Abstract
Objectives. We investigated the relationship between the presence of in-home piped water and wastewater services and hospitalization rates for respiratory tract, skin, and gastrointestinal tract infections in rural Alaska.
Methods. We determined in-home water service and hospitalizations for selected infectious diseases among Alaska Natives by region during 2000 to 2004. Within 1 region, infant respiratory hospitalizations and skin infections for all ages were compared by village-level water services.
Results. Regions with a lower proportion of home water service had significantly higher hospitalization rates for pneumonia and influenza (rate ratio [RR] = 2.5), skin or soft tissue infection (RR = 1.9), and respiratory syncytial virus (RR = 3.4 among those younger than 5 years) than did higher-service regions. Within 1 region, infants from villages with less than 10% of homes served had higher hospitalization rates for pneumonia (RR = 1.3) and respiratory syncytial virus (RR = 1.2) than did infants from villages with more than 80% served. Outpatient Staphylococcus aureus infections (RR = 5.1, all ages) and skin infection hospitalizations (RR = 2.7, all ages) were higher in low-service than in high-service villages.
Conclusions. Higher respiratory and skin infection rates were associated with a lack of in-home water service. This disparity should be addressed through sanitation infrastructure improvements.
Modern sanitation services (potable drinking water and safe wastewater disposal) are a cornerstone of public health progress and have contributed to decreased infectious disease morbidity and mortality. In 1950, 64.5% of US homes had complete sanitation services (a flush toilet, shower or bath, and kitchen sink).1 This increased to 93.1% by 1970 and to 99.4% by 2000.2,3
In 2000, 93.7% of Alaskan homes had complete sanitation, which ranked Alaska last among US states.3 In rural Alaska, where the vast majority of people are Alaska Natives, a much higher proportion lack basic sanitation facilities. Providing in-home sanitation services is difficult in remote villages where small, isolated populations live in a harsh, cold climate. Although many rural village homes lack in-home water service, nearly all villages have access to safe drinking water.4 Significant gains in health status indicators have occurred among rural Alaska Natives; however, the ongoing disparity in sanitation services remains unsolved in most of rural Alaska. Furthermore, there is a disparity in infectious disease hospitalizations among Alaska Natives compared with the general US population.5 To our knowledge, there are no evaluations of the health effects of a lack of modern sanitation services for rural Alaskans.
Alaska village residents who live without pressurized in-home water service typically obtain water from a community-based water point and bring it home in 5-gallon (19-L) plastic containers. As of 2000, one third of rural Alaska residents obtained water this way.4 Although water is available in centralized locations, some families must travel long distances or cross rivers to obtain safe water. This distribution method makes it difficult to obtain adequate amounts of water needed for basic consumption and hygiene practices.6 Alaska homes lacking pressurized in-home water service also lack flush toilets. Residents use outhouses or in-home waste containers commonly known as “honeybuckets” that require manual removal to a centralized waste disposal site or lagoon. Sanitation infrastructure is provided to rural Alaskans by state- and federally funded programs that have provided service first where the greatest number of homes could be served at the lowest cost.
Although it has long been recognized that access to modern sanitation services can reduce morbidity and mortality from gastrointestinal illnesses, recent data have established the important role of adequate water supplies for preventing respiratory diseases.7–9 The value of adequate supplies of safe water has been attributed to the prevention of both waterborne diseases, in which the pathogen can be ingested from contaminated water, and water-washed disease, in which hygienic practices such as handwashing and bathing play a role.10 We sought to describe the relationship between in-home water and wastewater service and the risks of waterborne and water-washed infectious diseases in rural Alaska. We used existing sanitation service data for rural Alaska along with hospital discharge records, a respiratory disease surveillance system, and a skin infection outbreak investigation to explore whether improved sanitation service was associated with improved health status among rural Alaska Native people.
METHODS
Population
The approximately 120 000 Alaska Natives are descendants of the indigenous population and represent 19% of Alaskans. Approximately 60% of Alaska Natives live in rural or remote villages. Of the approximately 170 rural villages, most have fewer than 300 residents, and the vast majority are Alaska Natives. Most villages are not accessible by road; travel between villages is mainly by airplane, snowmobile, or boat. Health care services are administered by regional Alaska Native–managed tribal health organizations, with some statewide facilities and services shared and coadministered, such as the referral medical center in Anchorage.
Sanitation Services
The Rural Alaska Housing Sanitation Inventory documented water and wastewater service in rural villages from July 2001 through April 2004. Each home was evaluated, and a statewide database was created. We defined “served” homes as having pressurized, in-home water service including piped water service from a municipal system or on-site well and septic tank or drain field systems, or “closed haul” systems in which water is delivered to storage tanks and distributed throughout the home via internally pressurized plumbing. For the latter, wastewater from flush toilets is held in a storage tank and periodically evacuated by a pump truck. We used data from 6 predominantly rural regions that were defined according to the boundaries of the tribal health care organizations. We defined “high-service” regions as those in which 80% or more of homes had service and “low-service” as those in which less than 80% were served.
Water service data for 1 region (region A) were used in a village-level analysis. Because water improvements are ongoing, we excluded from analysis villages in which more than 50% of homes had new water service from 1999 through 2004 (5 villages with 2740 persons, or 11.6% of the region's population). We categorized the remaining 47 villages into tertiles according to the proportion of homes served. We analyzed region A's largest town separately because it has near-complete water service and a population approximately 5 times larger than that of the next-largest village. Household size and income data were obtained from the 2000 US Census.11
Regional Disease Rates
Hospital discharge data for the fiscal years 2000 to 2004 for Alaska Natives in Alaska were obtained from the Indian Health Service's (IHS's) Direct and Contract Health Service inpatient data set.12 These data include patient discharge records from IHS-operated, tribally operated, and community hospitals that were contracted with IHS or with tribes to provide health care services to eligible persons.13 We selected hospitalizations for the 6 predominantly rural regions and urban Anchorage. Three regions were excluded because of small hospital discharge numbers.
Hospital discharges were selected for infectious gastroenteritis, pneumonia or influenza, skin or soft tissue infection, and methicillin-resistant Staphylococcus aureus (MRSA) infections for all ages, and respiratory syncytial virus (RSV) for children younger than 5 years. A record was selected if 1 of these diseases was listed among the first 6 discharge diagnoses according to the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM).14
The definitions of infectious gastroenteritis included diarrhea of determined etiology (bacterial: 001–005, 008.0–008.5, excluding 003.2; parasitic: 006–007, excluding 006.3–006.6; and viral: 008.6–008.8) and diarrhea of undetermined etiology (presumed infectious: 009.0–009.3). Pneumonia and influenza were identified with codes 480 to 487. Skin and soft tissue infections were identified with codes 680 to 682, 684, 686.8, and 686.9. Hospitalizations for MRSA were selected by code V09.0 (infection with microorganisms resistant to penicillins) among codes 038.11 (S aureus septicemia), 482.41 (other bacterial pneumonia caused by S aureus), and 041.11 (S aureus bacterial infection in conditions classified elsewhere and of unspecified site). Infection with RSV was defined as codes 480.1, 079.6, and 466.11. Because patient identifiers were not available, repeated hospitalizations could not be excluded.
Hospitalization rates were calculated per 10 000 persons per year for region of residence. The IHS fiscal year 2002 user population estimates (released March 2002) were used as the denominator. The user population included all Alaska Natives who had received IHS-funded health care at least once over the previous 3 years.12 We calculated age group–specific rates, categorizing age as younger than 1 year, 1 to 4 years, 5 to 19 years, 20 to 44 years, 45 to 64 years, and 65 years or older. Rate ratios with 95% confidence intervals (CIs) were calculated with Poisson regression analysis.15 Age adjustment with the direct method for the user population of Alaska did not substantially change the rates and is not reported.
Disease Rates Within Region A
We conducted ongoing surveillance of hospitalizations for acute lower respiratory tract infections (LRTIs) among children for region A and selected 1999 to 2004 to examine rates by village.16,17 We abstracted clinical and laboratory information from the computerized medical records for children younger than 1 year hospitalized at the regional hospital, or in Anchorage or who received contracted medical care at a nontribal hospital. We obtained for each hospitalization the birth dates, admission and discharge rates, ICD-9-CM diagnosis codes and narrative, and RSV test result. We merged duplicate hospitalization data on patients transferred to another hospital. A child was classified as having pneumonia if the discharge diagnoses included 1 of ICD-9-CM codes 480.1, 485, 486, or 507. Infection with RSV was defined as a hospitalized child younger than 1 year with acute LRTI and a nasopharyngeal aspirate positive for RSV by culture or a rapid identification method (enzyme immunoassay or direct fluorescent antibody). The majority of RSV testing was performed with Directogen (Bectin Dickenson, Cockeysville, MD). Comparable data for all Alaska Natives and for the US general population were obtained from published sources.17,18
Skin infection hospitalization data were obtained from an outbreak investigation in region A.19 We included hospitalizations for skin infections from July 1, 1999, through June 30, 2000, and used ICD-9-CM codes (680.0–682.9) to include carbuncle, furuncle, and cellulitis. The regional hospital laboratory was used to identify all confirmed S aureus cultures from skin infections for the same period. The MRSA infections were defined by a minimum inhibitory concentration of oxacillin at 2 μg/mL or greater. Clinical samples obtained at village-based clinics must be transported to the regional hospital for culture and confirmation, introducing a potential diagnostic access bias. To avoid overestimating infection rates in the 10 villages closest to the regional hospital, whose residents might seek care directly at the hospital-based clinics and hence be diagnosed more often, we excluded from analysis persons from these villages. Population denominators were obtained from the 2000 Census.11
The χ2 test for trend was used to compare hospitalization rates for villages with differing levels of water service. We adjusted for a potential confounder (number of persons per household) with the Cochran–Mantel Haenszel test comparing high-service to low-service villages.
RESULTS
Rural In-Home Water Service
We obtained water service data from 128 villages and a total of 12 480 homes in the 6 regions. Overall, 73% of homes had in-home water service (range by region: 57% to 100%). Wastewater service was present in 71% of homes; the percentages by region were similar to the proportion of homes with water service by region (Table 1). The high-service regions had 91% of homes with in-home water service compared with 61% of homes in the low-service regions.
TABLE 1.
In-Home Water and Wastewater Service to Homes, by Region: Alaska, 2000
| Region | American Indian/Alaska Native Population,a No. | Communities Surveyed, No. | Homes Surveyed, No. | Homes With Water Service, No. (%) | Homes With Wastewater Service, No. (%) |
| High service | |||||
| F | 5 409 | 25 | 1 555 | 1 387 (89) | 1 349 (87) |
| E | 12 370 | 26 | 2 834 | 2 499 (88) | 2 403 (85) |
| D | 4 518 | 4 | 368 | 368 (100) | 368 (100) |
| Low service | |||||
| C | 6 867 | 10 | 834 | 626 (75) | 627 (75) |
| B | 7 274 | 14 | 1 376 | 782 (57) | 751 (55) |
| A | 20 714 | 49 | 5 513 | 3 360 (61) | 3 328 (60) |
| Total | 57 152 | 128 | 12 480 | 9 022 (73) | 8 826 (71) |
Data from the 2000 US Census.11
Regional Hospitalization Rates and Water Service
Hospitalization rates by region for the 5 infectious disease categories varied by water service level (Table 2). The RSV hospitalization rate for children younger than 5 years was higher in the low-service regions than in the high-service regions (rate ratio [RR] = 3.4; 95% CI = 3.0, 3.8). For all ages, rates for pneumonia and influenza (RR = 2.5; 95% CI = 2.4, 2.7), skin or soft tissue infection (RR = 1.9; 95% CI = 1.8, 2.1), and MRSA infection (RR = 4.5; 95% CI = 3.6, 5.7) hospitalizations were also higher for low-service regions.
TABLE 2.
Hospitalization Rates per 100 000 for Specific Infections and the Proportion With In-Home Water Service, by Region: Alaska, 2000–2004
| Region | Water-Served Homes, % | Infectious Diarrhea, Hospitalization Rate (No.) | RSV,a Hospitalization Rate (No.) | Pneumonia or Influenz,ab Hospitalization Rate (No.) | Skin or Soft Tissue Infection,b Hospitalization Rate (No.) | MRSA Infection, Hospitalization Rate (No.) |
| Urban Anchorage | 100 | 7.14 (106) | 78.5 (130) | 63.24 (939) | 50.71 (753) | 5.25 (78) |
| High-service region | ||||||
| F | 89 | 5.80 (16) | 148.29 (39) | 85.93 (237) | 47.86 (132) | 2.90 (8) |
| E | 88 | 9.73 (70) | 29.76 (15) | 42.12 (303) | 26.0 (187) | 1.25 (9) |
| D | 100 | 6.43 (14) | 214.69 (57) | 98.26 (214) | 41.32 (90) | 1.38 (3) |
| Total high-service regions | 91 | 7.64 (206) | 90.1 (241) | 62.8 (1693) | 43.07 (1162) | 3.63 (98) |
| Low-service region | ||||||
| C | 75 | 5.78 (20) | 136.42 (59) | 100.87 (349) | 34.10 (118) | 0.58 (2) |
| B | 57 | 4.06 (16) | 129.48 (56) | 90.82 (358) | 39.07 (154) | 1.27 (5) |
| A | 61 | 8.72 (96) | 314.48 (481) | 199.82 (2200) | 113.62 (1251) | 26.70 (294) |
| Total low-service regions | 61 | 7.17 (132) | 248.90 (596) | 157.89 (2907) | 82.72 (1523) | 16.34 (301) |
| Rate ratioc (95% CI) | 0.94 (0.78, 1.17) | 2.81 (2.42, 3.26) | 2.54 (2.39, 2.70) | 1.93 (1.79, 2.08) | 4.51 (3.59, 5.66) |
Note. RSV = respiratory syncytial virus; MRSA = methicillin-resistant Staphylococcus aureus; CI = confidence interval. Number is the total number of hospitalizations for that disease.
Respiratory syncytial virus, for hospitalizations among children younger than 5 years.
Three pneumonia- or influenza-associated hospitalizations and 8 skin- or soft tissue–infection hospitalizations did not have community of residence available.
High- vs low-service regions.
Hospitalization rates for infectious diarrhea did not differ between high- and low-service regions (RR = 0.94; 95% CI = 0.78, 1.2). Diarrhea of undetermined etiology as the only diarrhea-coded diagnosis was reported for only 4.2% of the diarrhea hospitalizations, and the removal of this diagnosis did not affect the overall rate comparison.
Higher pneumonia and influenza hospitalization rates seen among the low-service regions were seen in each age group; however, the overall excess rate was greatest among the very young and the elderly (Table 3). The hospitalization rate among children younger than 1 year was 5 times higher in low-service regions than in the high-service regions. For children aged 1 to 4 years and persons 65 years or older, the rates were at least 2 times higher in the low-service regions than in the high-service regions.
TABLE 3.
Age-Specific Hospitalization Rates for Pneumonia and Influenza and Proportion With In-Home Water Service, by Region and In-Home Water Service Level: Alaska, 2000–2004
| Service Unit | Age <1 Year, Hospitalization Rate (No.) | Age 1–4 Years, Hospitalization Rate (No.) | Age 5–19 Years, Hospitalization Rate (No.) | Age 20–44 Years, Hospitalization Rate (No.) | Age 45–64 Years, Hospitalization Rate (No.) | Age ≥65 Years, Hospitalization Rate (No.) |
| Urban Anchorage | 246.69 (81) | 76.11 (100) | 13.57 (60) | 31.8 (179) | 112.4 (286) | 384.11 (233) |
| High-service region | ||||||
| F | 989.47 (47) | 143.85 (31) | 15.22 (14) | 19.16 (17) | 97.21 (47) | 397.06 (81) |
| E | 333.33 (20) | 51.80 (23) | 8.03 (18) | 15.46 (40) | 64.02 (83) | 211.37 (119) |
| D | 750.00 (42) | 190.93 (40) | 9.39 (7) | 27.30 (19) | 102.19 (35) | 552.53 (71) |
| Total high-service regions | 386.30 (190) | 88.87 (194) | 11.89 (99) | 26.0 (255) | 96.65 (451) | 335.53 (504) |
| Low-service region | ||||||
| C | 988.64 (87) | 194.48 (67) | 17.88 (23) | 32.32 (35) | 73.83 (33) | 492.89 (104) |
| B | 1435.48 (89) | 124.16 (46) | 20.38 (26) | 27.50 (37) | 89.07 (55) | 399.24 (105) |
| A | 2549.75 (756) | 317.11 (391) | 22.83 (90) | 34.4 (117) | 139.88 (205) | 954.58 (641) |
| Total low-service regions | 2087.35 (932) | 258.73 (504) | 21.4 (139) | 32.4 (189) | 115.81 (293) | 742.03 (850) |
| Rate ratioa (95% CI) | 6.57 (5.58, 7.72) | 2.96 (2.51, 3.50) | 1.80 (1.39, 2.33) | 1.25 (1.03, 1.51) | 1.20 (1.04, 1.39) | 2.31 (2.06, 2.59) |
Note. CI = confidence interval. Number is the total number of hospitalizations for that disease.
High- vs low-service regions.
Water Service in Region A
In region A, 61% of homes had water service, but service was not uniformly distributed throughout the region (Table 4). Water service was available in less than 10% of homes for 20 villages (30% of population), in 10% to 79% for 13 villages (20% of population), and in 80% or more for 14 villages (27% of population). The largest town, with 23% of the region's population, had 99.5% of homes with water service. With the exclusion of the largest town, the other groups of villages were similar in persons per household and mean household income. Villages with less than 10% of homes served had a slightly lower median population than those with a greater proportion of homes served. The population ranges overlapped for all 3 groups, and the largest difference in median village population between groups was 181 persons.
TABLE 4.
Village Demographic Characteristics and Annualized Rates of Respiratory Disease Hospitalizations (Children Younger Than 1 Year) and Soft Tissue Infections (All Ages), by Percentage With In-Home Water Service for Region A: Alaska, 1999–2004, and 1999–2000
| Percentage of Community With In-Home
Water Service |
P |
|||||
| Characteristic | < 10 | 10–79 | ≥ 80 | 100 | For Trenda | ≥ 80% vs 100% |
| Population (% of total) | 6956 (30) | 4743 (20) | 6415 (27) | 5459 (23) | ||
| Number of villages | 20 | 13 | 14 | 1b | ||
| Median village population (range) | 312 (49–1042) | 370 (96–651) | 493 (202–832) | 5459 | .31 | Not tested |
| Average no. persons per homec | 4.7 | 4.2 | 4.2 | 4.2 | .09 | Not tested |
| Average household income, $ per year | 30 633 | 28 393 | 31 160 | 57 321 | .87 | Not tested |
| Infections, hospitalization rate (no.d) | ||||||
| All LRTI | 351 (338) | 304 (121) | 282 (218) | 227 (141) | .002 | .02 |
| Pneumonia LRTI | 238 (229) | 201 (80) | 185 (143) | 130 (81) | .007 | .006 |
| RSV-positive | 140 (135) | 118 (47) | 113 (87) | 93 (58) | .08 | .24 |
| Pneumonia RSV | 78 (75) | 63 (25) | 63 (49) | 51 (32) | .23 | .34 |
| Staphylococcus aureus infection, any | 13.8 (55) | 10.8 (43) | 2.7 (17) | 8.4 (46) | <.001 | <.002 |
| MRSA infection, any | 11.3 (45) | 7.3 (29) | 1.6 (10) | 5.5 (30) | <.001 | .01 |
| Hospitalized for skin infection | 12.8 (89) | 9.6 (45) | 4.8 (30) | 5.5 (30) | <.001 | .61 |
Notes. LRTI = lower respiratory tract infection; RSV = respiratory syncytial virus; MRSA = methicillin-resistant Staphylococcus aureus.
Trend among villages excluding largest town in region.
Largest town in region.
Averages are weighted by village population size.
Number is the total number of hospitalizations for that disease.
Hospitalization Rates and Water Service in Region A
Among the regions, the highest hospitalization rates for each of the diagnoses were among persons in region A (Table 2). In particular, pneumonia and influenza hospitalization rates among the region's infants (2550 per 10 000) were more than 2 times higher than the rates for any other region (Table 3). On average, more than 25% of the birth cohort was hospitalized with this diagnosis yearly.
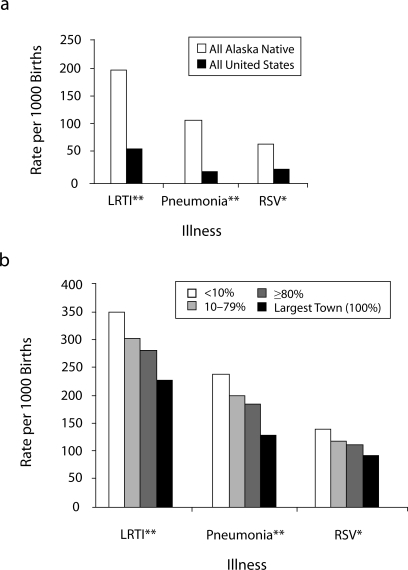
Hospitalization rates for infants with LRTI, pneumonia, and RSV were highest among infants in villages with the lowest level of in-home water service compared with those in other villages (Table 4). Also, we noted a trend of lower hospitalization rates for infants from villages with increasing proportions of homes served by in-home water service (Figure 1). This trend was highly significant for hospitalizations because of LRTI (P = .002) and LRTI with pneumonia (P = .007) and was present, but not statistically significant, for RSV infections and RSV pneumonia.
FIGURE 1.
Hospitalization rate among infants for lower respiratory tract infections (LRTI), pneumonia, and respiratory syncytial virus (RSV) in region A compared with all Alaska Native and US infants, by percentage of village homes with water service: Alaska, 1999–2004.
Note. Comparison rates for all Alaska Natives and all United States from references 17 and 18.
aRegion A's largest town had water service in almost all homes and was analyzed separately. *P = .08 for trend, region A; **P < .05 for trend, region A.
Relative hospitalization rates of infants from the lowest-service compared with those from the highest-service villages were as follows: LRTI (RR = 1.2; 95% CI = 1.1, 1.4), pneumonia (RR = 1.3; 95% CI = 1.1, 1.5), and RSV (RR = 1.2; 95% CI = 1.0, 1.6). These rate ratios were similar after adjustment for the number of household members. Compared with the overall US infant population, infants in the lowest-service villages had a 5-times-higher rate of both LRTI and RSV hospitalizations and an 11-times-higher hospitalization rate for pneumonia.
Region A had the highest rate of hospitalization for skin and soft tissue infections and for MRSA infections (Table 2). Within this region, we observed a significant trend of increased disease rates associated with lower levels of in-home water service for infections caused by S aureus, MRSA, and hospitalizations for skin infections (P < .001 for each; Table 4). The risk of skin infections was substantially higher among persons from villages with the least water service than for those villages with the highest water service for each of S aureus infections (RR = 5.1; 95% CI = 3.0, 8.7), MRSA infections (RR = 7.1; 95% CI = 3.6, 14.0), and skin infection hospitalizations (RR = 2.7; 95% CI = 1.8, 4.1; P < .001 for each comparison).
DISCUSSION
This is the first study to associate the absence of in-home water service with an increased risk of lower respiratory tract and skin infections among Alaska Natives. Using aggregated data from regions across Alaska, we found that hospitalization rates for pneumonia and influenza, skin and soft tissue infections, MRSA infections, and childhood RSV were 2 to 4 times higher in regions with a low proportion of homes with water service than in regions with a high proportion of homes with water service. Although suggestive, this relation was not entirely consistent and was influenced greatly by high hospitalization rates within region A. However, within region A, we undertook a closer look at disease rates by village-level water service and found that villages with the lowest level of water service (less than 10% of homes served) had the highest hospitalization rates for respiratory infections among infants and for skin and soft tissue infections among persons of all ages. The hospitalization rates demonstrated a typical dose–response group relation in which lower rates were related to progressively higher levels of in-home water service.
Because of the study design, these data fall short of establishing a causal relation between water service and infectious disease risks. However, the strength of the association, the dose–response group relation within region A, and the biological plausibility all support the conclusion that pressurized, in-home water service is an important determinant of health status and contributes to reducing transmission of these communicable diseases.
The infectious diarrheal hospitalization rate among Alaska Natives was similar to that among the general US population and did not differ significantly by water service.20,21 This may seem unexpected because high diarrheal disease rates are seen in other populations that lack in-home water and wastewater service. However, gastrointestinal disease morbidity and mortality among American Indian and Alaska Native populations has been declining since the 1950s.13,20 The current low rates are likely because of the availability of safe drinking water in nearly all villages (even those with no in-home water service); the relatively cold source water, which does not support propagation of waterborne bacterial pathogens; and the population's overall good nutritional status.
The diarrheal hospitalization rates were in stark contrast to the disparities noted for respiratory and skin infection rates in lower– water service villages. Particularly disturbing was the 5-times higher rate of LRTI hospitalizations and 11-times higher rate of pneumonia hospitalizations among infants in low-service villages in region A compared with the general US population.17,18 Because infant pneumonias in region A have been identified as a precursor to chronic respiratory diseases such as bronchiectasis and chronic productive cough, many of these children will likely have ongoing health problems because of these infections.22,23
Because respiratory and skin infections are not typically contracted through water, the higher rates in low-water-service villages may appear paradoxical. This is best explained by the important role water plays in preventing respiratory and skin infections through handwashing and other personal hygienic measures.24 It is known that the availability of pressurized, in-home water service increases both water consumption and hygiene practices.6,25 Thus, the availability of potable water appears to have stabilized waterborne disease rates in Alaska, but it is the water-washed diseases that remain health threats for villages lacking in-home water service. Our findings are consistent with other studies that have shown an association between handwashing and respiratory infectious diseases.7–9
Limitations
Some limitations should be considered when one interprets these data. Because of the study design, we cannot be certain that these associations arose from a cause-and-effect relationship. Water service may be a marker for other factors related to these health outcomes. When comparing regions, we could not control for factors such as income, village size, and crowding that might have confounded the associations. However, within region A, these characteristics were either similar across villages or were accounted for. The sanitation survey preceded some of the illness data; thus, some relevant water service improvements may not have been included. This could have led to overestimation of water service differences. In the region A analysis we accounted for this by removing villages that had received water service improvements over the study period. Finally, our study did not include data on outpatient respiratory or gastroenteric infections, personal hygiene practices, water quality, water quantity, or the different water delivery systems in use.
Conclusions and Recommendations
In 1954, Public Law 83-568 established the US Public Health Service Indian health program (later named the Indian Health Service) as responsible for improving the health of Alaska Native people. At that time, infectious diseases caused 46% of all Alaska Native deaths. Providing potable water and safe wastewater disposal services for Alaska Native communities was a primary objective.26
The IHS, along with the State of Alaska, US Environmental Protection Agency (EPA), US Department of Agriculture Rural Development Program, and Alaska's Tribal Health Organizations, has worked to increase the proportion of rural Alaska homes with modern sanitation service from less than 10% in 1950 to 84% in 2006 (W. Griffith, Village Safe Water Program, written communication, April 2006).
Sanitation improvements have been credited with contributing to the dramatic improvements in Alaska Natives’ health.4 However, substantial progress must be made to bring sanitation service in rural Alaska up to the modern standard enjoyed by 99.4% of the US population. The EPA has established the goal of providing modern sanitation services for 94% of rural Alaskan homes by 2011 (D. Wagner, Alaska EPA Drinking Water Program, written communication, April 2006). Even if this can be achieved, it will leave many rural Alaskans with substandard water and sanitation facilities.
Our study indicated that in-home water service is an important determinant of health in rural Alaska communities. Lower levels of water services were associated with a higher burden of hospitalizations for pneumonia and influenza, skin infections, and LRTIs. This finding was suggested by data in region-to-region analyses and is strongly supported by the village comparisons within region A.
These health disparities were borne disproportionately by Alaska Native infants, children, and elderly who resided in low-water-service villages. Of particular concern was that up to one fourth of region A infants were hospitalized annually for respiratory infections.
Further prospective studies could assess the relative contributions of hygienic practices, the volume of water used, and the water distribution system while accounting for potential confounding factors and the economic benefits of in-home water service for prevention of infectious diseases. Although those data would be helpful, we believe that the long-recognized value of in-home service along with the data from our study are convincing enough that programs should proceed with adequate support toward a goal of providing modern water and sanitation service to each home in rural Alaska villages.
Acknowledgments
We acknowledge Ryan DeLuche, Sue Ehrhart, and Edna Paisano of the Indian Health Service for their technical assistance; the staff who collected the Rural Alaska Housing Sanitation Inventory data; and the staff at the hospitals and clinics in the Alaska Native Health Services.
Human Participant Protection
Institutional review board approval for this study was obtained from the Centers for Disease Control and Prevention and the Alaska Area institutional review board of the Indian Health Service for the respiratory hospitalization data in region A. The hospital discharge administrative and disease outbreak data were determined to be exempt from review because they lacked patient identifiers and were obtained in a public health response to a disease outbreak, respectively.
Peer Reviewed
Note. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributors
T. W. Hennessy originated the study, supervised its conduct, conducted analyses, and was the principal writer. T. Ritter originated the study, analyzed water use data, and wrote sections of the article. R. C. Holman conducted analyses related to water use and regional hospitalization rates and assisted with writing. D. L. Bruden conducted analyses related to water use and disease rates within region A and assisted with writing. K. L. Yorita was involved in data preparation and analysis for water use and regional hospitalization rates and assisted with writing. L. Bulkow provided population data and oversaw the statistical analyses. J. E. Cheek originated the study. R. J. Singleton provided and analyzed data on respiratory hospitalization, assisted with writing, and participated in interpretation of the data. J. Smith was involved in the design and oversaw the water use data acquisition and analysis.
References
- 1.US Census Bureau. Census of Housing, 1950. Available at: http://www.census.gov/hhes/www/housing/census/historic/plumbing.html. Accessed January 27, 2008.
- 2.US Census Bureau. Census of Housing, 1970. Available at: http://www.census.gov/hhes/www/housing/census/historic/plumbing.html. Accessed January 27, 2008.
- 3.US Census Bureau. US Summary, 2000. Available at: http://www.census.gov/prod/2002pubs/c2kprof00-us.pdf. Accessed January 27, 2008.
- 4. Sanitation 2000: Water and Wastewater in Rural Alaska . Anchorage: Alaska Dept of Environmental Conservation, Village Safe Water Program; 2000 [Google Scholar]
- 5.Holman RC, Curns AT, Kaufman SF, Cheek JE, Pinner RW, Schonberger LB. Trends in infectious disease hospitalizations among American Indians and Alaska Natives. Am J Public Health 2001;91:425–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard G, Bartram J. Domestic Water Quantity, Service Level and Health . Geneva, Switzerland: World Health Organization; 2003:17–19 Available at: http://www.who.int/water_sanitation_health/diseases/WSH03.02.pdf. Accessed January 28, 2008 [Google Scholar]
- 7.Luby SP, Agboatwalla M, Feikin DR, et al. Effect of handwashing on child health: a randomised controlled trial. Lancet 2005;366:225–233 [DOI] [PubMed] [Google Scholar]
- 8.Ryan MA, Christian RS, Wohlrabe J. Handwashing and respiratory illness among young adults in military training. Am J Prev Med 2001;21:79–83 [DOI] [PubMed] [Google Scholar]
- 9.Fung IC, Cairncross S. Effectiveness of handwashing in preventing SARS: a review. Trop Med Int Health 2006;11:1749–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White GF, Bradley DJ, White AU. Drawers of Water: Domestic Water Use in East Africa . Chicago, IL: University of Chicago Press; 1972 [PMC free article] [PubMed] [Google Scholar]
- 11.Alaska Department of Labor and Workforce Development Census and Geographic Information Network, Research and Analysis Section . Available at: http://almis.labor.state.ak.us/?PAGEID=67&SUBID=114. Accessed October 24, 2005
- 12. Albuquerque, NM: Indian Health Service; 2005 [Google Scholar]
- 13. Rockville, MD: Indian Health Service; 2004 [Google Scholar]
- 14. Washington, DC: US Dept of Health and Human Services; 2005 [Google Scholar]
- 15.Kleinbaum DG, Kupper LL, Muller KE, Nizam A. Applied Regression Analysis and Other Multivariable Methods . Pacific Grove, CA: Duxbury Press; 1998 [Google Scholar]
- 16.Singleton RJ, Bruden D, Bulkow LR, Varney G, Butler JC. Decline in respiratory syncytial virus hospitalizations in a region with high hospitalization rates and prolonged season. Pediatr Infect Dis J 2006;25:1116–1122 [DOI] [PubMed] [Google Scholar]
- 17.Peck AJ, Holman RC, Curns AT, et al. Lower respiratory tract infections among American Indian and Alaska Native children and the general population of U.S. children. Pediatr Infect Dis J 2005;24:342–351 [DOI] [PubMed] [Google Scholar]
- 18.Holman RC, Curns AT, Cheek JE, et al. Respiratory syncytial virus hospitalizations among American Indian and Alaska Native infants and the general United States infant population. Pediatrics 2004;114:437–444 [DOI] [PubMed] [Google Scholar]
- 19.Baggett HC, Hennessy TW, Leman R, et al. An outbreak of community-onset methicillin-resistant Staphylococcus aureus skin infections in southwestern Alaska. Infect Control Hosp Epidemiol 2003;24:397–402 [DOI] [PubMed] [Google Scholar]
- 20.Holman RC, Parashar UD, Clarke MJ, Kaufman SF, Glass RI. Trends in diarrhea-associated hospitalizations among American Indian and Alaska Native children, 1980–1995. Pediatrics 1999;103:E11. [DOI] [PubMed] [Google Scholar]
- 21.Mounts AW, Holman RC, Clarke MJ, Bresee JS, Glass RI. Trends in hospitalizations associated with gastroenteritis among adults in the United States, 1979–1995. Epidemiol Infect 1999;123:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redding G, Singleton R, Lewis T, et al. Early radiographic and clinical features associated with bronchiectasis in children. Pediatr Pulmonol 2004;37:297–304 [DOI] [PubMed] [Google Scholar]
- 23.Singleton RJ, Redding GJ, Lewis TC, et al. Sequelae of severe respiratory syncytial virus infection in infancy and early childhood among Alaska Native children. Pediatrics 2003;112:285–290 [DOI] [PubMed] [Google Scholar]
- 24.Cairncross S, Valdmanis V. Water supply, sanitation and hygiene promotion. In: Jamison DT, Breman JG, Measham AR, et al., eds. Disease Control Priorities in Developing Countries 2nd ed.New York, NY: Oxford University Press; 2006:771–792 [Google Scholar]
- 25.Curtis V, Kanki B, Mertens T, et al. Potties, pits and pipes: explaining hygiene behavior in Burkina Faso. Soc Sci Med 1995;41:383–393 [DOI] [PubMed] [Google Scholar]
- 26.Alaska Dept of Health and Social Services Health Status in Alaska . Available at: http://www.hss.state.ak.us/dph/targets/PDFs/history2000.pdf. Accessed December 26, 2006