El costo medio durante ese periodo es por tanto de entre US$ 3800 y 4500 millones anuales. El costo medio para África es de US$ 1700 millones y US$ 2200 millones anuales en los escenarios optimista y pesimista, respectivamente; fuera de África, los costos correspondientes son de US$ 2100 millones y US$ 2400 millones.
Conclusión
Si bien no deberían utilizarse como modelo para la planificación en los países, estas estimaciones proporcionan una indicación sobre la magnitud y el alcance de los recursos necesarios y pueden ayudar a los donantes a colaborar para alcanzar una meta mundial y focalizar la financiación en los países más necesitados. El análisis destaca la necesidad de allegar muchos más recursos para alcanzar los objetivos y metas establecidos por la comunidad internacional para la lucha antimalárica.
ملخص
الغرض
استەدفت ەذە الدراسة توفير معلومات للمجتمع الدولي حول الموارد المالية المقدرة اللازمة للنەوض بأنشطة مكافحة الملاريا من أجل تحقيق الأەداف الدولية، بما في ذلك معلومات حول المخصصات المالية بحسب البلد، والسنة، والتدخل اللازم، مع الإشارة إلى فجوة التمويل الحالية.
الطريقة
تم استخدام نموذج لحساب التكاليف بغرض تقدير التكاليف الكلية اللازمة للنەوض بمجموعة من التدخلات والخدمات الداعمة وأنشطة تعزيز البرامج، الموصى بەا على نطاق واسع، في البلدان الأشد معاناة من توطن الملاريا والبالغ عددەا 81 بلداً. وتم تقييم تصورين باستخدام افتراضات مختلفة حول تأثير التدخلات على مدى الحاجة إلى التشخيص والمعالجة. وتمت مقارنة الإنفاق والتمويل الصحيين لمكافحة الملاريا مع الاحتياجات المقدَّرة.
الموجودات
تبلغ الاحتياجات الكلية اللازمة لمكافحة الملاريا للحقبة 2006 – 2015 من 38 إلى 45 بليون دولار. ويبلغ متوسط التكاليف خلال ەذە المدة من 3.8 إلى 4.5 بليون دولار في السنة. ويتراوح متوسط التكاليف لأفريقيا من 1.7 بليون إلى 2.2 بليون دولار في السنة، بحسب التصور (السيناريو) المتفائل والمتشائم على الترتيب. أما خارج أفريقيا فتبلغ التكاليف المقابلة من 2.1 إلى 2.4 بليون دولار.
الاستنتاج
برغم أن ەذە التقديرات لا ينبغي أن تُستخدم كنموذج للتخطيط على المستوى القطري، إلا أنەا تمثل مؤشراً على حجم ونطاق الموارد اللازمة، ويمكنەا أيضاً أن تساعد المانحين على التعاون من أجل بلوغ مستوى قياسي عالمي، ومن أجل توجيە الأموال إلى البلدان الأشد احتياجاً. ويبرز التحليل مدى الحاجة إلى المزيد والمزيد من الموارد لتحقيق الأەداف والغايات التي حددەا المجتمع الدولي لمكافحة الملاريا.
Introduction
Globally, there are more than a million malaria-related deaths each year. About four-fifths of these are in Africa.1
Effective interventions that reduce death and illness from malaria are still not widely accessible in most malaria-endemic countries. The World Health Assembly in 2005 urged Member States to establish policies and operational plans to ensure that at least 80% of those at risk of, or suffering from, malaria benefit by 2010 from major preventive and curative interventions, so as to ensure a reduction in the burden of malaria of at least 50% by 2010 and 75% by 2015.2 These targets are echoed in the Roll Back Malaria Partnership Global Strategic Plan 2005-2015.3 The United Nations Millennium Declaration set a target to halt and begin to reverse the global incidence of malaria by 2015.4
Achieving these targets will require additional financial resources. Comparison of estimated costs with present investments should help accelerate mobilization of funds and identify important country-level gaps.
This paper presents the methods used to construct a model for estimating the total financial costs of scaling up malaria control over 2006-2015 to achieve internationally agreed objectives and targets for the 81 most heavily affected malaria-endemic countries of the world’s 107 malaria-endemic countries and territories. Pessimistic and optimistic scenarios with different assumptions about the effect of interventions on the needs for diagnosis and treatment provide upper and lower bounds of the estimation.
The exercise includes a set of widely recommended interventions. Besides commodities and distribution costs, we included costs for necessary health system strengthening activities (programme costs in Figures 1-4), especially for community health workers, training, communication, operational research and monitoring and evaluation. We did not include costs for running health facilities since the bulk of interventions will be delivered at the peripheral level, and effective prevention and treatment of malaria should reduce the number of severe malaria cases requiring hospitalization. While we included the costs of technical assistance for national programmes, we did not consider those required at international level for managing such assistance, monitoring and evaluation, and research and development.
The analysis estimates the total cost of scaling up malaria control in each country, including the costs of existing levels of interventions. The needs calculated are then compared to current health expenditures and funding for malaria control by country.
Methods
A detailed description, including assumptions and calculations, is available in the working paper Methodology for estimating the costs of global malaria control (2006-15), at http://www.who.int/malaria/costing.
To arrive at the cost estimates, we selected countries for the analysis, estimated the population in need of each intervention, prepared scale-up scenarios, and calculated country-specific costs. All costs are calculated in 2006 US$.
Countries
The 81 countries included (listed in Table 3, available at: http://www.who.int/bulletin) are those which have significant populations at risk of Plasmodium falciparum malaria. The remaining malaria-endemic countries in the world are mainly affected by vivax malaria. The malaria risk there is highly variable, making the estimation of needs for prevention difficult. The inclusion of these countries could skew the estimates towards addressing problems which are not central to achieving the Millennium Development Goals. While the importance of vivax malaria should not be underestimated and its control may be challenging, these countries, with few exceptions, do not need external financial support for malaria control. Based on these criteria, all endemic countries in Africa south of the Sahara (but no country in North Africa) have been included. In the following, therefore, “Africa” refers to sub-Saharan Africa.
Table 3. Average estimated per-capita needs for malaria control in 2006 versus most recent per-capita total and government expenditure on health (US$).
| Country | Average estimated needs for malaria control per capita, 2006 | Per-capita total expenditure on health at average exchange rate, 2003 | Per-capita government expenditure on health at average exchange rate, 2003 |
|---|---|---|---|
| Africa | |||
| Angola | 3.74 | 26 | 22 |
| Benin | 2.77 | 20 | 9 |
| Botswana | 1.79 | 232 | 135 |
| Burkina Faso | 2.20 | 19 | 9 |
| Burundi | 2.32 | 3 | 1 |
| Cameroon | 2.27 | 37 | 11 |
| Cape Verde | 1.35 | 78 | 57 |
| Central African Republic | 2.46 | 12 | 5 |
| Chad | 2.27 | 16 | 7 |
| Comoros | 2.49 | 11 | 6 |
| Congo | 3.15 | 19 | 12 |
| Côte d’Ivoire | 7.10 | 28 | 8 |
| Democratic Republic of the Congo | 0.71 | 4 | 1 |
| Djibouti | 5.39 | 47 | 31 |
| Equatorial Guinea | 3.53 | 96 | 65 |
| Eritrea | 2.47 | 8 | 4 |
| Ethiopia | 1.84 | 5 | 3 |
| Gabon | 4.12 | 196 | 130 |
| Gambia | 2.19 | 21 | 8 |
| Ghana | 2.22 | 16 | 5 |
| Guinea | 2.37 | 22 | 4 |
| Guinea-Bissau | 2.43 | 9 | 4 |
| Kenya | 2.52 | 20 | 8 |
| Liberia | 2.35 | 6 | 4 |
| Madagascar | 2.86 | 8 | 5 |
| Malawi | 2.32 | 13 | 5 |
| Mali | 2.51 | 16 | 9 |
| Mauritania | 4.41 | 17 | 13 |
| Mozambique | 2.42 | 12 | 7 |
| Namibia | 2.58 | 145 | 101 |
| Niger | 2.60 | 9 | 5 |
| Nigeria | 2.33 | 22 | 6 |
| Rwanda | 1.77 | 7 | 3 |
| Sao Tome and Principe | 4.37 | 34 | 29 |
| Senegal | 2.82 | 29 | 12 |
| Sierra Leone | 2.16 | 7 | 4 |
| Somalia | 3.84 | n/a | n/a |
| South Africa | 1.25 | 295 | 114 |
| Sudan | 2.62 | 21 | 9 |
| Swaziland | 2.17 | 107 | 61 |
| Togo | 2.67 | 16 | 4 |
| Uganda | 2.13 | 18 | 5 |
| United Rep. of Tanzania | 2.08 | 12 | 7 |
| Zambia | 2.64 | 21 | 11 |
| Zimbabwe | 2.02 | 40 | 14 |
| Median | 2.43 | 19 | 8 |
| Asia and Oceania | |||
| Afghanistan | 1.09 | 11 | 4 |
| Bangladesh | 1.43 | 14 | 4 |
| Bhutan | 1.21 | 10 | 9 |
| Cambodia | 0.44 | 33 | 6 |
| China | 0.07 | 61 | 22 |
| India | 0.67 | 27 | 7 |
| Indonesia | 1.14 | 30 | 11 |
| Iran | 0.16 | 131 | 62 |
| Lao People’s Dem. Rep. | 1.49 | 11 | 4 |
| Malaysia | 0.45 | 163 | 95 |
| Myanmar | 1.17 | 394 | 77 |
| Nepal | 1.91 | 12 | 3 |
| Pakistan | 0.33 | 13 | 4 |
| Papua New Guinea | 2.43 | 23 | 20 |
| Philippines | 0.22 | 31 | 14 |
| Solomon Islands | 4.41 | 28 | 26 |
| Sri Lanka | 0.81 | 31 | 14 |
| Thailand | 1.23 | 76 | 47 |
| Timor-Leste | 3.21 | 39 | 30 |
| Vanuatu | 4.69 | 54 | 40 |
| Viet Nam | 0.61 | 26 | 7 |
| Yemen | 1.57 | 32 | 13 |
| Median | 1.16 | 30.5 | 13.5 |
| Americas | |||
| Bolivia | 1.28 | 61 | 39 |
| Brazil | 0.50 | 212 | 96 |
| Colombia | 0.74 | 138 | 116 |
| Dominican Republic | 1.22 | 132 | 44 |
| Ecuador | 0.50 | 109 | 42 |
| El Salvador | 1.93 | 183 | 84 |
| Guatemala | 0.84 | 112 | 44 |
| Guyana | 0.87 | 53 | 44 |
| Haiti | 1.83 | 26 | 10 |
| Honduras | 1.61 | 72 | 41 |
| Nicaragua | 0.82 | 60 | 29 |
| Paraguay | 0.78 | 75 | 24 |
| Peru | 0.76 | 98 | 47 |
| Suriname | 2.32 | 182 | 83 |
| Median | 0.86 | 104 | 44 |
| Global median | 2.16 | 26.5 | 11 |
Population figures for 2006 were calculated using the United Nations Population Division medium variants estimates of total population in 2003 and annual population growth rate over 2000–2005.
Epidemiological estimates
The proportion of people in each country exposed to a particular class of endemicity was assigned using sources ranging from climatic/environmental modelling5 to clinical reporting of incidence (see http://www.mara.org.za/; http://www.paho.org/english/hcp/hct/mal/malaria.htm; http://www.searo.who.int/EN/Section10/Section21.htm; http://www.wpro.who.int/sites/mvp/epidemiology/malaria/). In countries where epidemiological data was unavailable, estimates were prepared using data from countries with similar epidemiological conditions but better health reporting. Population data and growth rates were obtained from United Nations Population Division 2004 projections, interpolated to yearly estimates using MortPack software.6
Calculating country-specific costs
Commodity prices were derived primarily from “Sources and prices of selected products for the prevention, diagnosis and treatment of malaria.”7 We did not take into consideration the future price reductions likely to occur as a result of increased demand and production, nor possible increases due to the need to deploy novel medicines and insecticides because of resistance. Costs for malaria control interventions per country in a given year are estimated as unit cost (commodity plus delivery) multiplied by target population living in endemic areas for prevention, and by incidence of clinical episodes, for curative care. The scale and costs of other inputs were derived from typical programmes and budgets, including those described in successful proposals to the Global Fund to Fight AIDS, Tuberculosis and Malaria (see http://www.theglobalfund.org). Other expenses were based on country-specific estimates or were derived independently8 (see http://www.dcp2.org/file/24/wp9.pdf).
Interventions and services
Vector control
We estimated the costs for provision of long-lasting insecticidal nets (LLINs) to all people living in endemic areas9 at the rate of one net per two people, with replacement after three years. Other vector control methods, especially indoor residual spraying, may be substituted in certain areas, using the LLIN cost estimate as a rough equivalent in cost per person protected. Actual cost differences may vary in either direction;10 however, the long-term cost of LLINs is lower than that determined for conventional insecticide-treated mosquito nets in comparative studies.
Intermittent preventive therapy (IPT)
We costed provision of IPT using sulfadoxine-pyrimethamine (SP), distributed by ante-natal care services, with three treatment courses (see http://www.afro.who.int/malaria/publications/malaria_in_pregnancy_092004.pdf) given to all pregnant women living in Africa in regions with moderate to intense transmission. Age-specific fertility rates reported by the UN Population Division in the 2003 World Fertility Report were used to determine the number of pregnancies expected annually.
Rapid diagnostic tests (RDTs)
We assumed that RDTs would be used for all patients with malaria-like illness to detect P. falciparum in all areas with significant transmission of the parasite, except in children under five years in Africa up to 2010. WHO currently does not recommend using RDTs in this age-group in areas of intense transmission (see http://www.who.int/malaria/docs/ReportLABdiagnosis-web.pdf).11
Artemisinin-based combination therapies (ACTs)
ACTs were assumed to be the first-line treatment. The average cost of treatment was calculated for each of three age groups and multiplied by the annual expected number of fevers suspected to be malaria. In hyper- and holo-endemic areas: 0-4 years: 4, 5-14 years: 2, above 14 years: 1 episode per person; in meso-endemic areas, the corresponding rates were 2, 1 and 1; and in hypo-endemic areas, 1, 0.5 and 0.5.
Severe and complicated malaria
We assumed incidence rates of severe malaria ranging from 0.005 to 0.04 per person per year depending on endemicity and age-group. A median cost of US$ 29.50 for managing a single severe malaria case was derived from surveys in Africa (see http://www.who.int/malaria/cmc_upload/0/000/016/330/multicenter.pdf). This cost includes therapeutics and laboratory tests, but not transport and pre- and post-hospitalization costs.
Epidemic prevention and response
Resources for malaria epidemic prevention and control were estimated for areas with unstable P. falciparum malaria. In sub-Saharan Africa, the MARA-linked datasets were consulted to determine countries and populations at epidemic risk. To identify countries beyond Africa, we used reports in peer-reviewed journals12 as well as government and WHO regional office sources.
Costs were estimated for a “surveillance package” including training, computers and software, and for an “intervention package” including supplies, equipment and IRS operations to prevent or curb epidemics. Also costed were supplemental supplies of ACTs as well as the increased need for management of severe malaria.
Strengthening health infrastructure
We grouped countries according to the need for augmentation of infrastructure, based on the classifications described by the WHO Commission on Macroeconomics and Health.13 For each group, we defined sets of trained personnel and equipment necessary for management, monitoring and evaluation, improvement of microscopy services, enhancement of transport capacity and strengthening supply management and logistics.
Training for staff and community health workers
Many of the interventions represent new policies and procedures that will require training in treatment, diagnosis, delivery of preventive interventions, supervision, management and operational research. Estimates include costs of training of epidemiologists and entomologists, health service staff and community health workers.
Communication
We provide estimates for producing and communicating information to communities on malaria prevention, early recognition of symptoms and the need to seek prompt treatment.
Monitoring, evaluation and operational research
Estimates of the cost of monitoring and evaluation include routine assessment of surveillance data captured through health information systems, periodic surveys of health facilities in some countries, population surveys and studies on drug and insecticide resistance.
Scale-up and impact of implementation on costs
Coverage of most interventions is expected to increase gradually to 95% or 100% in 2015 in accordance with internationally agreed targets. For severe malaria management, “coverage” was considered to be 100% throughout, because an episode of severe disease almost inevitably incurs costs on families and/or health services. For programme costs, complete coverage was assumed from the outset, reflecting the need for staff and infrastructure for scale up of control. Consideration was given to supply chain constraints affecting ACTs in the first two years.
Costs were evaluated in two scenarios: one with a pessimistic set of assumptions, in which the effect of interventions on malaria incidence and thereby the needs for diagnosis and treatment is less than would be expected from field trials, and one with an optimistic set of assumptions, where needs for diagnosis and treatment decrease to a greater extent. Estimates of impact in the two scenarios were based on evidence where available,14 and on consensus among the authors. In the pessimistic scenario, vector control (exemplified by LLINs) at 80% coverage would reduce the need for RDTs, ACTs and severe malaria management by 50%, and in the optimistic scenario, by 75%. In the pessimistic scenario, 100% coverage with RDTs would reduce the need for ACTs by 25% in Africa and 50% elsewhere; in the optimistic scenario, the corresponding reductions would be 50% and 75%. In both scenarios, 100% coverage with ACTs would reduce severe malaria costs by 50%. For all these interventions, lower coverage levels would result in proportionally lower impacts.
Data on malaria financing
We extracted data on domestic annual funding for malaria control15 and on annual per capita total and government expenditure on health16 by country, where this information was available. These figures were then compared to the average estimated needs for funding for malaria control in each country.
Results
The summation of the baseline estimates for the 81 countries for 2005 resulted in 660 million persons in falciparum malaria-endemic areas in Africa and 1.240 billion in Asia and the Americas. The annual number of malaria-like fever episodes was 1.064 billion for Africa and 399 million for Asia and the Americas; severe episodes were estimated at 10.7 million a year for Africa and 3.3 million for Asia and the Americas.
Table 1 shows the cost of scaling up malaria control programmes worldwide to reach internationally agreed targets for coverage of malaria control. A total of US$ 38 billion (optimistic scenario) to US$ 45 billion (pessimistic scenario) will be required from 2006 to 2015; on average, US$ 3.8 to US$ 4.5 billion per year. The average annual costs for Africa are US$ 1.7 billion and US$ 2.2 billion in the optimistic and pessimistic scenarios, respectively; outside Africa, the corresponding costs are US$ 2.1 billion and US$ 2.4 billion.
Table 1. Estimated costs for scaling up malaria control interventions, 2006–2015.
| Year | Estimated cost (US$ billion) |
||||||
|---|---|---|---|---|---|---|---|
| Pessimistic scenario |
Optimistic scenario |
||||||
| Africa | Asia, Oceania, Americas | Total | Africa | Asia, Oceania, Americas | Total | ||
| 2006 | 1.689 | 1.842 | 3.531 | 1.671 | 1.835 | 3.506 | |
| 2007 | 1.774 | 2.045 | 3.819 | 1.686 | 1.972 | 3.658 | |
| 2008 | 1.854 | 2.018 | 3.872 | 1.657 | 1.857 | 3.514 | |
| 2009 | 2.076 | 2.440 | 4.516 | 1.724 | 2.159 | 3.883 | |
| 2010 | 1.991 | 2.263 | 4.254 | 1.576 | 1.932 | 3.508 | |
| 2011 | 2.151 | 2.338 | 4.489 | 1.687 | 1.973 | 3.661 | |
| 2012 | 2.575 | 2.760 | 5.335 | 1.990 | 2.389 | 4.380 | |
| 2013 | 2.445 | 2.497 | 4.942 | 1.797 | 2.092 | 3.889 | |
| 2014 | 2.362 | 2.430 | 4.792 | 1.662 | 2.000 | 3.662 | |
| 2015 | 2.700 | 2.960 | 5.660 | 1.957 | 2.511 | 4.468 | |
| Total | 21.617 | 23.593 | 45.210 | 17.407 | 20.720 | 38.129 | |
| Average/year | 2.162 | 2.359 | 4.521 | 1.741 | 2.072 | 3.813 | |
| Percent | 47.8 | 52.2 | 100 | 45.7 | 54.3 | 100 | |
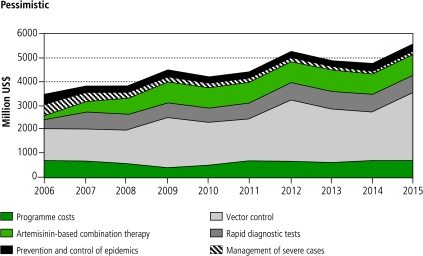
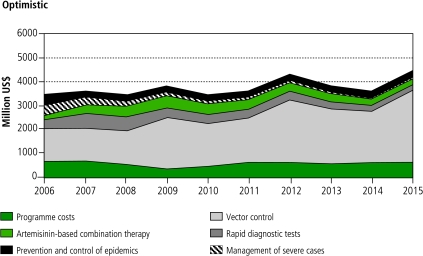
Figures 1 and 2 show the costs of specific interventions and programme costs over the 10-year period. In the two scenarios, the initial costs are identical. Vector control costs are dominant, increasing over time as a result of increasing coverage and population growth. While in the pessimistic scenario (Figure 1) case management costs are relatively constant after initial scale-up, in the optimistic scenario (Figure 2) they undergo a marked decline, especially after 2010. In both scenarios, the largest costs occur in 2012 and 2015. These peaks are mainly due to the periodic replacement cycles for LLINs. In reality, they would probably be smoothed by variable rates of scale up in individual countries.
Fig. 1.
Estimated global malaria control intervention and programme costs from 2006–2015 according to the pessimistic scenarioa
a The costs associated with intermittent preventive treatment in pregnancy are very small compared to the costs for other interventions, and therefore are not visible in the figure.
Fig. 2.
Estimated global malaria control intervention and programme costs from 2006–2015 according to the optimistic scenarioa
a The costs associated with intermittent preventive treatment in pregnancy are very small compared to the costs for other interventions, and therefore are not visible in the figure.
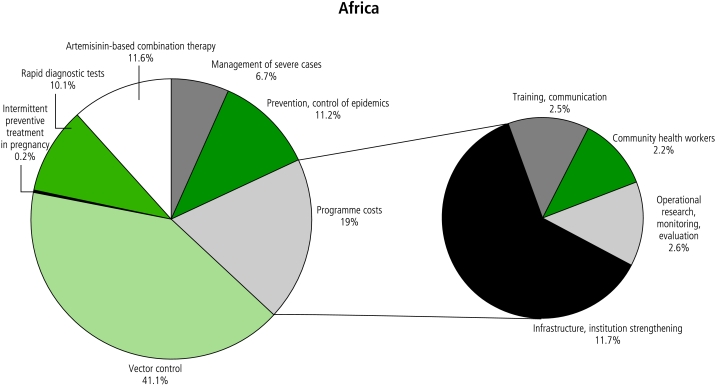
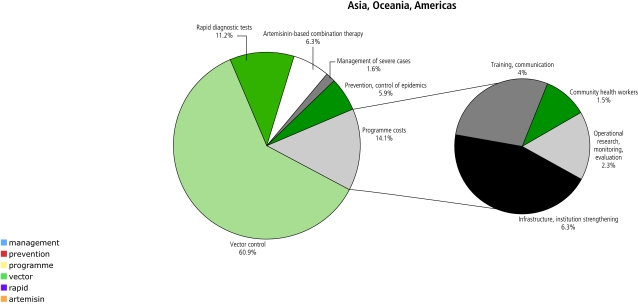
Figures 3 and 4 compare the distribution of expenditures by intervention and type of programme cost in Africa and the rest of the world. Outside Africa, vector control costs are more dominant relative to case management costs because of the larger populations and lower malaria incidence rates. Infrastructure and institutional strengthening costs are higher in Africa, while training costs are higher outside Africa due to large populations needing interventions and higher human resource costs.
Fig. 3.
Allocation to different interventions and types of programme costs in the optimistic scenario in Africa, averaged over the years 2006–2015
Fig. 4.
Allocation to different interventions and types of programme costs in the optimistic scenario in Asia, Oceania and the Americas, averaged over the years 2006–2015
Country-by-country comparisons of resources needed for malaria control and those available from national sources demonstrate large gaps in nearly all countries (see Table 2, available at: http://www.who.int/bulletin). Only approximately 4.6% of estimated needed resources are available from domestic sources in the African countries, and 9.2% in the countries outside Africa. Estimates of available resources should be treated with caution, however, due to the difficulty of isolating malaria funding within the government health budget and of estimating malaria funding from nongovernment sources.
Table 2. Comparison of available and needed domestic funding for malaria control (US$ million), for countries for which data is available.
| Country | Domestic annual funding, latest year for which data is available (2000–2003)15 | Estimated annual funding needs 2006–2010 (average of pessimistic and optimistic scenarios) | Estimated funding gap – domestic funding |
|---|---|---|---|
| Africa | |||
| Angola | 1.080 | 54.484 | 53.404 |
| Botswana | 0.432 | 3.218 | 2.786 |
| Burkina Faso | 0.096 | 33.467 | 33.371 |
| Burundi | 0.030 | 18.444 | 18.414 |
| Cameroon | 9.678 | 40.442 | 30.764 |
| Central African Republic | 0.179 | 10.359 | 10.180 |
| Chad | 0.028 | 24.101 | 24.073 |
| Comoros | 0.104 | 2.188 | 2.084 |
| Côte d’Ivoire | 0.167 | 42.984 | 42.817 |
| Eritrea | 0.098 | 11.855 | 11.757 |
| Ethiopia | 4.971 | 151.319 | 146.348 |
| Kenya | 0.082 | 89.910 | 89.828 |
| Madagascar | 5.358 | 56.114 | 50.756 |
| Malawi | 22.238 | 31.764 | 9.526 |
| Mali | 1.007 | 38.200 | 37.193 |
| Mauritania | 0.132 | 14.618 | 14.486 |
| Mozambique | 0.256 | 50.449 | 50.193 |
| Namibia | 0.573 | 5.159 | 4.586 |
| Nigeria | 3.530 | 323.381 | 319.851 |
| Rwanda | 0.120 | 17.033 | 16.913 |
| Sao Tome & Principe | 0.039 | 0.784 | 0.745 |
| Senegal | 2.100 | 31.347 | 29.247 |
| Somalia | 0.160 | 45.141 | 44.981 |
| South Africa | 8.300 | 59.057 | 50.757 |
| Swaziland | 0.450 | 2.463 | 2.013 |
| Sudan | 2.600 | 100.439 | 97.839 |
| Togo | 0.100 | 12.808 | 11.808 |
| Uganda | 0.385 | 64.868 | 64.483 |
| United Republic of Tanzania | 0.500 | 87.160 | 86.660 |
| Subtotal | 64.793 | 1423.556 | 1358.763 |
| Percent of estimated need | 4.6% | 100% | 95.4% |
| Asia, Oceania and Americas | |||
| Bangladesh | 0.232 | 233.829 | 233.597 |
| Bolivia | 0.918 | 11.315 | 10.397 |
| Brazil | 40.696 | 68.946 | 28.25 |
| Colombia | 13.050 | 32.294 | 19.244 |
| Dominican Republic | 1.221 | 11.596 | 10.375 |
| El Salvador | 4.555 | 12.108 | 7.553 |
| Ecuador | 3.816 | 6.237 | 2.421 |
| Guatemala | 0.703 | 9.737 | 9.034 |
| Guyana | 0.800 | 0.673 | -0.127 |
| Honduras | 0.081 | 11.227 | 11.146 |
| India | 49.100 | 802.709 | 753.609 |
| Indonesia | 0.045 | 278.458 | 278.413 |
| Islamic Republic of Iran | 6.206 | 10.055 | 3.849 |
| Lao People’s Democratic Republic | 0.369 | 8.846 | 8.477 |
| Malaysia | 0.927 | 11.971 | 11.044 |
| Myanmar | 23.041 | 63.772 | 40.731 |
| Nicaragua | 0.333 | 4.258 | 3.925 |
| Pakistan | 0.492 | 60.538 | 60.046 |
| Papua New Guinea | 1.450 | 15.341 | 13.891 |
| Paraguay | 5.412 | 4.292 | -1.120 |
| Peru | 4.110 | 128.450 | 124.340 |
| Philippines | 0.062 | 19.866 | 19.804 |
| Sri Lanka | 1.481 | 16.691 | 15.210 |
| Suriname | 0.161 | 0.761 | 0.600 |
| Thailand | 18.700 | 80.399 | 61.699 |
| Viet Nam | 4.537 | 54.581 | 50.044 |
| Yemen | 2.000 | 36.454 | 34.454 |
| Subtotal | 184.498 | 1995.404 | 1810.906 |
| Percent of estimated need | 9.2% | 100% | 90.8% |
Table 3 (available at: http://www.who.int/bulletin) shows that, in some countries, particularly in Asia, the Americas and southern Africa, current levels of health expenditure could, with some adjustment, cover malaria control needs. In others, mainly in Africa, estimated needs constitute over two-thirds of total annual health expenditures; much greater external funding will be necessary to fill these gaps.
Discussion
Considering population growth, our estimate for populations in endemic areas in Africa is close to other recent estimates,17 which are based on the same climate-based distribution model. The estimate of malaria-like fever episodes for Africa is lower than that of Snow et al., especially for adults,18 but it is higher than that of a field study in southern Ghana19 and is based on a model which has proved useful for WHO’s country-level work for supply planning in Africa. Outside Africa, estimation is fraught with greater uncertainty, because of the enormous epidemiological variability. Our estimate of population in areas endemic for P. falciparum outside Africa is about two-thirds of that of Snow et al.20 This is not surprising, because we have used a more eclectic approach to identify populations that need protection by continuous vector control. Our calculation of malaria-like fevers is also more uncertain beyond Africa, where widely applicable data are scarce. We estimated that 75% of all severe cases occur in Africa, which corresponds well to current estimates of the distribution of falciparum malaria,15 but our total estimate of severe cases is high (3-5%) compared to global estimates of falciparum malaria (see http://www.who.int/malaria/docs/incidence_estimations2.pdf), pointing to the need for population-based studies of this problem.
Although Africa’s malaria burden is higher than that of the rest of the world, the total costs are higher for Asia and the Americas due to the enormous size of the populations estimated to need vector control coverage. In many countries effective control over some years may interrupt transmission in areas with low transmission potential so that vector control could be replaced by surveillance, greatly reducing costs. Likewise, in countries with intense malaria transmission, increasing urbanization, combined with integrated vector management, could lead to reductions in malaria burden and thus in both preventive and curative expenditures. Especially in areas of low to moderate transmission, the widespread use of ACTs could help reduce transmission. We have not attempted to model this due to lack of good data.
The high allocation to RDTs is meaningful, because as malaria incidence decreases, the costs of diagnosis relative to those of treatment should increase.
Some limitations of our analysis deserve mention. The numbers reported for the optimistic and pessimistic scenarios are not intended to represent an absolute “ceiling” or “floor” for the cost of malaria control. Synergistic interactions could reduce the amount of resources required to achieve goals. In areas of particular vulnerability or opportunity, it may be possible to adopt a more accelerated and costly programme, while in other locales, the targets assumed in this analysis may be too ambitious.
For country-level planning, it is essential to assess systemic strengths and weaknesses, and to regularly review performance to adjust the rhythm of financial inputs. Our projected allocations to health system strengthening constitute 16-21% of total costs. The real needs would vary greatly by country depending on health system characteristics. For example, where there is high coverage of government services, the substantial financing estimated for community workers could be allocated instead to support delivery through public health facilities.
The exclusion of vector source reduction methods from this analysis does not reflect their value, but rather their complexity. The training component in this costing exercise is intended in part to build the capacity of managers and entomologists to develop locally appropriate long-term strategies.
Our results highlight the incongruity between goals and targets for malaria control set by the international community and the resources that are available to combat the disease. International funding has increased in recent years, with estimated annual contributions to malaria control from development agencies rising to US$ 600 million in 2004 from less than US$ 50 million in 2000 (see http://www.rbm.who.int/docs/hlsp_report.pdf). In 2005, estimated disbursements for malaria from bilateral donors, WHO and the Global Fund were approximately US$ 841 million. New major funding initiatives launched by the World Bank and the United States of America in 2005 suggest that resources for malaria control will continue to increase.
However, current international funding for malaria control represents approximately 20% of estimated total needs for gradual scale up. The continuity of funding is also of concern. It is unlikely that malaria control efforts will lead to the elimination of malaria in the countries included in this analysis. Therefore, high levels of coverage of curative and particularly preventive interventions will need to be maintained beyond 2015 in most places.
It is also important to monitor funding for malaria from all sources, including the private sector. To ensure long-term sustainability and national ownership of malaria control programmes, domestic funding should account for an ever-increasing proportion of total malaria spending.
Due to the generalizations needed to execute such a broad global costing, these estimates should not be used as a template for country-level planning. Nor are our estimates of commodity needs meant to be used as forecasting figures for industry. However, the estimates may be useful as benchmarks against which to assess planned inputs or global commodity need estimations. ■
Acknowledgements
We are grateful to Olusoji Adeyi of the World Bank and the Roll Back Malaria Partnership Working Group on Financing and Resources for organizing a review of an earlier version of this paper. Tania Dmytraczenko of Abt Associates, Philip Musgrove of Project Hope, Owen Smith of the World Bank and Eve Worrall of Liverpool Associates in Tropical Health made very helpful comments.
Footnotes
Funding: WHO’s Global Malaria Programme funded Anthony Kiszewski to do most of the analyses and consolidate the paper.
Competing interests: None declared.
References
- 1.Rowe AK, Rowe SY, Snow RW, Korenromp EL, Schellenberg JR, Stein C, et al. The burden of malaria mortality among African children in the year 2000. Int J Epidemiol. 2006;35:691–704. doi: 10.1093/ije/dyl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Resolution WHA. 58.2. Malaria control. In: Fifty-eighth World Health Assembly, Resolutions and Decisions Annex. Geneva: WHO; 2005. Available at: http://www.who.int/gb/ebwha/pdf_files/WHA58/WHA58_2-en.pdf
- 3.Global Partnership to Roll Back Malaria. The African Summit on Roll Back Malaria, Abuja, Nigeria, 25 April 2000. Geneva: WHO; 2000 (WHO/CDS/RBM/2000.17). Available at: http://whqlibdoc.who.int/hq/2000/WHO_CDS_RBM_2000.17.pdf
- 4.Millennium Declaration. New York: United Nations; 2000 (A/RES/55/2). Available at: http://www.un.org/millennium/declaration/ares552e.htm
- 5.Craig MH, Snow RW, le Sueur D. A climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitol Today. 1999;15:105–11. doi: 10.1016/S0169-4758(99)01396-4. [DOI] [PubMed] [Google Scholar]
- 6.World population prospects: the 2004 revision. New York: United Nations; 2005. [Google Scholar]
- 7.Sources and prices of selected products for the prevention, diagnosis and treatment of malaria. Geneva: WHO, UNICEF, Population Services International, Management Sciences for Health; 2004.
- 8.Johns B, Adam T, Evans DB. Enhancing the comparability of costing methods: cross-country variability in the prices of non-traded inputs to health programmes. Cost Eff Resour Alloc. 2006;4:8. doi: 10.1186/1478-7547-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Resolution WHA. 58.2. Malaria control. In: Fifty-eighth World Health Assembly, Resolutions and Decisions Annex. Geneva: WHO; 2005. [Google Scholar]
- 10.Guyatt HL, Kinnear J, Burini M, Snow RW. A comparative cost analysis of insecticide-treated nets and indoor residual spraying in highland Kenya. Health Policy Plan. 2002;17:144–53. doi: 10.1093/heapol/17.2.144. [DOI] [PubMed] [Google Scholar]
- 11.Guidelines for the treatment of malaria. Geneva: WHO; 2006.
- 12.Kiszewski AE, Teklehaimanot A. A review of the clinical and epidemiological burdens of epidemic malaria. Am J Trop Med Hyg. 2004;71(Suppl 2):128–35. [PubMed] [Google Scholar]
- 13.Commission on Macroeconomics and Health. Macroeconomics and health: investing in health for economic development. Geneva: WHO; 2001. [Google Scholar]
- 14.Murphy C, Ringheim K, Woldehanna S, Volmink J, editors. Reducing malaria’s burden: evidence of effectiveness for decision-makers. Washington: Global Health Council; 2003. [Google Scholar]
- 15.World malaria report. Geneva: WHO/UNICEF; 2005.
- 16.World health report 2006: Working together for health. Geneva: WHO; 2006.
- 17.Hay SI, Guerra CA, Tatem AJ, Atkinson PM, Snow RW. Urbanization, malaria transmission and disease burden in Africa. Nat Rev Microbiol. 2005;3:84–93. doi: 10.1038/nrmicro1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snow RW, Eckert E, Teklehaimanot A. Estimating the needs for artesunate-based combination therapy for malaria case-management in Africa. Trends Parasitol. 2003;19:363–9. doi: 10.1016/S1471-4922(03)00168-5. [DOI] [PubMed] [Google Scholar]
- 19.Agyepong IA, Kangeya-Kayonda J. Providing practical estimates of malaria for health planners in resource-poor countries. Am J Trop Med Hyg. 2004;71(Suppl 2):162–7. [PubMed] [Google Scholar]
- 20.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–7. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]