Abstract
Objective
Generally, lead poisoning is not considered a significant environmental hazard for children in rural areas of developing countries. With a prospectively designed policy experiment, the research community and the government are conducting a broad-based investigation to introduce and evaluate the impact of health policy reforms on children in a rural area of the Philippines – the Quality Improvement Demonstration Study (QIDS). As part of this study, we researched lead exposure in children under the age of five.
Methods
We sampled a population of children from the Visayas region in the central Philippines, covering approximately one third of the country’s geographical area. From December 2003 to September 2004, the survey collected blood lead levels (BLL) together with demographic, socioeconomic and child health data points. Supplemental field-testing among a sub-sample of the most exposed children assessed the sources of environmental lead exposure.
Findings
Among children in this study, 21% (601 of 2861 children) had BLL greater than10 µg/dl. BLL were associated independently with age, haemoglobin concentration, water source, roofing material, expenditures and history of breastfeeding. A follow-up assessment of possible environmental exposures among the sub-sample of children with elevated BLL revealed no single or predominant exposure source. Instead, there appear to be multiple potential sources, such as fossil-fuel combustion, lead paint (in or around 38% of homes) and household items.
Conclusion
Elevated BLL are common among children in the Visayas, and may signify an under-recognized threat to children living in rural areas of other developing nations. This setting has varied environmental sources of lead. Observed correlates of BLL may be of clinical, environmental and public health utility to identify and mitigate the consequences of lead toxicity.
Résumé
Objectif
En général, l’intoxication par le plomb n’est pas considérée comme un danger environnemental important dans les zones rurales des pays en développement. En appliquant une stratégie expérimentale de nature prospective, la communauté des chercheurs et les pouvoirs publics mènent une enquête de grande ampleur [Quality Improvement Demonstration Study (QIDS)] pour connaître et évaluer les effets des réformes de la politique sanitaire sur les enfants d’une zone rurale des Philippines. Dans le cadre de cette étude, nous avons étudié l’exposition au plomb des enfants de moins de cinq ans.
Méthodes
Nous avons constitué un échantillon à partir d’une population vivant dans la région de Visayas au centre des Philippines, qui couvre approximativement un tiers de la surface du pays. De décembre 2003 à septembre 2004, l’enquête a recueilli les résultats de dosages sanguins du plomb (plombémies) chez des enfants en association avec des données démographiques, socioéconomiques et sanitaires concernant ces enfants. Des études sur le terrain supplémentaires sur un sous-échantillon composé des enfants les plus exposés ont permis d’évaluer les sources de l’exposition environnementale au plomb.
Résultats
Parmi les enfants sujets de cette étude, 21% (601 sur 2861) présentaient une plombémie supérieure à 10 µg/dl. La plombémie était associée de manière indépendante à l’âge, au taux d’hémoglobine, à la source d’approvisionnement en eau, au matériau de toiture, aux dépenses et aux antécédents en matière d’allaitement au sein. Une évaluation de type suivi des expositions environnementales potentielles parmi le sous-échantillon d’enfants dont la plombémie était élevée n’a fait apparaître aucune source d’exposition unique ou principale. Au contraire, il semble que les sources potentielles soient multiples : combustion de combustible fossile, peinture au plomb (à l’intérieur ou autour de 38% environ des habitations) et biens d’équipement ménagers.
Conclusion
La présence d’une plombémie élevée est courante chez les enfants de la région de Visayas et peut représenter une menace non reconnue pour les enfants vivant dans des zones rurales d’autres nations en développement. Les Philippines abritent diverses sources environnementales de plomb. Les corrélations observées concernant la plombémie peuvent être utiles sur le plan clinique, environnemental et sanitaire pour identifier et atténuer les conséquences de l’exposition toxique au plomb.
Resumen
Objetivo
Generalmente se considera que la intoxicación por plomo no constituye un peligro ambiental importante para niños residentes en zonas rurales de los países en desarrollo. Utilizando un experimento de políticas diseñado de forma prospectiva, investigadores y gobierno están llevando a cabo una amplia investigación (el Quality Improvement Demonstration Study: QIDS) para introducir reformas de la política sanitaria y evaluar sus repercusiones en los niños de una zona rural de Filipinas. Como parte de ese estudio, hemos investigado la exposición al plomo en menores de cinco años.
Métodos
Se muestreó una población de niños de la región de Visayas, en el centro de Filipinas, que representa aproximadamente un tercio de la superficie del país. Desde diciembre de 2003 hasta septiembre de 2004 se midieron las concentraciones sanguíneas de plomo (CSP) y se registraron las características demográficas, socioeconómicas y sanitarias de los niños. En un trabajo de campo complementario se examinó una submuestra de los niños más expuestos para investigar las fuentes de la exposición ambiental al plomo.
Resultados
El 21% de los niños estudiados (601 de 2861) presentaron CSP superiores a 10 µg/dl. Las CSP se asociaron de forma independiente con la edad, la concentración de hemoglobina, la fuente de agua, el material de los tejados, los gastos y los antecedentes de lactancia materna. Un estudio de seguimiento de las posibles exposiciones ambientales llevado a cabo en la submuestra de niños con CSP elevadas no reveló ninguna fuente de exposición única ni predominante. Por el contrario, parecía haber múltiples fuentes potenciales, tales como el consumo de combustibles fósiles, las pinturas con plomo (presentes dentro o en el entorno del 38% de los hogares) y los enseres domésticos.
Conclusión
Las CSP elevadas son frecuentes en los niños de la región de Visayas y pueden suponer una amenaza subestimada para los niños residentes en zonas rurales de países en desarrollo. En este entorno fueron varias las fuentes ambientales de plomo. Los correlatos de las CSP elevadas pueden ser útiles desde el punto de vista clínico, medioambiental y de salud pública para identificar y mitigar las consecuencias de la toxicidad del plomo.
ملخص
الغرض
بشكل عام لا يُعتبر التسمم بالرصاص من المخاطر البيئية الەامة التي تەدد الأطفال في المناطق الريفية للبلدان النامية. ويجري مجتمع البحوث والحكومة في الفلبين، من خلال تجربة صُمِّمت بشكل استباقي لتقييم السياسات، استقصاءً عريضاً لتنفيذ إصلاحات في السياسات الصحية وتقييم تأثيرەا على الأطفال في منطقة ريفية في الفلبين. وأطلق على ەذە الدراسة اسم الدراسة الإيضاحية لتحسين الجودة. وقمنا في إطار ەذە الدراسة ببحث مدى تعرُّض الأطفال الذين ەم دون سن الخامسة للرصاص.
الطريقة
تم أخذ عينة من أطفال منطقة فيزاياس الواقعة في وسط الفلبين، والتي تبلغ مساحتەا حوالي ثُلث المساحة الجغرافية للبلد. وتم في إطار المسح، الذي امتد من كانون الأول/ديسمبر 2003 إلى أيلول/سبتمبر 2004، تقييم مستويات الرصاص في عينات الدم، إضافةً إلى جمع بيانات ديمغرافية واجتماعية اقتصادية، وبيانات حول صحة الأطفال. وأُجريت اختبارات ميدانية تكميلية لعينة فرعية من الأطفال الأكثر تعرضاً للرصاص، لتقييم مصادر التعرُّض للرصاص في البيئة.
الموجودات
بيَّنت الدراسة أن مستوى الرصاص في دم 21% من الأطفال المشاركين في الدراسة (أي 601 طفل من جملة 2861 طفلاً) يزيد على 10 مكروغرام/ديسي لتر. وقد ارتبطت مستويات الرصاص في الدم ارتباطاً مستقلاً بالعمر، وتركيز الەيموغلوبين، ومصدر المياە، والمواد المستخدمة في الأسطح، ومستوى الإنفاق، وتاريخ الأطفال في الإرضاع من الثدي. وبيَّن تقييم تكميلي للتعرض البيئي المحتمل بين عينة فرعية من الأطفال ذوي مستوى الرصاص المرتفع في الدم، عدم وجود مصدر وحيد أو رئيسي للتعرُّض. وإنما بيَّن التقييم وجود عدة مصادر محتملة، مثل احتراق الوقود الأُحفوري، والطلاء المحتوي على الرصاص (في حوالي 38% من المنازل)، والمواد المستخدمة في المنازل.
الاستنتاج
يشيع ارتفاع نسبة الرصاص في دم أطفال منطقة فيزاياس، وقد يدل ذلك على تەديد غير مدرك حق إدراكە للأطفال الذين يعيشون في أرياف البلدان النامية الأخرى. وتتميز المناطق الريفية باختلاف المصادر البيئية للرصاص. وقد تكون العوامل المرتبطة بمستوى الرصاص في الدم ذات فائدة سريرية وبيئية وصحية، لتحديد عواقب التسمم بالرصاص والتخفيف من آثارە.
Introduction
Lead poisoning is one of the most significant environmental health threats children face. Even low levels of lead exposure are associated with impairment of childhood cognitive function1 and abnormal infant behaviour.2
Over the past 30 years regulatory and environmental reforms in the developed world have significantly ameliorated lead pollution among children. In developing countries, the extent of environmental lead, and ways to reduce and prevent exposures are only beginning to be understood.3 These nations have many competing health challenges and lead poisoning may be considered less important when there is uncertainty about population prevalence rates and the various correlates and sources of lead poisoning. Regulations designed to limit lead pollution are less common and less likely to be enforced compared with developed countries.4
The few investigations carried out in developing countries have studied urban populations. These indicate that the problem may be greater than realized. One cohort study of children in Mumbai and Delhi, India, reported a correlation between blood lead levels (BLL) and age, standard of living, height/weight percentile and parity.5 Around the Philippines capital Metro Manila, several small investigations found children’s elevated BLL associated with proximity to lead acid battery recycling or repair activities,6 consumption of certain foods7 and playground soil.8 In Karachi, children’s BLL were linked to proximity to the city centre, application of surma (a traditional eye make-up), parental illiteracy, paternal lead exposure at the workplace and hand-to-mouth activity.9
The Quality Improvement Demonstration Study (QIDS) is a large population-based study focused on health policy reforms’ impact on the health status and health-care utilization of children under five living in the Visayas. The baseline QIDS survey collected biomarkers, including blood lead, along with demographic, socioeconomic and child health data points. Data were collected from over 2800 children allowing for assessment of BLL in this population and analysis of the correlates.
This paper describes the prevalence of lead poisoning among these children living in a rural area that covers about one third of the Philippines. We explore the correlations of lead toxicity in this population and describe an environmental investigation to characterize an unexpectedly common toxic health hazard.
Methods
This study was approved by the ethics committees at the University of California, San Francisco, and the University of the Philippines, Diliman. The children’s parents or guardians provided written informed consent before enrolment. The sections below detail the methodological approach taken in our investigation’s three phases: description of the prevalence of lead poisoning in this population, analysis of the correlates of BLL and environmental exposure assessment to identify the potential sources of lead.
Study subjects
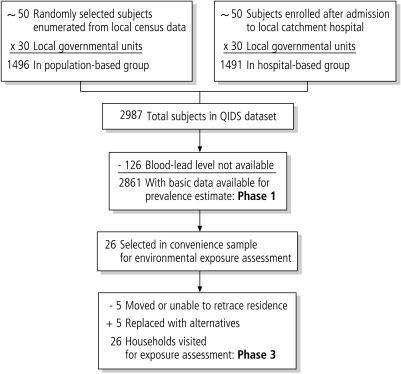
Children aged between six months and five years were eligible for inclusion in QIDS. A stratified random sample was used: approximately 100 children from each of the 30 identified health districts were interviewed. Half were selected randomly from households using the current National Statistics Office census frame; the other half had been discharged from hospital within the past four to eight weeks. All answered the same questionnaire. Both separate and combined models were run. The discharged patients had an average of 8% lower levels of lead. For the analysis, we use the combined sample controlling for this covariate in the models reported in the results section. Hospital-based children were tested for lead on the day of discharge; the randomly selected children were tested during a home visit. The analysis population is illustrated in Fig. 1.
Fig. 1.
Assembly of analysis datasets
Study variables
Detailed information about socioeconomic and health status was obtained using household survey instruments. These queried household characteristics, expenditure, sources of water, sanitation, labour force participation and education. Health measures included a general (child) health questionnaire, birth history, anthropometrics and blood tests for haemoglobin, folate and lead. Medical technicians collected the household surveys and physical health measures; project leaders monitored data collection methods to ensure quality and uniformity.
Medical technicians obtained whole venous blood samples using standard techniques. Lead samples were analysed at a central laboratory using the LeadCare analyser, approved for use in clinical measurement of blood lead concentration by the United States of America’s Food and Drug Administration in 1997.10 Previous field work demonstrates good correlation (r = 0.829 among 51 subjects) between values obtained using the LeadCare device and the gold standard measure, atomic absorption spectroscopy (AAS).11 Accordingly, our study carried out AAS analysis on a sample subset to provide overall calibration and adjustments of the reported LeadCare results. Elevated BLL were defined as greater than 10 μg/dl, conforming to current US Centers for Disease Control and Prevention (CDC) and WHO guidelines.12,13
Analysis of BLL correlates using QIDS database
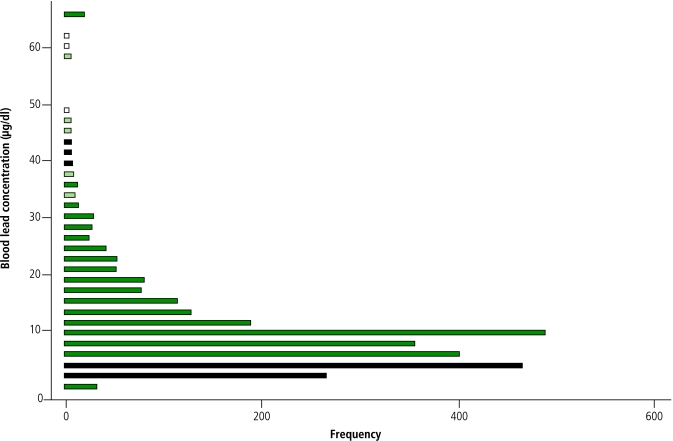
All children in the project who had complete household survey data and measured BLL were included in an exploratory analysis to determine the correlates. We logarithmically transformed BLL to normalize the distribution (Fig. 2). Stata software (version 7.0) was used to perform linear regression analyses accounting for multiple independent variables. Regression assumptions were used to assess models’ compliance and standard regression diagnostic techniques included an assessment of the impact of outliers; all data points were retained.
Fig. 2.
Measured blood lead concentrations (µg/dl) in the QIDS baseline cohort
Note: Cluster of values at 65 μg/ml represents upper detection limit of LeadCare device.
Environmental exposure assessment
To increase the likelihood of identifying environmental lead sources, we generated a convenience sample of 100 children with the highest BLL in the QIDS cohort – 50 of these lived in two provinces. Of these 50 children, 26 with the highest lead levels were selected for an environmental exposure assessment. We were unable to locate 5 subjects, and replaced them with alternatives from the original list of 50. All the subjects’ guardians agreed to participate in this assessment. This sample frame yielded subjects with initial lead levels that ranged between 35.7 μg/dl and >65 μg/dl.
We used a structured survey to investigate specific exposures that might contain lead. Environmental sources were assessed by performing assays using LeadCheck swabs; these colorimetric markers qualitatively indicate lead presence in suspect materials. Testing protocols for each material were adapted from protocols developed by Hybrivet Systems in conjunction with the CDC.
Household materials tested for lead were: drinking water, soil, household dust (using dust wipes), paint (interior and exterior), candy wrappers, solder from canned foods, any items made of lead, petrol and motor oil. Any environmental samples that tested positive as well as samples of dust, soil and water were sampled quantitatively by AAS analysis. The sample collection protocols were coordinated with a contracted AAS laboratory that followed methods published by the US Department of Housing and Urban Development.14
Results
Lead toxicity prevalence
A total of 2861 children were tested for BLL. Of these, 601 (21%) had BLL > 10 μg/dl. The cohort’s mean BLL was 6.9 μg/dl; the median concentration was 4.1 μg/dl (Fig. 2). Leyte had the highest mean (8.5 μg/dl) among the 11 provinces included in the study. BLL were significantly lower in Camiguin, Capiz, Negros Occidental and Siquijor.
BLL correlates
Environmental and clinical correlates with elevated BLL – such as roof construction material, water source, blood haemoglobin concentration, blood folate concentration, age, sex, birth weight, history of breastfeeding and of prematurity – were assessed to determine possible associations. We also investigated a variety of socioeconomic factors including household income, yearly and selected household expenditures, source group (population versus hospital-based) and home province (a community-level variable). After adjustment for covariates, our model showed that roof material, water source, haemoglobin, a history of breastfeeding, age, expenditure on cell phones and home province were all associated significantly with increasing BLL (P < 0.05). Age showed a significant quadratic relationship, indicating that lead levels initially increased with age until 30-36 months, then began to decrease (P < 0.001). No other significant nonlinear relationships were found (Table 1, available at: http://www.who.int/bulletin/volumes/85/9/06.036137/en/index.html).
Table 1. Household and individual factors associated with blood-lead levels (BLL).
| Characteristics | No. of children | Mean blood level μg/dl | Multiple regression model |
|||
|---|---|---|---|---|---|---|
| Coefficienta (95% CI) | P-value | |||||
| Roof construction material | ||||||
| Strong | 760 | 6.3 | 0.13 | 0.323 | 0.323 | |
| Light | 800 | 8.1 | 0.36 | 0.009 | 0.009b | |
| Mixed but predominantly strong | 633 | 6.6 | 0.17 | 0.206 | 0.206 | |
| Mixed but predominantly light | 563 | 6.6 | 0.16 | 0.219 | 0.219 | |
| Mixed but predominantly salvaged | 32 | 6.6 | 0.54 | 0.010 | 0.010c | |
| Salvaged/makeshift | 60 | 4.7 | (referent category) | |||
| Water source | ||||||
| Own use, tubed/piped well | 178 | 5.9 | –0.16 | 0.052 | 0.052 | |
| Shared, tubed/piped well | 649 | 6.8 | –0.10 | 0.048 | 0.048c | |
| Dug well | 577 | 6.5 | –0.03 | 0.510 | 0.510 | |
| Spring, river, stream etc. | 134 | 6.9 | 0.05 | 0.554 | 0.554 | |
| Rain | 20 | 10.1 | 0.54 | 0.039 | 0.039c | |
| Peddler | 9 | 3.1 | –0.17 | 0.529 | 0.529 | |
| Others | 45 | 9.2 | 0.17 | 0.296 | 0.296 | |
| Communal water system | 1238 | 6.9 | (referent category) | |||
| Blood haemoglobin concentration | –0.04 | (–0.07, –0.02) | 0.001b | |||
| <11 g/dl | 672 | 7.8 | ||||
| 11–13 g/dl | 1622 | 6.8 | ||||
| >13 g/dl | 523 | 6.3 | ||||
| Blood folate concentration | –0.0001 | (–0.0003, 0.0002) | 0.583 | |||
| <1.20 ng/ml | 548 | 6.6 | ||||
| 1.20–1.40 ng/ml | 1294 | 7.2 | ||||
| >2.40 ng/ml | 1018 | 6.3 | ||||
| HOME percentage score | –0.01 | (–0,02, –0.01) | <0.00b | |||
| Low (<50%) | 2503 | 6.7 | ||||
| Middle (50-75%) | 12 | 7.0 | ||||
| High (>75%) | 2 | 2.2 | ||||
| Age | 0.03 | 0.000 | <0.001b | |||
| 6 months to 1 year | 443 | 5.9 | ||||
| 1 year | 1061 | 6.7 | ||||
| 2 years | 644 | 7.5 | ||||
| 3 years | 455 | 7.4 | ||||
| 4 years | 250 | 7.6 | ||||
| Age² | –0.0004 | 0.001 | <0.001b | |||
| Sex | –0.09 | 0.015 | 0.015c | |||
| Female | 1330 | 6.5 | ||||
| Male | 1530 | 7.3 | (referent category) | |||
| Birth weight | 0.00003 | (–0.00001, 0.00007) | 0.144 | |||
| <2500 g | 419 | 6.7 | ||||
| 2500–3500 g | 1293 | 6.6 | ||||
| >3500 g | 475 | 7.2 | ||||
| Breastfeeding | 0.22 | (0.09, 0.34) | 0.001c | |||
| History of breastfeeding | 2576 | 7.1 | ||||
| No breastfeeding | 274 | 5.7 | (referent category) | |||
| Urbanity | –0.10 | (–0.18, –0.02) | 0.019c | |||
| Rural | 1953 | 7.0 | ||||
| Urban | 907 | 6.8 | (referent category) | |||
| Income proxy | 0.15 | (0.03, 0.27) | 0.013c | |||
| Owns mobile phone | 535 | 7.1 | ||||
| Does not own mobile phone | 2303 | 6.9 | (referent category) | |||
| Household type | –0.08 | (–0.16, –0.002) | 0.044c | |||
| Follow-home | 1425 | 6.9 | ||||
| Random | 1435 | 6.9 | (referent category) | |||
CI, confidence interval. a These coefficients indicate percentage changes of the blood-lead level for every unit change of the regressors. A coefficient of 0.10 indicates that 1 unit increase in a continuous explanatory variable results in an estimated 10% increase in BLL. Similarly, a coefficient of 0.10 for a categorical variable indicates that BLL for the associated category is estimated to be 10% higher than that for the reference category. Individual p-values for categorical variables are derived from the t-statistics of individual regression terms. b Statistical significance at P = <0.001 level. c Statistical significance at P = <0.05 level.
Clinically, we observed that the blood haemoglobin concentration was inversely associated with BLL – 1 g/dl increase in haemoglobin = 3% decrease in BLL (95% confidence interval, CI: 1, 5%; P = 0.043). A history of breastfeeding was associated with a 19% increase in BLL (95% CI: 5, 35%; P = 0.006); females showed an 8% (95% CI: 2, 14%; P = 0.016), marginally significant, decrease in BLL.
Environmental exposure assessment
A structured survey among individuals with very elevated BLL identified multiple possible sources of lead exposure (Table 2, available at: http://www.who.int/bulletin/volumes/85/9/06.036137/en/index.html). For example, all families had potential sources in food and traditional remedies. Tests in households and nearby environments detected lead in soil, paint, household dust, fishing weights, canned fish and fossil fuels. The presence of lead in all the positive field samples was confirmed using the quantitative methods described (Table 3).
Table 2. Reported prevalence of potential lead exposure sources (n=26).
| Potential exposure source | Households reporting presence (%) |
|---|---|
| Water system with lead piping/solder | 3 (12) |
| Soil | 26 (100) |
| Traditional remedies | 13 (50) |
| Canned food | 23 (88) |
| Fish | 26 (100) |
| Wrapped candy | 26 (100) |
| Painted home/other painted buildings | 16 (62) |
| Family lives near road designated as a highway | 7 (27) |
| Family owns vehicle | 10 (38) |
| Family sells petrol | 0 (0) |
| Family has auto batteries in house | 1 (4) |
| Family melts/recycles lead | 1 (4) |
| Someone in family paints | 4 (15) |
| Someone in family makes fishing weights | 1 (4) |
| Occupational exposure: manufacturing | 1 (4) |
| Occupational exposure: mining | 0 (0) |
| Occupational exposure: fertilizers and pesticides | 5 (19) |
Table 3. Summary of lead content of environmental samples.
| Item | Samples tested | Lead concentration | Hazard limita |
|---|---|---|---|
| Soil | 2 | < 10–93 ppm | 420 ppm |
| Paint chips | 10 | 530–120 000 ppm | 5000 ppm |
| Dust wipes | 7 | < 10–110 µg/ft² | 100 µg/ft² |
| Canned tuna | 2 | 0.14–0.16 ppm | 0.2 ppm |
| Candy wrapper | 1 | < 42 ppm | 0 ppm |
| Petrol | 2 | 4.6–24.6 ppm | 13 ppm |
| Motor oil | 2 | 13.8–15.4 ppm | (not regulated) |
| Fishing weights | 3 | 89–100% | (not regulated) |
a Current hazard limits as determined by applicable United States of America regulatory agencies (Department of Housing and Urban Development, Environmental Protection Agency, Centers for Disease Control and Prevention, Food and Drug Administration and Department of Agriculture) or Philippine regulatory agency (e.g. Department of Environment and Natural Resources for petrol).
Paint
Interior and exterior paint was found in 10 out of 26 households (38%) and tested for lead. All 10 paint chip samples had detectable amounts of lead; 3 (12%) exceeded the 0.5% allowable limit.14 In nearly all instances, paint was chipped and/or peeling. We also tested two local schools and three hospitals. Lead paint was found on interior and exterior walls and trim in one school, and on walls, trim, cribs and beds in all three hospitals.
Water
The primary source of drinking water tested negative in all 26 households. We learned of widespread upgrades to the water system in the study area with the highest lead levels (Leyte) between initial study visits and follow-up exposure assessment visits.
Soil and dust
Qualitative tests on composite soil samples from each household were all negative. Two control samples were sent for quantitative AAS testing: one was negative, the other had a detectable level of 93 ppm (420 ppm allowable limit).15 No dust samples tested positive in qualitative sampling. Four of seven dust wipes submitted for quantitative AAS testing had detectable lead levels. The two highest lead-dust concentrations were found in samples obtained from the floor under a child’s bed where fishing weights were stored; both exceeded the recognized hazard threshold.14
Fishing weights
Four sets of fishing weights were found, and all tested positive (at least 89% by weight) for lead. These were stored under a child’s bed in one household and were melted down for shaping in the kitchen of another.
Canned tuna
Two locally obtained samples of canned tuna were tested. Both contained detectable levels of lead at 0.14 ppm and 0.16 ppm respectively, just below the allowable limit of 0.2 ppm.16
Fossil fuels
Samples of petrol (one premium, one regular) and motor oil were obtained in Leyte, the province with the highest number of children with elevated BLL. The Philippine Department of Energy has set the maximum allowable limit for lead in petrol at 13 ppm.17 There is no established maximum for motor oil, specifically 2T motor oil commonly added to the fuel mixture in two-stroke engines, motorcycles and tricycles (motorcycles with sidecars). The premium petrol sample had a lead concentration of 4.6 ppm; regular contained 24.6 ppm. The motor oil samples contained 13.8 ppm and 15.4 ppm of lead respectively. Lead in petrol was first regulated in the Philippines in 1993 and reduced to its current allowable level of 0.13 g/L on 31 December 2000.17 Government experts believe that residual lead oxide remains in dust, soil and water, especially in areas with high traffic volumes.
Discussion
We report that 21% of 2861 children living in the rural Philippines had elevated levels of lead in whole blood. This is a previously unreported environmental hazard for children living in the Visayas. Smaller studies have found elevated BLL in developing countries, but these were in urban areas where exposure to industrial paint sources and fossil-fuel burning are relatively high.5,9,18
Elevated lead levels in rural settings have not been reported widely or characterized as a problem for developing countries. Yet the associations between elevated lead levels and socioeconomic factors are different from those in more affluent countries.19 The combination of elevated lead and high poverty in our population is a particular concern. The average annual income in our sample was US$ 1179; the lowest quintile earned less than US$ 460 (based on direct assessment of 2004 annual expenditures). Constrained public resources in the Philippines, and other developing countries, make it unlikely that surveillance or screening will occur unless awareness is increased and the consequences of lead toxicity are understood. This paper looks at the prevalence, associations and possible causes of lead exposure, but further analyses will be needed to assess the consequences of these exposures.
The inverse gradient between haemoglobin concentration and BLL (Table 1) is another important finding. Some reports suggest that this is the clinical consequence of parallel iron and lead uptake in iron-deficient children.20 Related studies have demonstrated that children with iron deficiency anaemia are at increased risk of developing lead poisoning.21 This effect may be mediated through a common absorptive receptor; intestinal iron ligands may also bind lead.20 Another possible mechanism for the observed association is confounding, since both lead poisoning and iron deficiency are associated with a variety of circumstances related to lower socioeconomic status. However, this study found that the relationship persisted despite adjustment for multiple confounders, suggesting that the association is more likely to be biological.
The association between breastfeeding and a significant increase in BLL (7.1 μg/dl mean BLL in breastfed children; 5.7 μg/dl mean BLL with no history of breastfeeding) also may have a biological basis. Previous research has indicated that lead may be transferred via breast milk.22 In sub-group analyses, the association between breastfeeding and BLL was strongest among the youngest children, those most likely to be breastfeeding. This indicates that breastfeeding remains a significant predictor of increased BLL in a population ranging from 6 months to 5 years of age.
Concurrently collected data from the broad-based (QIDS) population survey link roofing material and water source with elevated lead levels. Roofing materials serve as a means of collecting rainwater and thus potential ingestion. Alternatively, both roofing materials and water are proxies for expenditure, which is also significant in our study. Our confirmation testing did not find significant lead levels in rainwater or in any roofing material. However, lead levels in paint, petrol and motor oil were far higher than regulatory standards. The fact that lead levels in the petroleum products were above mandated limits indicates that aerosolized lead is likely to be an important source. This indicates the difficulty of enforcing environmental regulations within the country.
The positive association between lead levels and income proxies, such as roofing and water sources, further suggests that ingested or inhaled lead from paints and fossil fuel consumption may be an important source of the widespread exposure we observed. For example, we know that relatively better-off families are more likely to live along major thoroughfares, use motorcycles and tricycles for transportation, paint their houses and not breastfeed their newborns. For lead exposure this suggests that it is safer to be very poor rather than relatively affluent if you live in the countryside.
This study has important limitations. The cross-sectional nature of the sample limits any causal inferences, and the non-random environmental assay also is a limitation. We are confident there is no single overwhelming source of exposure, but follow-up assessments are needed to identify all potential sources.
Several lead sources identified in this study appear open to intervention. Over a third of the households (as well as public hospitals and schools) contain lead-based paint, often in poor condition. As the sale of lead-based home paint is not regulated in the Philippines, this offers the opportunity for targeted interventions at national and local levels.
The prevalence of lead toxicity could be reported to the public and preventive messages about BLL directed towards at-risk populations. The relationships between BLL and blood haemoglobin concentration or the lack of breastfeeding could be exploited. Our findings and others suggest that iron supplementation may have the double benefit of reducing anaemia and decreasing gastrointestinal lead uptake.23 At the very least, anaemia should be considered a clinical risk factor for elevated BLL and its presence may warrant screening.
Breast milk is widely considered to be the optimal mode of nutrient delivery to term infants.24 The health benefits for this population clearly outweigh any benefit from lowering BLL by reducing breastfeeding. This association indicates the importance of limiting maternal lead exposure and eventual child exposure through some of the simple measures described.
Lead is an anthropogenic neurotoxicant now dispersed widely around the globe. Its presence in toxic concentrations among child populations seems to closely follow economic and industrial development.25 This study population had minimal direct exposure to industry but extensive exposure to lead-containing products brought into the region. Lead pollution also may be an indicator of wider environmental degradation in rural areas of developing nations. Accordingly, careful environmental, societal and economic assessments of environmental lead exposure are important. Development and implementation of comprehensive strategies are matters for global and local policy.
Lead poisoning is a threat to the potential cognitive development of children living in poor, rural areas. Other threats are malnutrition and chronic poverty, poor hygiene, incomplete access to healthcare and limited educational opportunity. Yet in comparison with such large and complex problems, lead exposures may be relatively straightforward to mitigate, and greater awareness and further investigation are warranted. Successful childhood lead-poisoning prevention programmes in other countries can serve as models for regulating anthropogenic lead pollution and enforcing existing laws. ■
Ethics committee approval
The QIDS study protocol and the lead evaluation were approved by the Committee on Human Research, University of California, San Francisco (approval number H10609-19947-03) and by the University of the Philippines School of Economics (UPEcon) Institutional Review Board (FWA number 000005371). All study participation was strictly voluntary.
Footnotes
Funding & acknowledgements: The QIDS study is investigator-initiated, funded by a US NIH R01 grant and by a grant from the Philippine Health Insurance Corporation. The exposure assessment phase was funded by a grant from James Strand. We thank the many technicians, research assistants and others who gathered data for the QIDS study and the administrators and clinicians at participating district hospitals.
Competing interests: None declared.
References
- 1.Canfield RL, Henderson CR, Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003;348:1517–26. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendelsohn AL, Dreyer BP, Fierman AH, Rosen CM, Legano LA, Kruger HA, et al. Low-level lead exposure and cognitive development in early childhood. J Dev Behav Pediatr. 1999;20:425–31. doi: 10.1097/00004703-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Romieu I, Lacasana M, McConnell R.and theLead Research Group of the Pan-American Health Organization. Lead exposure in Latin America and the Caribbean. Environ Health Perspect 1997105398–405. 10.2307/3433336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The global dimensions of lead poisoning: an initial analysis. Washington: Alliance to End Childhood Lead Poisoning and Environmental Defense Fund; 1994.
- 5.Jain NB, Hu H. Childhood correlates of blood lead levels in Mumbai and Delhi. Environ Health Perspect. 2006;114:466–70. doi: 10.1289/ehp.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suplido ML, Ong CN. Lead exposure among small-scale battery recyclers, automobile radiator mechanics, and their children in Manila, the Philippines. Environ Res. 2000;82:231–8. doi: 10.1006/enrs.1999.4024. [DOI] [PubMed] [Google Scholar]
- 7.Zhang ZW, Subida RD, Agetano MG, Nakatsuka H, Inoguchi N, Wantabe T, et al. Nonoccupational exposure of adult women in Manila, the Philippines, to lead and cadmium. Sci Total Environ. 1998;215:157–65. doi: 10.1016/S0048-9697(98)00118-1. [DOI] [PubMed] [Google Scholar]
- 8.Sharma K, Reutergardh LB. Exposure of preschoolers to lead in the Makati area of Metro Manila, the Philippines. Environ Res. 2000;83:322–32. doi: 10.1006/enrs.2000.4064. [DOI] [PubMed] [Google Scholar]
- 9.Rahbar MH, White F, Agboatwalla M, Hozhabri S, Luby S. Factors associated with elevated blood lead concentrations in Karachi, Pakistan. Bull World Health Organ. 2002;80:769–75. [PMC free article] [PubMed] [Google Scholar]
- 10.FDA approves simpler, more accessible lead poisoning test kit. HHS News. 10 Sept 1997; p97-31. Available at: http://www.fda.gov/bbs/topics/NEWS/NEW00590.html
- 11.Counter SA, Buchanan LH, Laurell G, Ortega F. Field screening of blood lead levels in remote Andean villages. Neurotoxicology. 1998;19:871–7. [PubMed] [Google Scholar]
- 12.Preventing lead poisoning in young children. Atlanta: US Centers for Disease Control; October 1991.
- 13.International Programme on Chemical Safety (IPCS). Inorganic lead. Environmental health criteria 165. Geneva: WHO; 1995. [Google Scholar]
- 14.Guidelines for the evaluation and abatement of lead-based hazards in housing. US Department of Housing and Urban Development; June 1995. Available at: http://www.hud.gov/offices/lead/guidelines/hudguidelines/index.cfm
- 15.National Resources Conservation Service. Soil quality - urban technical note no. 3. Heavy metal soil contamination. Washington: US Department of Agriculture; 2000. Available at: http://soils.usda.gov/sqi/files/u03d.pdf
- 16.Emerging international contaminant issues: development of codex alimentarus standards to address the issues. Food Safety Magazine. Feb-Mar 2000. Available at: http://www.cfsan.fda.gov/~cjm/codexfa2.html
- 17.2002 national air quality status report. Quezon City: Philippines Department of Energy and Natural Resources; 8 December 2003.
- 18.Heinze I, Gross R, Stehle P, Dillon D. Assessment of lead exposure in schoolchildren from Jakarta. Environ Health Perspect. 1998;106:499–501. doi: 10.2307/3434183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee R, Lee H, Yoo I, Kim SR. Trend of blood lead levels in children in an industrial complex and its suburban area in Ulsan, Korea. Int Arch Occup Environ Health. 2002;75:507–10. doi: 10.1007/s00420-002-0333-5. [DOI] [PubMed] [Google Scholar]
- 20.Barton JC, Conrad ME, Nuby S, Harrison L. Effects of iron on the absorption and retention of lead. J Lab Clin Med. 1978;92:536–47. [PubMed] [Google Scholar]
- 21.Wright RO, Tsaih SW, Schwartz J, Wright RJ, Hu H. Association between iron deficiency and blood lead level in a longitudinal analysis of children followed in an urban primary care clinic. J Pediatr. 2003;142:9–14. doi: 10.1067/mpd.2003.mpd0344. [DOI] [PubMed] [Google Scholar]
- 22.Ettinger AS, Tellez-Rojo MM, Amarasiriwardena C, Bellinger D, Peterson K, Schwartz J, et al. Effect of breast milk lead on infant blood lead levels at 1 month of age. Environ Health Perspect. 2004;112:1381–5. doi: 10.1289/ehp.6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmermann MB, Muthayya S, Moretti D, Kurpad A, Hurrell RF. Iron fortification reduces blood lead levels in children in Bangalore, India. Pediatrics. 2006;117:2014–21. doi: 10.1542/peds.2005-2440. [DOI] [PubMed] [Google Scholar]
- 24.Postpartum care of the mother and newborn: report of a technical working group. Geneva: WHO; 1998. Available at: http://www.who.int/reproductive- health/publications/msm_98_3/index.html
- 25.Flegal AR, Smith DR. Lead levels in preindustrial humans. N Engl J Med. 1992;326:1293–4. [PubMed] [Google Scholar]