Abstract
The purpose of this study was to develop and test a novel culture model for studying fibroblast migration in 3-D collagen matrices.
Human corneal fibroblasts were seeded within dense, randomly oriented compressed collagen matrices. A 6 mm diameter button of this cell-seeded matrix was placed in the middle of an acellular, less dense outer collagen matrix. These constructs were cultured for 1, 3, 5 or 7 days in serum-free media, 10% fetal bovine serum (FBS), or 50 ng/ml PDGF. Constructs were then fixed and labeled with AlexaFluor 546 phalloidin (for f-actin) and TOTO-3 (for nuclei). Cell-matrix interactions were assessed using a combination of fluorescent and reflected light confocal imaging.
Human corneal fibroblasts in serum-free media showed minimal migration into the outer (non-compressed) matrix. In contrast, culture in serum or PDGF stimulated cell migration. Cell-induced collagen matrix reorganization in the outer matrix could be directly visualized using reflected light imaging, and was highest following culture in 10% FBS. Cellular contraction in 10% FBS often led to alignment of cells parallel to the outer edge of the inner matrix, similar to the pattern observed during corneal wound healing following incisional surgery.
Overall, this 3-D model allows the effects of different culture conditions on cell migration and matrix remodeling to be assessed simultaneously. In addition, the design allows for ECM density, geometry and mechanical constraints to be varied in a controlled fashion. These initial results demonstrate differences in cell and matrix patterning during migration in response to serum and PDGF.
Keywords: Extracellular Matrix, Corneal Fibroblasts, Cell Migration, Confocal Microscopy, PDGF
INTRODUCTION
Migration of activated corneal keratocytes (corneal fibroblasts) plays an important role in matrix patterning during developmental morphogenesis and is required for repopulation of wounded corneal tissue following injury or surgery (Jester et al. 1999; Netto et al. 2005). In some cases (e.g. following lacerating injury or incisional surgery), contractile force generation is needed to facilitate wound closure. In other circumstances (e.g. following refractive surgery), it is preferable to have corneal fibroblasts repopulate the wound space without remodeling the extracellular matrix or generating large forces, i.e. to assume a regenerative migratory phenotype as apposed to a contractile repair phenotype. Thus understanding how cell-matrix mechanical interactions are regulated during migration may facilitate the development of strategies to modulate key aspects of corneal healing in vivo.
While there are standard models available for measuring cell migration in 2-D culture, these models do not simulate the 3-D environment cells encounter in vivo. Furthermore, they do not allow assessment of cell-induced matrix reorganization, a key event in wound healing. In this study, we develop and test a novel experimental model for assessing the pattern and amount of cell migration within 3-D collagen matrices, as well as the differences in local matrix patterning produced by migrating cells in response to different culture conditions. In this model, a compressed collagen matrix containing cells (tissue equivalent) is placed within an acellular, lower density outer matrix. The pattern and amount of fibroblast migration from the inner to the outer matrix is assessed using fluorescent labeling of cells, and cell-induced matrix reorganization is evaluated using confocal reflection imaging. Since the inner matrix is generated by external compression (Brown et al. 2005), the cells are not required to be activated or contractile at the start of the experiment. In addition, the matrix geometry, cell numbers, and collagen density can be directly controlled. Overall, this model may provide a unique and flexible platform for investigating the migratory mechanics of fibroblasts and other cells following exposure to specific wound healing cytokines. Our initial results demonstrate differences in cell and matrix patterning during migration in response to serum (which activates Rho) and PDGF (which activates Rac).
MATERIALS & METHODS
Cell culture
A previously established, human corneal fibroblast (HTK) extended lifespan cell line was used in this study (Jester et al. 2003). HTK cells were maintained in serum-containing medium consisting of Dulbecco modified Eagle medium (DMEM; Gibco Invitrogen Cell Culture, Carlsbad, CA) supplemented with 1% penicillin/streptomycin/fungizone (BioWhittaker Inc, Walkersville, MD) and 10% fetal bovine serum (FBS; Sigma Chemical, St. Louis, MD).
Preparation of Nested Collagen Matrices
Nested collagen constructs were prepared using inner and outer matrices as described below.
Compressed Cellular Matrices (Inner matrices)
Rat tail type I collagen (10 ng/ml, BD Biosciences, San Jose, CA) was diluted using Acetic acid to final concentration of 3 mg/ml. 5.0 ml of the mixture was added to 0.5 ml of 10x MEM. After drop-wise neutralization with 1M sodium hydroxide, a suspension of 1 million HTK cells in 0.5 ml DMEM was added to the collagen mixture. The solution containing the cells and the collagen was poured into a 3×2×1cm well, and allowed to set for 1 hour at 37°C. Matrices were then compacted by a combination of compression and blotting using layers of mesh and paper sheets as previously described (Figure 1A) (Brown et al. 2005; Neel et al. 2006). Briefly, a 165μm thick stainless steel mesh (mesh size ∼ 300μm) and a layer of nylon mesh (∼50μm mesh size) were placed on a double layer of absorbent paper. The constructs were placed on the nylon mesh, covered with a second nylon mesh, and loaded with a 130g stainless steel block for 5 minutes at room temperature, leading to the formation of flat collagen sheet (∼150 μm thick). A 6 mm diameter “button” was cut out of this matrix using a trephine. In some experiments, the button was then placed in 0.5% 5-(4,6-dichlorotriazinyl) aminofluorescein (DTAF) in 0.2 M NaHCO3 for 1 minute to label the collagen. Pilot experiments demonstrated that DTAF had no effect on cell viability. All buttons were then washed in cell medium and placed inside the acellular matrix, described below.
Figure 1.
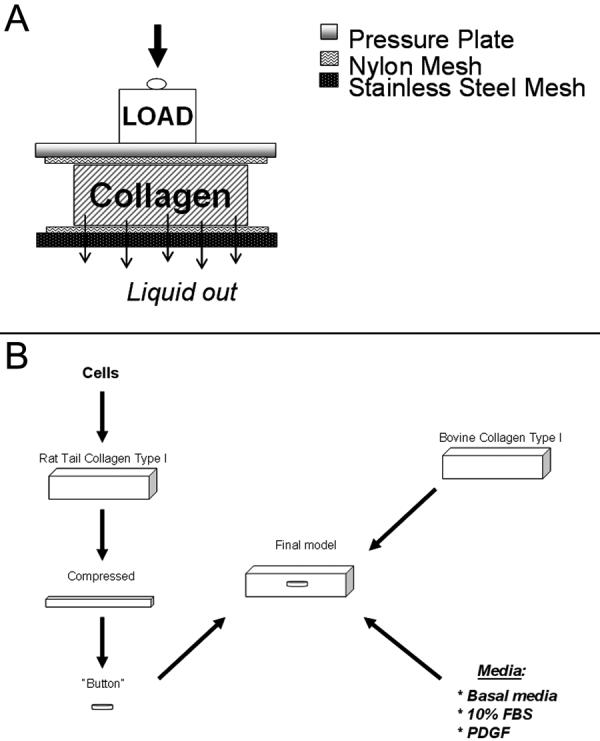
A. Illustration of the collagen compression procedure is shown. Cell-seeded collagen was placed between two nylon meshes and compressed using a load for 5 minutes, during which time liquid was allowed to exit through a stainless steel mesh at the bottom. B. Schematic of the process for constructing the migration model. Cells were seeded in rat tail collagen and compressed (as shown in A), and a 6 mm button was punched out and placed inside an acellular uncompressed matrix.
Acellular matrices (OUTER matrices)
Rectangular collagen constructs were prepared as described previously (Eastwood et al. 1998; Karamichos et al. 2007a; Mudera et al. 2000). Two ml of type I bovine dermal collagen (3 mg/ml, PureCol. Inamed Corp, Fremont, CA) was added to 0.2 ml of 10x MEM, neutralized with 1M sodium hydroxide, and poured into 4.4 × 2.0 cm Lab-Tek chambers (NUNC, Rochester, NY). Bovine collagen was used to allow imaging of matrix organization using confocal reflection microscopy. The compressed collagen button containing the cells was then placed in the middle of this acellular matrix (Figure 1B). Constructs were then allowed to set for 1 hour at 37°C. Serum-free media (basal media) was prepared using DMEM supplemented with 1% RPMI vitamin mix (Sigma-Aldrich, Saint Louis, MO), 100 μM nonessential amino acids (Invitrogen, Carlsbad, CA), 100 μg/mL ascorbic acid, and 1% penicillin/streptomycin/fungizone. Constructs were cultured in either basal media, basal media supplemented with 50ng/ml PDGF, or serum containing media (10% FBS). Two different mechanical constraints were used: 1) Attached (ATT) matrices in which constructs were covered with medium after 1 hour of incubation (to allow polymerization of the outer matrix), but remained attached to the bottom and sides of the well, and 2) unconstrained (UN) matrices in which constructs were released from the bottom of the well after polymerization, and allowed to float in medium.
F-Actin and DNA staining
At the end of each experiment (1, 3, 5, or 7 days) constructs were fixed in 4% formaldehyde for 1 hour and permeabilized with 0.5% Triton X-100 in phosphate buffer for 10 minutes. Cells were then incubated in phalloidin for 1 hour (1:50 dilution, Alexa Fluor 546, Molecular Probes, Eugene, OR) and then washed in PBS (3 times for 5 minutes). TOTO-3 iodide (1:1000; Molecular Probes) was then added to each construct to stain the cell nuclei. Constructs were incubated for 15 minutes and washed with PBS (3 times for 5 minutes).
Laser Confocal Microscopy
After staining, buttons were mounted onto 25 μm thick, 60 mm diameter, mylar petri dishes (Bachofer, Hamburg,Germany) for imaging. Fluorescent imaging was used for DTAF, F-actin and TOTO-3 whereas reflected light was used for collagen fibril imaging. Sequential scanning was used to prevent crosstalk between fluorophores. 3-D optical stacks (z-series) were acquired using confocal microscopy using both a 20x dry objective (2 μm steps) and a 63x water immersion objective (1μm steps). Overlapping stacks of images were collected using the 20X objective, from the edge of the inner matrix until the last cell was reached (Fig. 2). This was repeated for up to four different quadrants (north, east, south, west) for each construct. The use of the mylar Petri dishes and the fact that the inner matrices were placed near the bottom of the outer matrices (< 500 μm) insured that there was sufficient working distance to reach all of the migrating cells with the 20X objective in most cases.
Figure 2.
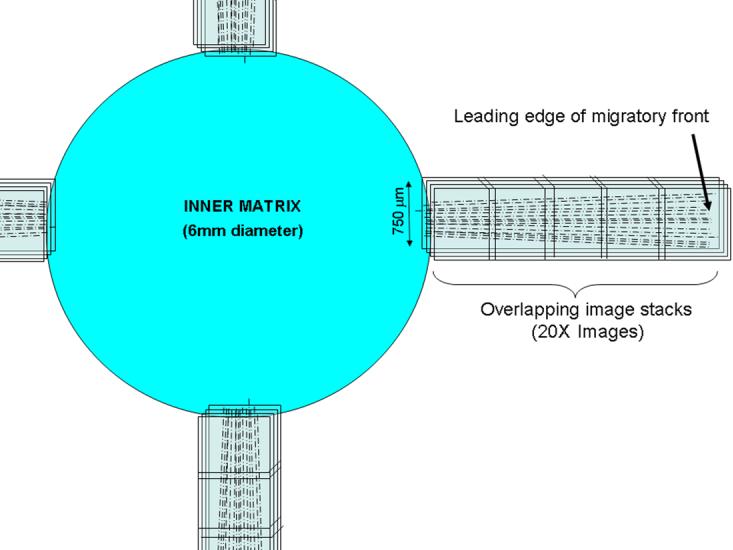
Schematic showing the pattern of 3-D image stacks collected in each sample using laser confocal microscopy.
Maximum intensity projection (MIP) images of phalloidin and TOTO-3 image stacks were created using Metamorph. Photoshop was then used to align overlapping MIP images, resulting in a 750 μm wide montage image for each quadrant. These images were used to measure the distance of the leading edge cells from the inner-outer matrix interface. These were the cells that had traveled the farthest from the interface; a straight line between the leading cell end and the interface was used for this distance measurement. To assess cell-induced matrix reorganization, 63X image stacks were collected both near the edge of the inner matrix, and at the leading edge of the migratory front.
Statistical analyses
Statistical analyses were performed using Sigmastat (version 3.1.1; Systat Software Inc., Point Richmont, CA). ANOVA was used to compare group means and differences were considered significant when P< 0.05.
RESULTS
Effect of Culture Conditions on the Pattern and Amount of Cell Migration
As a model for assessing cell migration, corneal fibroblasts were embedded in a compressed inner matrix, which was placed within in an uncompressed acellular outer matrix. At 24 hours, cells were observed at the edge of the inner matrix with only occasional pseudopodia partially extending into the outer matrix. Migration of entire cells into the outer matrix was not generally observed until 2-3 days after plating these constructs (Figure 3A-C). The edge of the inner matrix was easily discernable using confocal reflection imaging, as confirmed by using DTAF to label the collagen in the inner matrix. In some cases, the edge of the inner matrix was folded, and/or a gap was detected between the inner and outer matrices (these samples were excluded); however, in most cases the mechanical integrity of the constructs was maintained.
Figure 3.
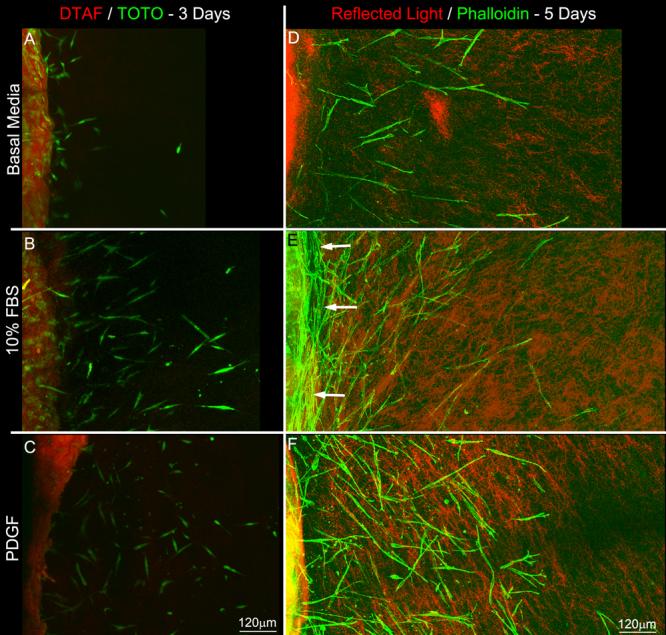
Maximum intensity projection images showing cell migration into the outer matrix. A-C) DTAF and TOTO-3 overlays collected after 3 days of culture in basal media (A), 10% FBS (B), and PDGF (C). D-F) Reflected light and phalloidin overlays collected after 5 days of culture in basal media (D), 10% FBS (E), and PDGF (F). Both serum and PDGF stimulated cell migration from the inner (left) into the outer (right) matrix. Note the alignment of cells along the inner-outer matrix interface after 5 days of culture in 10% FBS (E, arrows).
By 5 days, there were striking differences in the number of cells in the outer matrix, as well as the distance they traveled under different culture conditions (Figure 3D-F). Corneal fibroblasts in serum-free media showed minimal migration into the outer matrix. In contrast, culture in serum or PDGF stimulated cell migration. This increase was even more pronounced after 7 days (Figure 4). These responses were observed in both the ATT and UN models.
Figure 4.
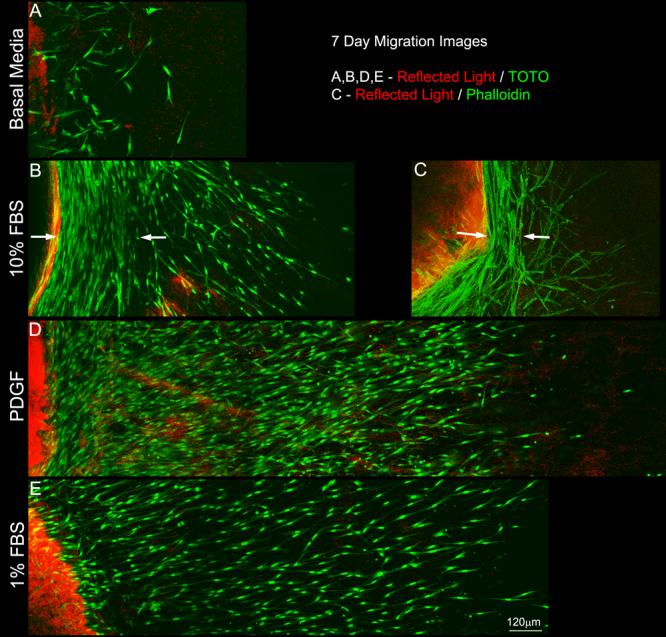
Maximum intensity projection images collected after 7 days of culture in basal media (A), 10% FBS (B and C), PDGF (D) and 1% FBS (E). Both serum and PDGF stimulated cell migration from the inner (left) into the outer (right) matrix. Note the dramatic alignment of cells along the inner-outer matrix interface following culture in 10%FBS (B and C, arrows), but not 1% FBS or PDGF..
We often observed cells aligned parallel with the outer edge of the inner matrix following culture in 10% FBS. This alignment was first observed at 5 days (Fig. 3E, arrows) and was more pronounced at 7 days (Figure 4B and C, arrows) in both ATT and UN models. These cells were on the outside edge of the inner matrix; thus they had already migrated into the uncompressed outer matrix. In contrast, we did not observe any consistent pattern of alignment of cells within the inner compressed matrix. In order to determine whether this effect was dependent on serum concentration, we performed 7 day experiments using 1% FBS. 1% FBS stimulated cell migration (Figure 4E), but most cells remained oriented perpendicular to the edge of the inner matrix, similar to PDGF.
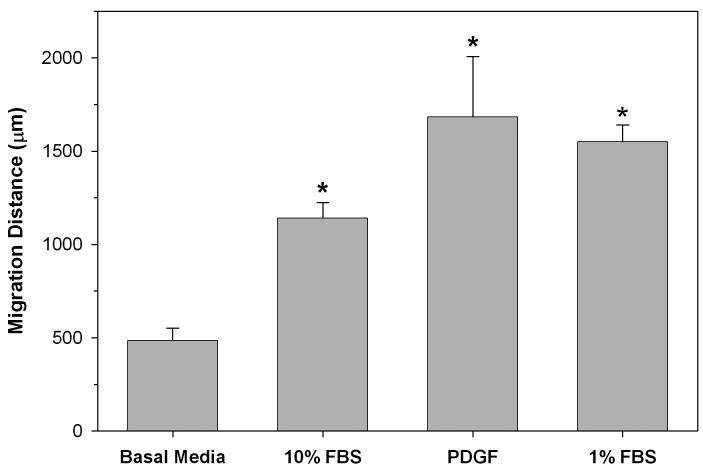
Figure 5 shows the average distance of the leading edge of cell migration from the edge of the inner matrix after 7 days of migration. Cells cultured in 1% FBS, 10% FBS and PDGF migrated significantly farther (p≤0.05) than those in basal media.
Figure 5.
Quantitative analysis of the distance cells traveled into the outer matrix under different culture conditions. The distances were measured from the interface to the leading edge of the migrating front.
Matrix Reorganization Produced During Cell Migration
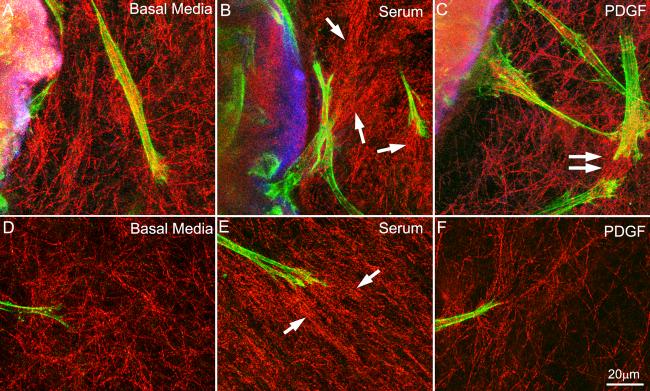
Local cell-matrix interactions were assessed using high magnification confocal reflection imaging at two different sites within the constructs: 1) At the interface between the inner and outer matrices, and 2) at the leading edge of the migration front. Figure 6 shows the cell-matrix interactions for all three conditions after 3 days in culture. Morphologically, cells under all conditions were generally bipolar and had pseudopodial processes, characteristics typical of corneal fibroblasts in 3-D matrices. At the interface between the inner and outer matrices, some compaction and alignment of ECM was observed under all conditions studied. However, the largest amount of cell-induced collagen reorganization was consistently observed following culture in media containing 10% FBS (Figure 6B, arrows), as compared to basal media (Figure 6A) and media containing PDGF (Figure 6C). Under all conditions, increased matrix alignment was often observed between neighboring cells (Figure 6C, double arrow), suggesting cell-cell mechanical enhancement of ECM reorganization. Cell-induced matrix organization was also analyzed at the furthest point from the inner matrix, i.e. at the leading edge. Increased matrix reorganization was again observed following culture in 10% FBS (Figure 6E, arrows), as compared with culture in basal media or PDGF. By 5 days, the matrix was often compacted to a point where individual collagen fibrils could not be easily distinguished using confocal reflection imaging, particularly under 10% FBS conditions.
Figure 6.
Color overlays of fluorescent images of phalloidin (green) and DTAF (blue), and confocal reflection images of collagen fibrils (red) following 3 days of culture in basal media (A and D), 10% FBS (B and E) and PDGF (C and F). A-C) At the interface between the inner and outer matrices, some compaction and alignment of ECM was observed under all conditions studied. However, the largest amount of cell-induced collagen reorganization was consistently observed following culture in media containing 10% FBS (B, arrows). D-F) Cells located near the furthest point from the inner matrix (i.e the leading edge). Increased matrix reorganization was again observed following culture in 10% FBS constructs (E, arrows), as compared with culture in basal media or PDGF. Under all conditions, increased matrix alignment was often observed between neighboring cells (for example C, double arrow), suggesting cell-cell mechanical enhancement of ECM reorganization.
Visual inspection of the unconstrained constructs revealed that following 5-7 days of culture in 10%FBS the outer matrix was often contracted and reduced in size along both the x and y axes. In contrast matrix size reduction was not observed following culture in basal media or PDGF. In the attached constructs, cells were often able to pull the matrix from the sides of the wall across the x-axis after 7 days of culture in 10%FBS, and create a visible “waist”. These samples were sometimes wrinkled and distorted along the bottom, suggesting partial detachment along this plane as well.
DISCUSSION
Stromal keratocytes play a central role in mediating the corneal response to lacerating injury or refractive surgery (Netto et al. 2005). During wound healing, quiescent corneal keratocytes differentiate into fibroblast and/or myofibroblast phenotypes that mediate cell migration, wound contraction and matrix remodeling (Jester et al. 1999). While there are standard models available for measuring the effects of growth factors on the rate of cell migration in 2-D culture, models for assessing cell migration within 3-D matrices are more limited. One approach is to embed tissue explants within acellular collagen matrices, and study cells as they migrate out (Sawhney and Howard 2002; Stopak and Harris 1982). However, the corneal stroma is maintained in a highly dehydrated state in vivo, and swells dramatically when explanted; thus this technique is problematic when using corneal explants and is highly dependent upon the source of the tissue. Another approach is to place cells on the surface of collagen matrices and measure their movement into the interior (Andresen et al. 2007; Andresen et al. 2000; Sabeth et al. 2004; Schor 1980), but this does not simulate the 3-D geometry of wound healing.
More recently, Grinnell and coworkers used a nested matrix approach for studying 3-D cell migration (Greiling and Clark 1997). In this model, human foreskin fibroblasts are stimulated to contract a collagen matrix, and this pre-contracted matrix is placed within a second acellular collagen matrix (Grinnell et al. 2006). Fibroblast migration into the outer matrix is then followed over time. This elegant model has provided novel insights into the regulation of dermal fibroblast migration by specific cytokines and mechanical signals (Grinnell et al. 2006; Jiang et al. 2008; Miron-Mendoza et al. 2008). However, since our ultimate goal is to study the transformation and migratory response of quiescent (i.e. non-contractile) corneal keratocytes to specific wound healing cytokines, we needed a model that did not require cellular pre-contraction of the inner matrix. The compressed collagen matrix model was selected because collagen is compacted by using external compression (Brown et al. 2005), thus the cells are not required to be contractile or activated at the start of the experiment. Furthermore, compressed matrices support differentiation of both activated fibroblasts and quiescent keratocytes (unpublished observation), and have a collagen concentration and mechanical stiffness which are similar to that of native corneal tissue (Neel et al. 2006). We also used a cell density in the compressed matrices (∼11,000 cells/mm3) that is of the same order as that in the human corneal stroma in vivo (Patel et al. 2001). Thus in this study, the compressed inner matrices were engineered to simulate key properties of in vivo corneal tissue. However, a key feature of our approach is that the cell density, geometry, and collagen density (of both the inner and outer matrices) can all be modulated during preparation in order to mimic the properties of other tissue types.
In our model, cell migration into the outer matrix was not generally observed until 2-3 days after plating the constructs. During this lag phase, cells were observed at the edge of the inner matrix with only occasional pseudopodia partially extending into the outer matrix. It is possible that this delay is due to the large difference in mechanical stiffness between the inner and outer matrices, since cells generally prefer to migrate from soft to stiff environments (Lo et al. 2000). It may also be related to the high density of collagen within the compacted inner matrix, through which the cells must migrate in order to reach the outer matrix. While the origins of this delay are currently unclear, it should be noted that a similar lag phase is observed during corneal wound healing in vivo, in which cells also migrate out of a mechanically stiff ECM (Jester et al. 1995; Jester et al. 1999). Further studies in which the cell and/or collagen density are varied in our model may clarify our understanding of this phenomenon. One advantage nesting a pre-contracted matrix is that there is a much shorter lag phase (8-16 hours) than in our model (Grinnell et al. 2006). This may be due to the activated state of the cells following contraction, or differences in overall matrix geometry and mechanical properties.
By 5 days, we found striking differences in the number of cells in the outer matrix as well as the distance they traveled under different culture conditions. Both serum and PDGF stimulated significant cell migration into the outer matrix, and this was even more pronounced after 7 days. PDGF is expressed in the human cornea, and stimulates migration of corneal fibroblasts plated on rigid substrates or on top of collagen matrices (Andresen et al. 2007; Andresen et al. 2000; Kim et al. 1999). In 3-D culture, PDGF activates Rac and stimulates corneal fibroblast spreading in single collagen matrices (Petroll et al. 2008a), as well as dermal fibroblast migration in nested collagen matrices (Grinnell et al. 2006). PDGF also stimulates proliferation of corneal fibroblasts (Kim et al. 1999), thus the number of cells observed in the outer matrix at five and seven days likely reflects a combination of both migratory and proliferative responses. Proliferation of corneal fibroblasts also occurs during in vivo wound healing (Jester et al. 1999; Netto et al. 2005).
While significant migration was also observed following culture in 10% FBS, important differences in the pattern of cell alignment were observed. Cells in 10% FBS tended to align parallel to the outer edge of the inner matrix beginning at five days; whereas cells cultured in PDGF were aligned perpendicular to the edge of the inner matrix. This alignment was even more pronounced after seven days. Interestingly, a similar pattern of cell alignment is observed during corneal wound contraction following incisional surgery (Petroll et al. 1993; Petroll et al. 1998); in these studies a force-balance analysis predicted that as cells generate contractile forces they pull themselves into alignment with the wound edge, due to its increased rigidity as compared to the ECM within the wound space. While serum contains several pro-migratory growth factors (including PDGF), it also contains factors such as lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P) which stimulate cell contractility through activation of the Rho/Rho Kinase pathway (Grinnell 2000; Jiang et al. 2008; Petroll et al. 2008b). Thus the shift in cell alignment observed at five and seven days may reflect a transition from a Rac-induced migratory phenotype to a Rho-induced contractile phenotype. In order to determine whether this effect was dependent on serum concentration, we performed 7 day experiments using 1% FBS. 1% FBS stimulated cell migration, but most cells remained oriented perpendicular to the edge of the inner matrix, similar to PDGF. Thus the balance between Rho and Rac activation appears to change as the serum concentration is increased, with Rho predominating at higher concentrations.
To directly assess cell-induced matrix reorganization during migration, confocal reflection imaging was used (Friedl and Brocker 2000; Friedl et al. 1997). After 3 days, some compaction and alignment of ECM was observed under all conditions studied. However, the largest amount of cell-induced collagen reorganization was consistently observed following culture in media containing 10% FBS, as compared to basal media or PDGF. The residual matrix reorganization observed following culture in serum-free media or PDGF is most likely due to a basal level of Rho-kinase activity in this corneal fibroblast cell line (Petroll et al. 2008a), that would not be expected in quiescent corneal keratocytes (Jester and Chang 2003).
It is important to note that visual inspection of the unconstrained constructs revealed that following 5-7 days of culture in 10% FBS the outer matrix was often contracted and reduced in size. In contrast matrix size reduction was not observed following culture in basal media or PDGF. In the attached constructs, cells were often able to detach the matrix from the sides of the wall across the x-axis after seven days of culture in 10% FBS, and create a visual “waist”. A similar waist has been reported in partially restrained matrices using contractile cells (Brown et al. 1998; Eastwood et al. 1998; Karamichos et al. 2007b).
Taken together, our results indicate that there is increased cell contractility and cell-induced matrix reorganization during migration in the presence of 10% FBS as compared to culture in PDGF. Thus PDGF may be a candidate for stimulating wound healing following refractive surgery in vivo, where it is preferable to have corneal fibroblasts repopulate the wound space without remodeling the extracellular matrix or generating large contractile forces; both of which can alter corneal clarity and/or refractive power.
In nested collagen matrices, dramatic differences in dermal fibroblast migration have been observed between ATT and UN conditions (Miron-Mendoza et al. 2008). In the current study, however, a similar pattern and amount of cell migration was observed under both conditions. The reason behind this is not entirely clear, but is likely related to the fact that the distance from matrix attachment points to the edge of the inner matrix was relatively large in our model, and thus the constraints may have little impact on the effective mechanical stiffness initially encountered by the cells. Furthermore, the collagen density of the outer matrix was higher in the current study (2.5 mg/ml vs. 1.5 mg/ml), providing increased rigidity even under UN conditions. Further investigation is clearly required to clarify the role of these and other variables on the mechanics of cell migration by varying these parameters in our model.
Acknowledgments
This study was supported in part by NIH EY013322, NIH infrastructure grant EY016664, and an unrestricted grant and Senior Scientific Investigator Award (WMP) from Research to Prevent Blindness, Inc., NY, NY.
REFERENCES
- Andresen JL, Ledet T, Ehlers N. Keratocyte migration and peptide growth factors: the effect of PDGF, bFGF, EGF, IGF-1, aFGF and TGF-beta on human keratocyte migration in a collagen gel. Curr Eye Res. 2007;16:605–613. doi: 10.1076/ceyr.16.6.605.5081. [DOI] [PubMed] [Google Scholar]
- Andresen JL, Ledet T, Hager H, Josephsen K, Ehlers N. The influence of corneal stromal matrix proteins on the migration of human corneal fibroblasts. Exp Eye Res. 2000;71:33–43. doi: 10.1006/exer.2000.0850. [DOI] [PubMed] [Google Scholar]
- Brown RA, Prajapati R, McGrouther DA, Yannas IV, Eastwood M. Tensional homeostasis in dermal fibroblasts: mechanical responses to mechanical loading in three-dimensional substrates. J. Cell. Physiol. 1998;175:323–332. doi: 10.1002/(SICI)1097-4652(199806)175:3<323::AID-JCP10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Brown RA, Wiseman M, Chuo C-B, Cheema U, Nazhat SN. Ultrarapid engineering of biomimetic materials and tissues: fabrication of nano- and microstructures by plastic compression. Adv Funct Mater. 2005;15:1762–1770. [Google Scholar]
- Eastwood M, Mudera VC, McGrouther DA, Brown RA. Effect of prcise mechanical loading on fibroblast populated collagen lattices: Morphological changes. Cell motil cytoskeleton. 1998;40:13–21. doi: 10.1002/(SICI)1097-0169(1998)40:1<13::AID-CM2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Friedl P, Brocker E-B. The biology of cell locomotion within three-dimensional extracellular matrix. Cell Mol Life Sci. 2000;57:41–64. doi: 10.1007/s000180050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Maaser K, Klein CE, Niggemann B, Krohne G, Zanker KS. Migration of highly aggressive MV3 melanoma cells in 3-dimensional collagen lattices results in local matrix reorganization and shedding of alpha2 and beta1 integrins and CD44. Cancer Res. 1997;57:2061–2070. [PubMed] [Google Scholar]
- Greiling D, Clark RA. Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci. 1997;100:861–870. doi: 10.1242/jcs.110.7.861. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast-collagen matrix contraction: growth-factor signalling and mechanical loading. Trends. Cell Biol. 2000;10:362–365. doi: 10.1016/s0962-8924(00)01802-x. [DOI] [PubMed] [Google Scholar]
- Grinnell F, Rocha LB, Iucu C, Rhee S, Jiang H. Nested collagen matrices: A new model to study migration of human fibroblast populations in three dimensions. Exp Cell Res. 2006;312:86–94. doi: 10.1016/j.yexcr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Jester JV, Chang J-H. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77:581–592. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- Jester JV, Huang J, Fisher S, Spiekerman J, Chang JH, Wright WE, Shay JW. Myofibroblast differentiation of normal human keratocytes and hTERT, extended-life, human corneal fibroblasts. Invest Ophthal Vis Sci. 2003;44:1850–1858. doi: 10.1167/iovs.02-0973. [DOI] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Barry PA, Cavanagh HD. Temporal, 3-dimensional, cellular anatomy of corneal wound tissue. J. Anat. 1995;186:301–311. [PMC free article] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of the myofibroblast. Prog Retinal Eye Res. 1999;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- Jiang H, Rhee S, Ho C-H, Grinnell F. Distinguishing fibroblst promigratory and procontractile growth factor environments in 3-D collagen matrices. FASEB J. 2008;22:2151–2160. doi: 10.1096/fj.07-097014. [DOI] [PubMed] [Google Scholar]
- Karamichos D, Brown RA, Mudera V. Complex dependence of substrate stiffness and serum concentration on cell-force generation. J Biomed Mater Res A. 2007a;78:407–415. doi: 10.1002/jbm.a.30814. [DOI] [PubMed] [Google Scholar]
- Karamichos D, Lakshman N, Petroll WM. Extracellular matrix mechanical properties regulate corneal fibroblast morphology and collagen remodeling in 3-D culture. Invest Ophthalmol Vis Sci. 2007b;48:5030–5037. doi: 10.1167/iovs.07-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W-J, Mohan RR, Mohan RR, Wilson SE. Effect of PDGF, IL-1α, and BMP2/4 on corneal fibroblast chemotaxis: expression of the platelet-derived growht factor system in the cornea. Invest Ophthalmol Vis Sci. 1999;40:1364–1372. [PubMed] [Google Scholar]
- Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophysical Journal. 2000;79(1):144–52. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron-Mendoza M, Seemann J, Grinnell F. Collagen fibril flow and tissue translocation coupled to fibroblast migration in 3D collagen matrices. Mol Biol Cell. 2008;19:2051–2058. doi: 10.1091/mbc.E07-09-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudera VC, Pleass R, Eastwood M, Tarnuzzer R, Schultz G, Khaw P, McGrouther DA, Brown RA. Molecular responses of human dermal fibroblasts to dual cues: contact guidance and mechanical load. Cell motil cytoskeleton. 2000;45:1–9. doi: 10.1002/(SICI)1097-0169(200001)45:1<1::AID-CM1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Neel EAA, Cheema U, Knowles JC, Brown RA, Nazhat SN. Use of multiple unconfined compression for control of collagen gel scaffold density and mechanical properties. Soft Matter. 2006;2:986–992. doi: 10.1039/b609784g. [DOI] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Ambrosio R, Hutcheon AEK, Zieske JD, Wilson SE. Wound healing in the cornea: A review of refractive surgery complications and new prospects for therapy. Cornea. 2005;24:509–522. doi: 10.1097/01.ico.0000151544.23360.17. [DOI] [PubMed] [Google Scholar]
- Patel SV, McLaren JW, Hodge DO, Bourne WM. Normal human keratocyte density and corneal thickness measurement by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci. 2001;42:333–339. [PubMed] [Google Scholar]
- Petroll WM, Cavanagh HD, Barry-Lane P, Andrews P, Jester JV. Quantitative analysis of stress fiber orientation during corneal wound contraction. J. Cell Sci. 1993;104:353–363. doi: 10.1242/jcs.104.2.353. [DOI] [PubMed] [Google Scholar]
- Petroll WM, Cavanagh HD, Jester JV. Assessment of stress fiber orientation during healing of radial keratotomy wounds using confocal microscopy. Scanning. 1998;20:74–82. doi: 10.1002/sca.1998.4950200202. [DOI] [PubMed] [Google Scholar]
- Petroll WM, Ma L, Kim A, Ly L, Vishwanath M. Dynamic assessment of fibroblast mechanical activity during Rac-induced cell spreading in 3-D culture. J Cell Physiol. 2008a;217:162–171. doi: 10.1002/jcp.21487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroll WM, Ma L, Ly L, Vishwanath M. Analysis of the pattern of sub-cellular force generation by corneal fibroblasts following Rho activation. Eye Contact Lens. 2008b;34:65–70. doi: 10.1097/ICL.0b013e3181580d5b. [DOI] [PubMed] [Google Scholar]
- Sabeth F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MTI-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawhney RK, Howard J. Slow local movements of collagen fibers by fibroblasts drive the rapid global self-organization of collagen gels. J Cell Biol. 2002;157:1083–1091. doi: 10.1083/jcb.200203069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor SL. Cell proliferation and migration on collagen substrata in vitro. J Cell Sci. 1980;41:159–175. doi: 10.1242/jcs.41.1.159. [DOI] [PubMed] [Google Scholar]
- Stopak D, Harris AK. Connective tissue morphogenesis by fibroblast traction. Dev Biol. 1982;90:383–398. doi: 10.1016/0012-1606(82)90388-8. [DOI] [PubMed] [Google Scholar]