Abstract
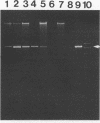
Proliferative bowel disease is an intestinal disorder of a variety of domestic animals associated with the presence of an intracellular Campylobacter-like organism (ICLO). We have identified the ICLO obtained from a ferret with proliferative colitis by 16S rRNA sequence analysis. In this ferret, proliferative bowel tissue containing the ICLO had translocated to the mesenteric lymph nodes, omentum, and liver. The 16S rRNA genes of the ICLO were amplified from an infected fragment of extraintestinal tissue by using universal prokaryotic primers. Approximately 1,480 bases of the amplified 16S rRNA gene were sequenced by cycle sequencing. Comparison of the sequence of the ICLO with those of over 400 bacteria in our data base indicated that the sequence of the ICLO was most closely related to that of Desulfovibrio desulfuricans (87.5% similarity). Phylogenetic analysis with 12 Desulfovibrio species and 20 species from related genera placed the ICLO in a subcluster within the genus Desulfovibrio with D. desulfuricans and 5 other Desulfovibrio species. We will refer to this organism as the intracellular Desulfovibrio organism (IDO). Specific primers were produced for PCR amplification of a 550-base fragment of the 16S rRNA gene of the IDO in proliferative intestinal tissue samples. This unique 550-base segment was amplified from samples of frozen intestinal tissue from nine ferrets and three hamsters with ICLO-associated disease but not in four intestinal tissue samples from animals without the ICLO-associated disease. The 550-base amplified products from the bowel tissues of one hamster and one ferret were fully sequenced. The ferret IDO partial sequence was identical to the previously determined 16S rRNA sequence over its length, and the hamster IDO sequence differed by a single base. The same intracellular organism has been identified in proliferative intestinal tissues of swine and that the organism has been successfully maintained in tissue culture. The availability of specific primers for PCR-based detection of this intracellular Desulfovibrio organism will aid in the determination of its role in the pathogenesis of proliferative bowel disease in a variety of infected hosts.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beerens H., Romond C. Sulfate-reducing anaerobic bacteria in human feces. Am J Clin Nutr. 1977 Nov;30(11):1770–1776. doi: 10.1093/ajcn/30.11.1770. [DOI] [PubMed] [Google Scholar]
- Biester H. E., Schwarte L. H. Intestinal Adenoma in Swine. Am J Pathol. 1931 Mar;7(2):175–185.6. [PMC free article] [PubMed] [Google Scholar]
- Devereux R., Delaney M., Widdel F., Stahl D. A. Natural relationships among sulfate-reducing eubacteria. J Bacteriol. 1989 Dec;171(12):6689–6695. doi: 10.1128/jb.171.12.6689-6695.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux R., He S. H., Doyle C. L., Orkland S., Stahl D. A., LeGall J., Whitman W. B. Diversity and origin of Desulfovibrio species: phylogenetic definition of a family. J Bacteriol. 1990 Jul;172(7):3609–3619. doi: 10.1128/jb.172.7.3609-3619.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhamel G. E., Wheeldon E. B. Intestinal adenomatosis in a foal. Vet Pathol. 1982 Jul;19(4):447–450. doi: 10.1177/030098588201900410. [DOI] [PubMed] [Google Scholar]
- Elwell M. R., Chapman A. L., Frenkel J. K. Duodenal hyperplasia in a guinea pig. Vet Pathol. 1981 Jan;18(1):136–139. doi: 10.1177/030098588101800120. [DOI] [PubMed] [Google Scholar]
- Fox J. G., Lawson G. H. Campylobacter-like omega intracellular antigen in proliferative colitis of ferrets. Lab Anim Sci. 1988 Feb;38(1):34–36. [PubMed] [Google Scholar]
- Fox J. G., Murphy J. C., Ackerman J. I., Prostak K. S., Gallagher C. A., Rambow V. J. Proliferative colitis in ferrets. Am J Vet Res. 1982 May;43(5):858–864. [PubMed] [Google Scholar]
- Fox J. G., Murphy J. C., Otto G., Pecquet-Goad M. E., Lawson G. H., Scott J. A. Proliferative colitis in ferrets: epithelial dysplasia and translocation. Vet Pathol. 1989 Nov;26(6):515–517. doi: 10.1177/030098588902600610. [DOI] [PubMed] [Google Scholar]
- Fox J. G., Stills H. F., Paster B. J., Dewhirst F. E., Yan L., Palley L., Prostak K. Antigenic specificity and morphologic characteristics of Chlamydia trachomatis, strain SFPD, isolated from hamsters with proliferative ileitis. Lab Anim Sci. 1993 Oct;43(5):405–410. [PubMed] [Google Scholar]
- Gebhart C. J., Barns S. M., McOrist S., Lin G. F., Lawson G. H. Ileal symbiont intracellularis, an obligate intracellular bacterium of porcine intestines showing a relationship to Desulfovibrio species. Int J Syst Bacteriol. 1993 Jul;43(3):533–538. doi: 10.1099/00207713-43-3-533. [DOI] [PubMed] [Google Scholar]
- Gibson G. R., Cummings J. H., Macfarlane G. T., Allison C., Segal I., Vorster H. H., Walker A. R. Alternative pathways for hydrogen disposal during fermentation in the human colon. Gut. 1990 Jun;31(6):679–683. doi: 10.1136/gut.31.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. R., Cummings J. H., Macfarlane G. T. Competition for hydrogen between sulphate-reducing bacteria and methanogenic bacteria from the human large intestine. J Appl Bacteriol. 1988 Sep;65(3):241–247. doi: 10.1111/j.1365-2672.1988.tb01891.x. [DOI] [PubMed] [Google Scholar]
- Gibson G. R., Cummings J. H., Macfarlane G. T. Use of a three-stage continuous culture system to study the effect of mucin on dissimilatory sulfate reduction and methanogenesis by mixed populations of human gut bacteria. Appl Environ Microbiol. 1988 Nov;54(11):2750–2755. doi: 10.1128/aem.54.11.2750-2755.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni S. J., Britschgi T. B., Moyer C. L., Field K. G. Genetic diversity in Sargasso Sea bacterioplankton. Nature. 1990 May 3;345(6270):60–63. doi: 10.1038/345060a0. [DOI] [PubMed] [Google Scholar]
- Hirono I., Kuhara K., Hosaka S., Tomizawa S., Golberg L. Induction of intestinal tumors in rats by dextran sulfate sodium. J Natl Cancer Inst. 1981 Mar;66(3):579–583. [PubMed] [Google Scholar]
- Howard B. H., Hungate R. E. Desulfovibrio of the sheep rumen. Appl Environ Microbiol. 1976 Oct;32(4):598–602. doi: 10.1128/aem.32.4.598-602.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby R. O., Johnson E. A. Transmissible ileal hyperplasia. Adv Exp Med Biol. 1981;134:267–289. doi: 10.1007/978-1-4757-0495-2_25. [DOI] [PubMed] [Google Scholar]
- Kusukawa N., Uemori T., Asada K., Kato I. Rapid and reliable protocol for direct sequencing of material amplified by the polymerase chain reaction. Biotechniques. 1990 Jul;9(1):66-8, 70, 72. [PubMed] [Google Scholar]
- Lawson G. H., McOrist S., Jasni S., Mackie R. A. Intracellular bacteria of porcine proliferative enteropathy: cultivation and maintenance in vitro. J Clin Microbiol. 1993 May;31(5):1136–1142. doi: 10.1128/jcm.31.5.1136-1142.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson G. H., Rowland A. C., MacIntyre N. Demonstration of a new intracellular antigen in porcine intestinal adenomatosis and hamster proliferative ileitis. Vet Microbiol. 1985 Jun;10(4):303–313. doi: 10.1016/0378-1135(85)90001-x. [DOI] [PubMed] [Google Scholar]
- Lovley D. R., Giovannoni S. J., White D. C., Champine J. E., Phillips E. J., Gorby Y. A., Goodwin S. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch Microbiol. 1993;159(4):336–344. doi: 10.1007/BF00290916. [DOI] [PubMed] [Google Scholar]
- Marcus R., Watt J. Seaweeds and ulcerative colitis in laboratory animals. Lancet. 1969 Aug 30;2(7618):489–490. doi: 10.1016/s0140-6736(69)90187-1. [DOI] [PubMed] [Google Scholar]
- McOrist S., Jasni S., Mackie R. A., MacIntyre N., Neef N., Lawson G. H. Reproduction of porcine proliferative enteropathy with pure cultures of ileal symbiont intracellularis. Infect Immun. 1993 Oct;61(10):4286–4292. doi: 10.1128/iai.61.10.4286-4292.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McOrist S., Lawson G. H. Reproduction of proliferative enteritis in gnotobiotic pigs. Res Vet Sci. 1989 Jan;46(1):27–33. [PubMed] [Google Scholar]
- Moon H. W., Cutlip R. C., Amtower W. C., Matthews P. J. Intraepithelial vibrio associated with acute typhlitis of young rabbits. Vet Pathol. 1974;11(4):313–326. doi: 10.1177/030098587401100404. [DOI] [PubMed] [Google Scholar]
- Relman D. A., Loutit J. S., Schmidt T. M., Falkow S., Tompkins L. S. The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N Engl J Med. 1990 Dec 6;323(23):1573–1580. doi: 10.1056/NEJM199012063232301. [DOI] [PubMed] [Google Scholar]
- Relman D. A., Schmidt T. M., MacDermott R. P., Falkow S. Identification of the uncultured bacillus of Whipple's disease. N Engl J Med. 1992 Jul 30;327(5):293–301. doi: 10.1056/NEJM199207303270501. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schoeb T. R., Fox J. G. Enterocecocolitis associated with intraepithelial Campylobacter-like bacteria in rabbits (Oryctolagus cuniculus). Vet Pathol. 1990 Mar;27(2):73–80. doi: 10.1177/030098589002700201. [DOI] [PubMed] [Google Scholar]
- Solnick J. V., O'Rourke J., Lee A., Paster B. J., Dewhirst F. E., Tompkins L. S. An uncultured gastric spiral organism is a newly identified Helicobacter in humans. J Infect Dis. 1993 Aug;168(2):379–385. doi: 10.1093/infdis/168.2.379. [DOI] [PubMed] [Google Scholar]
- Stills H. F., Jr Isolation of an intracellular bacterium from hamsters (Mesocricetus auratus) with proliferative ileitis and reproduction of the disease with a pure culture. Infect Immun. 1991 Sep;59(9):3227–3236. doi: 10.1128/iai.59.9.3227-3236.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier J. A., Keppler K. J. A note on the neighbor-joining algorithm of Saitou and Nei. Mol Biol Evol. 1988 Nov;5(6):729–731. doi: 10.1093/oxfordjournals.molbev.a040527. [DOI] [PubMed] [Google Scholar]
- Ward D. M., Weller R., Bateson M. M. 16S rRNA sequences reveal numerous uncultured microorganisms in a natural community. Nature. 1990 May 3;345(6270):63–65. doi: 10.1038/345063a0. [DOI] [PubMed] [Google Scholar]
- Watt J., Marcus R. Experimental ulcerative disease of the colon in animals. Gut. 1973 Jun;14(6):506–510. doi: 10.1136/gut.14.6.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt J., Marcus R. Ulceration of the colon in rabbits fed sulphated amylopectin. J Pharm Pharmacol. 1972 Jan;24(1):68–69. doi: 10.1111/j.2042-7158.1972.tb08868.x. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Fox J. G., Ho Y., Zhang L., Stills H. F., Jr, Smith T. F. Comparison of the major outer-membrane protein (MOMP) gene of mouse pneumonitis (MoPn) and hamster SFPD strains of Chlamydia trachomatis with other Chlamydia strains. Mol Biol Evol. 1993 Nov;10(6):1327–1342. doi: 10.1093/oxfordjournals.molbev.a040079. [DOI] [PubMed] [Google Scholar]