Abstract
Aims
This research examined the performance of a broad range of measures posited to relate to smoking craving.
Design
Heavy smokers and tobacco chippers, who were either deprived of smoking or not for 7 hours, were exposed to both smoking (a lit cigarette) and control cues.
Participants
Smokers not currently interested in trying to quit smoking (n = 127) were recruited. Heavy smokers (n = 67) averaged smoking at least 21 cigarettes/day and tobacco chippers (n = 60) averaged 1–5 cigarettes on at least 2 days/week.
Measurements
Measures included urge rating scales and magnitude estimations, a rating of affective valence, a behavioral choice task that assessed perceived reinforcement value of smoking, several smoking-related judgement tasks and a measure of cognitive resource allocation.
Findings
Results indicated that both deprivation state and smoker type tended to affect responses across these measurement domains.
Conclusions
Findings support the use of several novel measures of craving-related processes in smokers.
Introduction
Drug craving is often thought to be a core feature of addiction. Despite its importance, basic assumptions regarding the nature and assessment of craving remain in dispute (Sayette et al., 2000). A comprehensive assessment of craving requires articulation of this construct. We adapted from Baker, Morse & Sherman (1987) the following definition: cravings are emotional states reflecting the activation of motivational (drug-acquisitive) systems that have particular response patterns involving self-report, behavioral and cognitive correlates. Regardless of whether feelings, thoughts and actions related to craving are viewed as components or simply effects of craving (cf. Rankin, Hodgson & Stock-well, 1979; Baker et al., 1987), studying these processes, described herein as craving responses, promises to improve understanding of addiction from both clinical and conceptual perspectives (Zinser et al., 1999; Drummond et al., 2000).
Our chief aim was to examine several new measures of craving responses. Specifically, we tested the performance of a behavioral choice measure, two judgement measures and a novel measure of self-reported urge. We exposed both heavy smokers (HS) and light smoking tobacco chippers (TC) to a potent craving manipulation and a comprehensive craving response assessment battery.
In order to evaluate these measures, we needed to induce a robust craving state. A common craving manipulation has been drug cue exposure, in which participants are presented with stimuli associated with a particular drug. Across drugs, cue exposure has reliably elicited cravings in both addicts and heavy users (Carter & Tiffany, 1999). Nevertheless, urge reports have been low in many studies, often with ratings falling on the lower half of urge scales (Wertz & Sayette, 2001). A factor that seems to affect urge during cue exposure is whether a participant is seeking abstinence. Addicts not currently trying to quit report urges about twice as strong as do those undergoing cue exposure assessment at the start of treatment (Wertz & Sayette, 2001). A second factor that may affect smoking urge is the nature of the cue. Asking smokers to hold a lit cigarette appears to elicit robust cravings (e.g. Hutchison, Niaura & Swift, 1999; Sayette & Parrott, 1999) compared to when the cigarette is unlit (e.g. Juliano & Brandon, 1998). Accordingly, this study induced craving by exposing smokers not currently attempting cessation to the sight, smell and touch of a lit cigarette.
We aimed to elicit a wide range of cravings to evaluate our measures. We varied craving strength by manipulating two factors, the outcomes of which were predicted based on prior research. The first involved nicotine deprivation. Ideally one would assign smokers to nicotine deprived and completely non-deprived conditions. In practice it is difficult to create a totally non-deprived environment. Most studies require non-deprived participants to smoke at the outset. By the time they receive control and smoking cues and complete key measures, as much as 30–40 minutes have elapsed since smoking. For nicotine-dependent smokers, even this brief interval may be sufficient to create a mild deprivation state (Hughes, 1991). Thus, the present study included a nicotine-deprived and minimally deprived condition. There is evidence, however, that deprived smokers report stronger urges than minimally deprived smokers (Sayette & Hufford, 1994).
The second factor was smoking status. We recruited both nicotine-dependent, HSs and light smoking, non-dependent TCs. Typically, TCs smoke several days a week, and about four cigarettes on any given day (Shiffman et al., 1994). They smoke normally, absorb normal amounts of nicotine, eliminate nicotine normally and develop nicotine tolerance (Shiffman et al., 1990), yet they do not show signs of dependence (see Shiffman et al., 1994). A widely held view is that craving is primarily a product of dependence, reflecting some physiological “need” for nicotine. According to DSM-IV, for example, craving is “likely to be experienced by most if not all individuals with substance dependence”. Accordingly, in the present study, craving responses were expected to be stronger among HSs than TCs.
Craving response measurement
Self-reported urge
Despite their appeal, the utility of self-reports of craving can be compromised in smoking cue studies (Sayette et al., 2000). Typically smokers enter the laboratory in a nicotine-deprived state. After baseline ratings, they receive smoking cues and again report their urge. Although not especially sophisticated, a change in urge report from baseline to cue exposure often is considered the key measure of craving. Numerous studies indicate, however, that nicotine deprivation alone can produce high baseline urge levels (see Sayette et al., 2000). As such, there may be an inadequate range on the scale to quantify increases in urge elicited by cue exposure.
One approach to addressing potential ceiling effects is to use magnitude estimation, in which smokers rate their current urges relative to baseline levels (Sayette et al., 2000). A magnitude estimation rating has no maximum end-point. Smokers assign their initial urge level to a certain value, e.g. 10, which serves as a comparison for subsequent estimates. If craving has doubled due to cue exposure, then they would rate their new level as 20. Although magnitude estimation addresses potential ceiling effects, the absence of a maximum value can skew data distributions. Individuals also cannot report a zero baseline urge level. Finally, smokers may enter a study with varying levels of urge. Thus, subsequent values are based on comparison to different initial levels, which poses a problem for assessing individual differences (Green, Shaffer & Gilmore, 1993). By using both an urge rating scale and magnitude estimation, one can compute a composite measure in which initial pre-cue exposure baseline urge rating scale scores are multiplied by a subsequent cue exposure magnitude score. This analysis accounts for initial urge levels, as well as urge increase during cue exposure. The goal of the composite urge score was not to assess change in urge, but to measure accumulated urge produced by initial drug deprivation and subsequent cue exposure. This study included urge rating scale scores, magnitude estimation and composite score ratings of self-reported urge.
Behavioral choice measures
Recently, researchers have tried to quantify perceived reinforcement value of drugs by asking participants to choose between drug use and varying amounts of money (e.g. Griffiths et al., 1993; Perkins et al., 1994). Presumably, the greater value attributed to drug use the stronger the craving. These measures have not always proved sensitive to craving manipulations. One reason may be that the choice between drug use and money often is hypothetical, as drug is not actually available (e.g. Perkins, Grobe & Fonte, 1997; Bickel, Odum & Madden, 1999). Another concern is that there often is a delay before drug use can begin (e.g. 15 minutes; Griffiths et al., 1993). Yet research indicates that even small delays may sharply discount the perceived reinforcement value of drug use (e.g. Bickel et al., 1999). In this study, the behavioral choice task used a smoking reinforcer that was both real and immediate.
Affect
Nearly all theories of craving assume a relation between affect and craving. In some cases, cravings are even defined as affective states (Baker et al., 1987). Often the affect associated with craving is thought to be negative (e.g. Tiffany, 1992). Negative affect is especially likely in craving situations in which the drug is not available for use (e.g. Baker et al., 1987; Sayette & Hufford, 1995). To the degree that negative emotional states are associated with craving, then stronger cravings should be accompanied by more negative affect. In the present study, we collected a self-report measure of affective valence during smoking cue exposure.
Judgements
People continually evaluate themselves and their environment, and these judgements are crucial in determining behavior (see Sayette, 1999). Kunda’s (1990) motivated reasoning theory proposes that information becomes biased, such that one is more likely to reasonably choose to engage in a desired behavior. Motivation is thought to affect how relevant information is both generated and evaluated. Measures of generation and evaluation of drug-related information might broaden assessment of craving-related processes. Marlatt (1985) describes “bolstering tactics” during craving that distort the probability of drug use outcomes which enhance a drug’s attractiveness. Despite its theoretical importance, with few exceptions (e.g. Cooney et al., 1987) cue exposure studies rarely measure outcome expectancies (Niaura, 2000). Sayette & Hufford (1997) found that smokers generated more positive, but not negative, aspects of smoking during a high craving session, compared to a low craving session. Brandon, Wetter & Baker (1996) observed that smokers reporting stronger cravings evaluated smoking consequences in a more positive manner than did those reporting weaker cravings. We posited that craving would affect the likelihood of generating positive, relative to negative outcomes, during an exercise in which smokers listed everything they liked and disliked about smoking. In addition, we predicted that craving would affect the evaluation of smoking outcomes provided on a smoking consequence questionnaire, such that participants who were experiencing stronger cravings would judge positive outcomes to be relatively more probable than would those experiencing weaker cravings.
Response time
There is emerging consensus that craving is associated with a shift in non-automatic processing (Tiffany, 1990). One approach to assessing redistribution of limited-capacity cognitive resources during craving involves divided attention tasks, such as secondary response time (RT) tasks. The RT paradigm has identified the degree to which a primary task draws on cognitive resources, by recording performance decrements on a secondary RT task (Kerr, 1973). Across addictions, individuals have increased RT during peak craving periods (see Sayette et al., 2000). However, theories differ about whether RT and urge reports should correlate. If RT indexes drug motivation, then measures should correlate. If instead RT serves to index a redistribution of non-automatic resources independent of drug motivation, then the measures should be unrelated (Cepeda-Benito & Tiffany, 1996). Several smoking cue exposure studies assessing RT and urge report have found them to significantly correlate only during conditions that produced the strongest cravings (Sayette & Hufford, 1994, Experiments 1 and 2; Juliano & Brandon, 1998). Sayette & Hufford (1994), for example, found increases in urge and RT to correlate only when smokers were nicotine deprived and exposed to smoking cues (see also Franken, Kroon & Hendriks, 2000).
Overview of study
The present study examined several craving response measures by assessing four groups of smokers predicted to vary in the strength of their cravings. HSs and TCs who were either deprived of nicotine for at least 7 hours or minimally deprived were exposed to smoking cues. We used a robust smoking cue exposure manipulation and a conceptually derived assessment battery. We predicted that nicotine deprivation would affect craving responses in both groups of smokers, but especially in HSs. We expected the minimal nicotine deprivation condition to show slight craving responses in the HS, but none in the TC. Consequently, we predicted that our various measures of craving response would reveal main effects for both deprivation state and for smoking group, but did not expect deprivation state × smoking group interactions.
Method
Participants
Smokers (n = 60 male, 67 female) age 21–35 years were recruited through advertisements in newspapers and radio programs. Seventy-seven per cent of the sample was Caucasian, 17% African-American and 6% Hispanic or Asian-American. TC (n = 60) had to report smoking at least 2 days/week. On smoking days, they had to average 1–5 cigarettes/day. HS (n = 67) had to smoke an average of 21 or more cigarettes/day. Both groups had to report smoking at these rates for at least 24 continuous months (Shiffman et al., 1994). Participants were excluded if they reported a medical condition that contraindicated nicotine ethically, or if they were illiterate. Informed consent was obtained from all participants. Deprived HSs had to have a CO that did not exceed 16 p.p.m. and deprived TCs could not exceed 10 p.p.m. Table 1 presents participant characteristics by group. Groups did not differ on age, years of formal education or ethnic make-up (ps > 0.05).
Table 1.
Participant characteristics listed by group
| Tobacco chippers |
Heavy smokers |
|||
|---|---|---|---|---|
| Minimally-deprived | Deprived | Minimally-deprived | Deprived | |
| Age | 24.4 (4.2) | 23.8 (3.9) | 25.4 (4.3) | 25.2 (4.4) |
| Years formal education | 14.5 (1.8) | 14.8 (1.9) | 14.1 (1.5) | 14.4 (2.0) |
| Years smoking | 7.4 (4.2) | 6.0 (4.5) | 8.3 (5.3) | 9.4 (4.9) |
| Cigarettes/smoking day | 3.9 (1.3) | 3.6 (1.6) | 24.8 (5.4) | 24.9 (5.4) |
| Quit | 6.3 (2.6) | 6.7 (2.0) | 6.3 (2.3) | 6.5 (3.1) |
| CO #1 | 7.2 (5.8) | 3.9 (2.3) | 29.1 (13.1) | 9.4 (3.6) |
| CO #2 | 9.0 (5.6) | 3.7 (2.1) | 28.5 (12.1) | 8.6 (3.6) |
Mean and SD for age, number of years of formal education, number of years smoking, number of cigarettes smoked on a smoking day, number of previous quit attempts, carbon monoxide level at outset of study (CO #1), and carbon monoxide level after minimally deprived participants are permitted to smoke (CO #2).
As noted in Table 1, TCs reported smoking fewer cigarettes/day and fewer years of smoking than HSs. Analyses of CO at the outset of the study (CO#1) and then after the minimally deprived smokers were asked to smoke (CO#2) revealed main effects for group and for deprivation, and a group × deprivation interaction [Fs (1,123) > 33, ps < 0.0001]. Deprived TCs had the lowest CO levels, followed by minimally deprived TCs, deprived HSs and minimally deprived HSs.
Experimental design
Our main goal was to examine craving responses for groups of smokers predicted to experience varying levels of smoking motivation. Most of these responses could not be measured more than once due to carryover effects. Thus, affective valence, generation of smoking characteristics, evaluation of smoking consequences and a behavioral choice task (see below for details) were administered only after smoking cue exposure. Although both control and smoking cues were used, only urge ratings and RT were administered during both smoking cue and control exposure. These latter measures permitted comparisons to previous studies that assessed urge ratings during smoking and control cues (e.g. Sayette & Hufford, 1994). In addition, they provided a check to ensure that the main assessment battery was being administered during the time of peak craving.
This study used a mixed factorial design, with HS and TC randomly assigned to 7-hour nicotine-deprived or minimally deprived conditions. All participants were exposed to control and smoking cues. Order of cue exposure was fixed, with control cue exposure preceding smoking cue exposure. Counterbalancing was not used because urge ratings following drug cue exposure tend to remain high, making it difficult to interpret effects of any subsequent control exposure (e.g. Hutchison et al., 1999). Participants’ own cigarettes served as smoking cue to increase magnitude of reactivity. A small roll of electrical tape served as the control cue, as it was of similar size and weight to a cigarette yet unlikely to be associated with smoking cues (Sayette & Hufford, 1994).
Baseline assessment measures
To assess individual differences that may affect craving, data on age, gender, ethnicity, marital status, education and income were obtained. Smoking history and patterns also were assessed.
Craving response measures
Reported urge to smoke
Reported urge to smoke was assessed by a rating scale ranging from 0 (labeled “absolutely no urge to smoke at all”) to 100 (labeled “strongest urge to smoke I’ve ever experienced”) (Juliano & Brandon, 1998). [In many cases, it is preferable to assess craving report using a multi-item scale (Sayette et al., 2000). Although such an approach can improve reliability, however, it also is likely to increase reactivity relative to a single item scale (Juliano & Brandon, 1998). Reactivity is of particular concern when there are multiple administrations of a measure. Relying on single items may still work fairly well to capture cravings (Sayette et al., 2000)]. Participants also reported a magnitude estimation urge score, in which they compared their current feelings proportionately to their baseline urge, which they assigned the standard value of 10.
Affective valence
During smoking cue exposure, participants indicated how they felt on a scale ranging from 0 (labeled “I feel very bad right now”) to 10 (labeled “I feel very good right now”).
Response time (RT)
Participants responded to auditory tones (70 dB, 400 Hz) by pressing a mouse button as fast as possible before and during cue exposure (Sayette & Hufford, 1994).
Ad libitum characteristics of smoking (AD LIB: Sayette & Hufford, 1997)
Participants listed everything that they liked about smoking on half of a sheet of paper and everything they disliked on the other half, over a 3-minute period. They were told to list an item only once. An experimenter reviewed the form following its completion to ask about items that were illegible. This measure was administered only once, because it is susceptible to practice effects (Sayette & Hufford, 1997).
Smoking Consequences Questionnaire–Brief
(SCQ-B). Beliefs about possible consequences of smoking were rated on a scale ranging from −5 (extremely undesirable) to +5 (extremely desirable) and then on a 10-point scale to indicate the probability that they believed this consequence would occur (Copeland, Brandon & Quinn, 1995). Copeland et al. (1995) examined probability levels and the cross product of probability and desirability. Data provided more support for the validity of the probability version than the subjective utility version. We focused on probability judgements and used 24 items (space constraints preclude listing items, which are available from the first author), including both desirable and undesirable consequences, from Copeland et al.’s (1995) scale.
Behavioral choice task
Participants chose between immediate access to a cigarette and delayed access with financial compensation. They indicated the minimum amount of money they would accept in order to postpone smoking for 5 minutes. If this value was less than a previously set but undisclosed amount, they would receive the amount they requested in return for smoking delay. The critical variable was the minimum amount of money required to postpone smoking for 5 more minutes. To begin, participants who indicated that they wanted to smoke were asked if they would be willing to postpone smoking for 5 minutes for an additional $50. The experimenter continued to propose lower values until participants indicated that they would prefer to smoke immediately rather than accept that amount. At that point a value midway between the unacceptable sum and the lowest acceptable sum was offered by the experimenter, and this process was repeated until the exact “crossover point” (Griffiths, Rush & Puhala, 1996) was reached, which presumably reflected the participant’s minimum monetary value of delaying smoking. (All participants were then informed that they would be permitted to smoke immediately and would receive an additional $5.)
Procedure
Telephone screening
Smokers who responded to advertisements underwent a telephone interview to exclude those not meeting selection criteria. Eligible participants were asked to attend a 2-hour laboratory session for which they would be paid $40.00. Those assigned to the deprivation conditions were instructed to abstain from smoking for at least 7 hours, and told that breath samples would ensure that they had abstained. Participants were told to bring a pack of their preferred brand of cigarettes.
Laboratory set-up
Participants underwent cue exposure manipulations while seated behind a desk. On the desk was an intercom, and a mouse button used in the RT task. Facing the desk was a mounted video camera. Subjects were told that the camera and intercom facilitated communication and helped determine that instructions were understood. Next to the desk was a speaker connected to a computer in the next room, which was used to generate tones for the RT task.
Baseline assessment
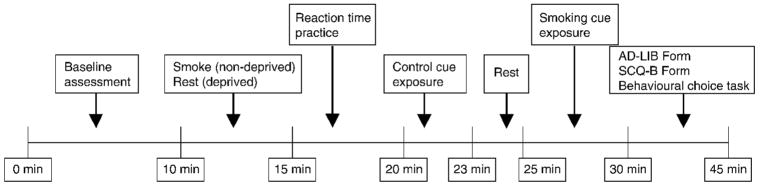
Figure 1 presents a time line of the procedures. Sessions began between 3.00 and 5.00 p.m. Upon arrival, written informed consent was obtained. To check compliance with deprivation instructions, participants reported the last time they smoked and provided a CO reading (#1). They presented their pack of cigarettes and lighter to the experimenter. Participants next completed baseline assessment, including an urge rating scale score (#1). At this time, all minimally deprived participants smoked a cigarette. During this 5-minute interval, deprived participants sat quietly. They then provided CO #2 and reported their urge to smoke (#2), using both the urge rating scale and magnitude estimation. From this point on, whenever reported urge measures occurred they were assessed using first the rating scale and then magnitude estimation.
Figure 1.
Schematic timeline of study procedures.
RT instructions and practice
Participants placed the index finger of the hand that they did not normally use to hold a cigarette when smoking, on a computer mouse button. They were told to press the button as fast as possible whenever they heard a computer-generated tone. They practiced responding to five tones while holding a pencil in their dominant hand, to approximate the task that was required during smoking cue exposure (Sayette & Hufford, 1994).
Control cue exposure
Participants were informed that they would be presented with a series of tones and a tray containing a plastic cover was placed on their desk. They were instructed not to touch the tray and to continue to press the button when they heard a tone. “Pre-exposure” tones sounded after 15 and 35 seconds. Twenty seconds later they were instructed to pick up the cover, which revealed a roll of tape. They were asked to hold the tape in their dominant hand and to look at it while continuing to monitor tones. Tones were presented 16 seconds and 34 seconds after they picked up the tape. Immediately after the second exposure tone, participants completed an urge rating (#3).
Cigarette cue exposure
Following a 2-minute rest, another covered tray was placed on the desk. Participants were again told not to touch the tray and to press the button when they heard a tone. They were presented with “pre-exposure” tones after 21 and 39 seconds, followed by an urge rating (#4). Sixteen seconds later they picked up the cover, revealing the pack of cigarettes along with a lighter and ashtray, and rated affective valence. They were instructed to remove a cigarette and light it without putting it in their mouths. Participants next were told to put down the lighter, hold the cigarette comfortably in their dominant hand, look at it without placing it in their mouths, and continue to monitor tones. Tones were presented 6 and 31 seconds after they finished lighting the cigarette. Immediately after the second tone, urge rating (#5) was administered. Seven seconds later, participants extinguished their cigarette in the ashtray. They then completed the AD LIB, SCQ-B, followed by the behavioral choice task. Finally, participants completed a form asking them about the study’s purpose, and were debriefed and paid $45.00 ($40 plus $5 from the behavioral choice task).
Data analysis
We tested the main and interactive effects of smoking group (HS vs. TC) and deprivation state (nicotine-deprived vs. minimally deprived) on the key craving response measures. Effect size estimates (d) are provided for significant main effects. As noted above, some measures (self-reported urge, RT) were completed during smoking cue and control cue exposure, while others were used once (after smoking cue exposure). Thus, analytical strategies differed across measures, as described below. Gender was unrelated to any of the measures, and was not included in analyses.
Results
Self-reported urge
Urge rating scale scores
Ratings were recorded at five points. Table 2 presents urge ratings throughout the study. A Group× Deprivation × Time repeated-measures ANOVA with urge ratings as a repeated variable revealed group, time and deprivation main effects, as well as Group × Deprivation and Time × Deprivation interactions [Fs >6, ps <0.001]. To test these interactions, urge at each time was analyzed in a Group × Deprivation ANOVA. At time 1 there were main effects for both independent variables (Fs >13.4, ps <0.001). A Group×Deprivation interaction also emerged, F (1, 122) = 10.1, p < 0.002). Time 1 ratings for deprived HSs differed from those in the other three groups, which were similar to each other (see Table 2).
Table 2.
Mean (SD) self-reported urge ratings among tobacco chippers and heavy smokers under minimally-deprived and deprived conditions
| Tobacco chippers |
Heavy smokers |
|||
|---|---|---|---|---|
| Minimally-deprived | Deprived | Minimally-deprived | Deprived | |
| Urge rating scale (time 1) | 18.9 (21.5)a | 22.2 (22.3)a | 20.8 (17.9)a | 48.1 (22.8)b |
| Urge rating scale (time 2) | 9.5 (14.1)a | 21.3 (18.6)b | 2.6 (5.5)a | 49.1 (20.2)c |
| Urge rating scale (time 3) | 11.7 (15.3)a | 23.0 (21.4)b | 10.1 (13.5)a | 48.6 (21.4)c |
| Urge rating scale (time 4) | 10.9 (14.4)a | 25.1 (23.2)b | 14.5 (19.1)a | 50.5 (21.6)c |
| Urge rating scale (time 5) | 24.3 (23.0)a | 44.2 (32.3)b | 34.1 (24.3)a,b | 70.7 (23.0)c |
| Change in urge (time 5 minus time 1) | 5.4 (20.1)a | 22.0 (19.1)b | 13.3 (23.6)a,b | 22.6 (18.1)b |
| Change in urge (time 5 minus time 2) | 14.8 (14.4)a | 22.9 (20.3)a,b | 30.2 (26.5)b | 21.6 (18.5)a,b |
| Magnitude estimation (time 2) | 6.2 (4.3)a | 10.7 (2.8)b | 4.3 (4.4)a | 12.0 (5.0)b |
| Magnitude estimation (time 3) | 7.0 (4.8)a | 11.7 (5.4)b | 7.4 (6.1)a | 12.5 (5.0)b |
| Magnitude estimation (time 4) | 7.0 (5.2)a | 12.5 (5.8)b | 8.8 (7.8)a | 14.3 (6.5)b |
| Magnitude estimation (time 5) | 16.0 (13.1)a | 24.6 (16.5)b | 23.3 (19.2)a, b | 28.2 (19.8)b |
| Urge composite (time 5) | 28.0 (33.5)a | 57.2 (66.9)a | 42.7 (61.5)a | 137.5 (135.8)b |
Time 1: post-initial instruction; time 2: post-smoke for the minimally deprived smokers; time 3: control cue exposure; time 4: pre-smoking cue baseline; time 5: smoking cue exposure. Values in each row with non-overlapping subscripts are significantly different from each other (p < 0.05). For purposes of illustration, untransformed values are presented for magnitude estimation and composite urge ratings. Only contrasts with transformed values are noted. Urge composite means (mean of the composite score of each subject) are not the product of the mean values of urge rating scale#1 and magnitude estimation time 5).
Time 2 tested the effects of smoking on urge ratings, as half the participants (those in minimally deprived conditions) were allowed to smoke. Main effects for Group and Deprivation and a Group×Deprivation interaction emerged (all Fs > 14, ps < 0.001). Table 2 shows Deprivation had a greater effect on HSs than on TCs, and that urge ratings were especially high for deprived HSs. These effects for Group, Deprivation and the Group × Deprivation interaction were maintained during the control exposure and the pre-cigarette exposure periods (times 3 and 4) (Fs > 9.3, ps < 0.005). During cigarette exposure (time 5), main effects appeared for both Group (d = 0.58) and Deprivation (d = 0.92) (Fs > 15.3, ps < 0.001), and there was a marginally significant interaction, F (1, 122) = 3.2, p < 0.08, such that Deprivation especially increased urge among HSs (see Table 2).
To test whether the minimally deprived conditions were equivalent for TCs and HSs during the interval between initial smoking and smoking cue exposure, a Group×Deprivation ×Time repeated-measures ANOVA was calculated with urge ratings during times 2–4 as the repeated measures variable. A main effect for Time, and a Group×Deprivation ×Time interaction appeared (Fs > 5.4, ps < 0.01). As shown in Table 2, during this time urge ratings did not change for minimally deprived TCs, but increased steadily for minimally deprived HSs.
Magnitude estimation of urge
Magnitude estimation ratings prior to smoking cue exposure (times 2–4) showed a main effect of Deprivation, with deprived smokers reporting higher levels than minimally deprived smokers (Fs > 22, ps < 0.0001). Magnitude estimations during smoking cue (time 5) were positively skewed, and a square root transformation was performed. A Group×Deprivation ANOVA revealed a main effect for Deprivation (d = 0.49) F (1, 122) = 8.5, p < 0. 01, and a marginally significant effect for Group (d = 0.31) F (1, 122) = 3.5, p < 0. 07. The Group × Deprivation interaction was not significant (F < 1) (see Table 2).
Composite urge score
We divided magnitude estimation score during cigarette exposure by 10, and then multiplied that value by the urge rating scale score at time 1. This value was square root transformed to address a positive skew. Main effects for Group (d = 0.63), Deprivation (d = 0.77) and a Group × Deprivation interaction emerged (Fs > 4.7, ps < 0. 04). Deprived smokers reported higher scores than minimally deprived smokers, HSs reported higher scores than TCs and deprived HSs reported especially high scores relative to the other groups (see Table 2).
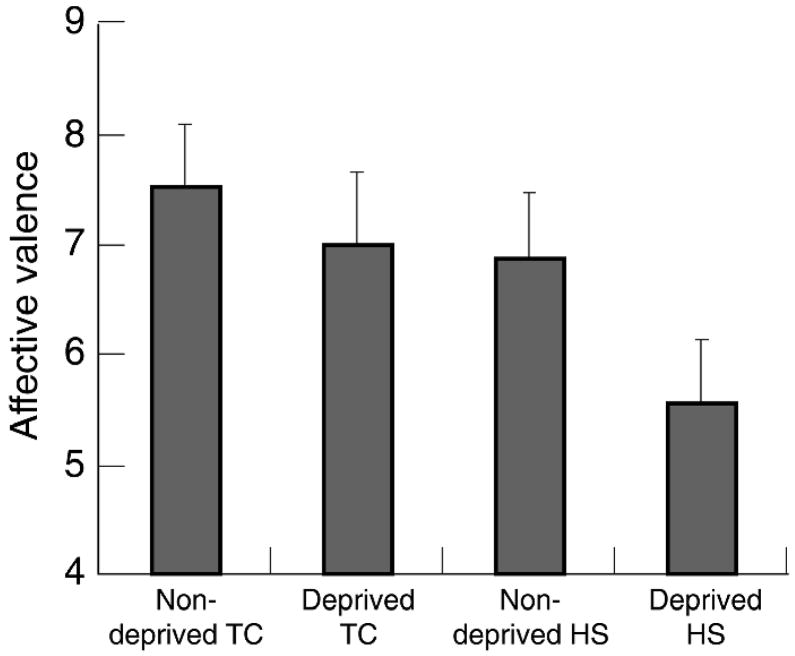
Affective valence
Figure 2 presents group ratings. A Group×Deprivation ANOVA showed TCs reported a more positive affective valence than HSs (d = 0.45) F (1, 122) = 6.8, p < 0. 02. There was also a marginally significant effect for Deprivation (d = 0.32), such that minimally deprived smokers reported more positive affective valence than did deprived smokers, F (1, 122) = 3.5, p < 0.06. The Group × Deprivation interaction was not significant (F < 1).
Figure 2.
Affective valence during smoking cue exposure. Scale ranged from 0 (labeled ‘I feel very bad right now’) to 10 (labeled ‘I feel very good right now’). Error bars represent standard errors. TC = tobacco chippers, HS = heavy smokers.
Behavioral choice
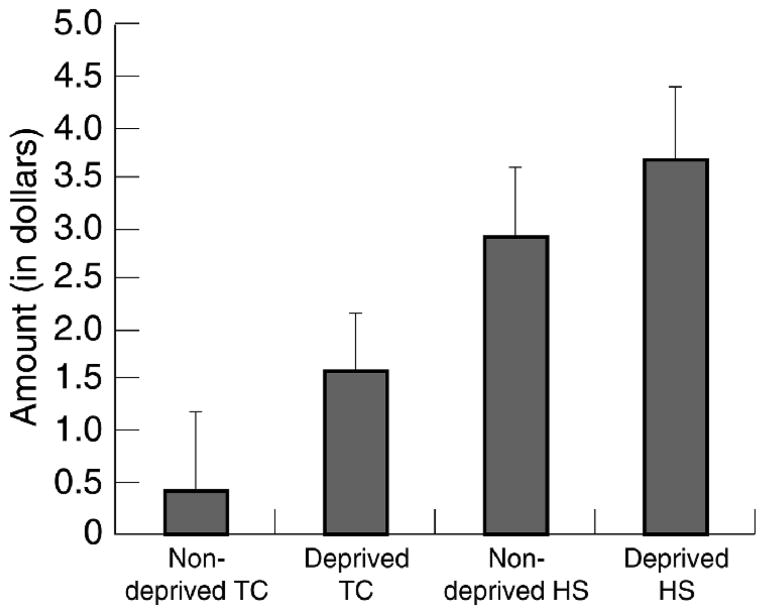
Due to a positive skew, analyses were performed on square root transformed values. An ANOVA revealed main effects for Group (d = 0.57) and Deprivation (d = 0.41)(Fs > 6, ps < 0.02). HSs needed more money to delay smoking than TCs, and deprived smokers required more money than did minimally deprived smokers. The Group × Deprivation interaction was not significant. Figure 3 shows that Deprived HSs required the most money, followed by minimally deprived HSs, deprived TCs and minimally deprived TCs.
Figure 3.
Monetary values on behavioral choice task. Error bars represent standard errors. TC = tobacco chippers. HS = heavy smokers. For purposes of illustration, untransformed values are presented.
SCQ-B
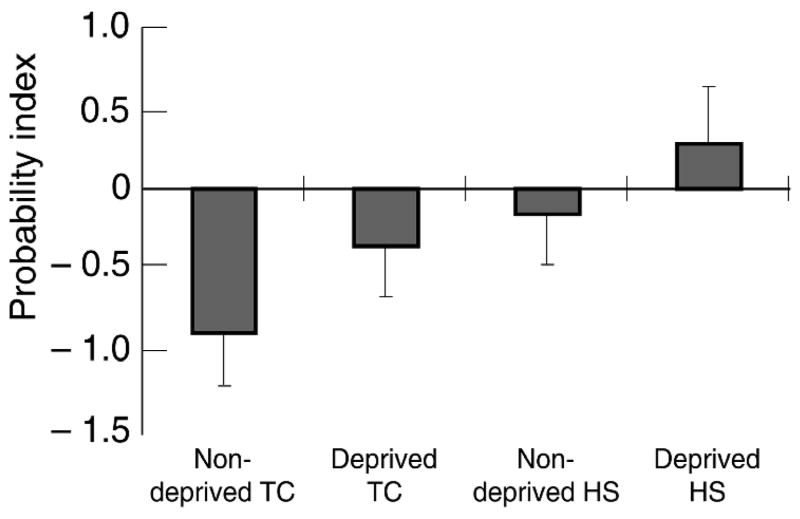
We examined the probability of positive consequences relative to negative ones by subtracting the mean probability value of negative from positive items. Figure 4 presents SCQ-B probability values for all groups. A main effect appeared for Group (d = 0.40) F (1, 120) = 5.1, p < 0.03, with HSs judging positive consequences to be relatively more probable than TCs. A marginally significant effect for Deprivation (d = 0.29), F (1, 120) = 2.8, p < 0.09 suggested that deprived smokers found positive consequences to be relatively more probable than did minimally deprived smokers. The Group × Deprivation interaction was not significant.
Figure 4.
SCQ-B probability judgements. Positive values indicate that desirable consequences of smoking were considered relatively more probable than were undesirable consequences. Error bars represent standard errors. TC = tobacco chippers, HS = heavy smokers. Schematic time-line of study procedures.
AD LIB
Two Group×Deprivation ANOVAs, with total positive items and total negative items generated as the dependent variables, revealed no main effects. A marginally significant interaction appeared for positive items (but not negative ones), such that Deprivation increased generation of positive items for HSs, but tended to decrease it for TCs, F (1, 123) = 3.3, p < 0.07.
RT
RT latencies to the two pre-exposure baseline tones, and the two exposure tones were averaged to compute mean baseline and exposure RTs, respectively. We next tested whether the two baselines preceding the control cue and smoking cue exposure were equivalent. A Group×Deprivation × Cue ANOVA with Cue as a within-subject variable and with baseline RTs as the dependent variable revealed a significant Cue effect, with baseline values higher before cigarette baseline (M = 333 ms, SD = 75) than before tape baseline (M = 320 ms, SD = 70), F (1, 118) = 5.8, p < 0.02. A Cue × Deprivation interaction, F (1, 118) = 7.0, p < 0.01 indicated that differences between the cigarette and control baselines were due to differences in deprived (M = 25.4 ms), but not minimally deprived (M = 0.5 ms) participants. No other baseline effects were significant.
Difference scores were calculated by subtracting mean baseline RT from the corresponding exposure means during each of the cue exposures. A Group × Deprivation × Cue repeated-measures ANOVA, with change from baseline RT to exposure RT as the dependent variable, revealed a Cue effect, F (1, 117) = 31.4, p < 0.0001, with RT increasing by 71.2 ms (SD = 75) during smoking and 26.7 ms (SD = 52) during control exposure. No other effects were significant, indicating that neither Group nor Deprivation affected RT increase during smoking cue exposure.
Correlations
Pearson correlations examined covariation across measures. To reduce the number of comparisons, only measures that were sensitive to deprivation and smoking group manipulations were included. Thus, the four measures were self-report urge (composite urge) during smoking cue exposure, behavioral choice, affective valence and probability judgement on the SCQ-B. (Magnitude estimation and urge rating scale values during time 5 were also sensitive to deprivation state and group status and were both highly correlated with composite urge. We chose composite urge for these analyses, as it was the most sensitive of our self-report measures to our manipulations.) Four of the six possible correlations were significant, with behavioral choice being correlated with (a) composite urge (r = 0.38, p < 0.001), (b) affective valence (r = 0.21, p < 0.02) and (c) SCQ-B judgements (r = 0.31, p < 0.001). Affect also was correlated with composite urge (r = 0.34, p < 0.001).
Because RT increase during smoking cue exposure was affected by neither smoking group nor deprivation state, it was not included in the correlation matrix presented above. Nevertheless, previous studies have found increases in RT and urge to correlate only in the experimental conditions eliciting the strongest urge. To compare the present data with prior findings, we examined for each experimental condition correlations between RT increases and the different measures of self-reported urge during cue exposure. Specifically, for each of the four groups, change in RT from baseline to cue exposure was correlated with four self-reported urge values: (1) cue exposure—rating scale urge scores; (2) change in rating scale urge scores from baseline to cue exposure; (3) cue exposure—magnitude estimation; and (4) cue exposure—composite urge. RT increase was not significantly related to any of the four urge measures for the deprived TC, minimally deprived TC or minimally deprived HS groups (all rs < 0.25, ps > 0.19). In contrast, among the highest craving group (deprived HSs), RT increase was significantly correlated with three of the four urge measures: rating scale urge (r = 0.40, p < 0.03); magnitude estimation (r = 0.44, p < 0.02); and composite urge (r = 0.51, p < 0.01).
Discussion
This study examined several measures of craving responses. A potent craving manipulation evaluated the effects of deprivation and smoking group status on self-reported urge, behavioral choice and judgement tasks. Results showed that several of these measures were sensitive to the effects of nicotine deprivation and smoking group status. We expected that all participants would experience their peak cravings during smoking cue exposure. To test this assumption, self-report urge ratings and RT data were examined, as both measures were collected at multiple points throughout the study. Both rating scale urges and RT increases were highest during smoking cue exposure. Thus, we are confident that our smoking cue exposure assessment battery was administered at the optimal point in the study. Moreover, across the four groups, mean urge values on the 0–100 rating scale during smoking cue exposure ranged from 24 (minimally deprived TCs) to 71 (deprived HSs), suggesting that the study offered a wide range of craving responding, which is important for examining the various craving response measures.
Self-reported urge
Because smoking cue exposure studies that rely on urge rating scales may be vulnerable to ceiling effects (Sayette et al., 2000), the present study included alternative approaches to urge ratings, which led to different conclusions. Regarding the urge rating scale, conclusions differ depending on whether one focuses on unadjusted rating scale values, or change in urge ratings adjusted for baseline levels. During smoking cue exposure, deprived HSs reported the strongest urges. (We also examined rating scale urges at time 5, covarying for initial time 1 values, and the pattern and significance of findings were identical to those reported using change scores.) Alternatively, when time 2 urge ratings were subtracted from smoking cue exposure urge ratings, change scores suggested that minimally deprived HSs were most reactive to the smoking cues. [Time 2 reflects the period often used as a baseline for smoking studies manipulating deprivation (e.g. Perkins et al., 1994).] This latter finding suggests that deprived HSs wish to smoke regardless of context, whereas minimally deprived HSs are more sensitive to their environment. This result may be confounded, however, by ceiling effects. Whereas almost all minimally deprived HSs were near the minimum on the urge scale prior to cue exposure, most deprived HSs rated their initial urge at least at the midpoint of the scale. Moreover, magnitude estimation during smoking cue exposure revealed that minimally deprived HSs did not report greater increases in urge than did either minimally deprived TCs, deprived HSs, or deprived TCs. Because initial standards (rating scale urge at time 1) differed across groups, a magnitude estimation method is not ideal. The composite score tried to account for these initial group differences to examine peak urge. When composite urge score was examined, a significant Deprivation × Group interaction emerged, such that deprived HSs appear most sensitive to the effects of deprivation during smoking cue exposure.
This composite measure best illustrates difficulties associated with ceiling effects and change scores using a traditional rating scale. Throughout the study, deprived HSs reported higher urge scores on the rating scale than did the other three groups, and thus were most likely to experience ceiling effects. Not surprisingly, for the two TC groups and the minimally deprived HS group, composite urges were similar to rating scale urges during smoking cue exposure. In contrast, the composite urge score was much larger than smoking cue exposure rating scale urges for the deprived HSs, suggesting that these smokers were unable to fully express their urge during smoking cue exposure using the 0–100 rating scale. Inclusion of composite urge provided a more comprehensive assessment than that found with either magnitude estimation or rating scales alone.
Affective valence
Consistent with results from the composite urge ratings, the affective valence data indicated that deprived HSs felt less positive than did the other three groups of smokers during smoking cue exposure. Although deprived HSs did not report a negative affect valence, our prediction that deprived HSs would feel worse than the other groups was supported. We restricted our affect measure to a single item in order to reduce reactivity associated with a lengthy assessment (Drummond et al., 2000; Sayette et al., 2000). Perhaps a more comprehensive assessment of affective valence would have produced lower values than found here.
Behavioral choice
The present data support the validity of our behavioral choice procedure. Depending on the smoking group, avoiding a 5-minute delay to smoke was worth between $0.51 and $3.70. Consistent with predictions, opportunity to smoke immediately was more highly valued by HSs than by TCs, and by deprived smokers more than minimally deprived smokers. This measure may have been sensitive to drug motivation because the opportunity to smoke was real (rather than hypothetical) and immediate (rather than delayed). This measure also differed from most drug choice tasks in that participants chose between drug use and the chance to earn extra money, rather than choosing between drug use and forfeiting money they already had been promised. Individuals may be more willing to part with money that they were not expecting to receive, which might explain the relatively large amounts that subjects were willing to turn down in the present study. Perkins et al. (1997), for example, found deprived regular smokers to be willing to pay (< $1) less than what even deprived TCs in the present study were willing to forgo.
RT
Both HSs and TCs responded slower during cigarette cues than control cues. This replicates prior studies (e.g. Sayette & Hufford, 1994; Sayette et al., 1994). In contrast to our previous research with smokers, however, we did not find effects for deprivation during cigarette exposure, as all smokers seemed to be distracted. It is not obvious to us why we did not replicate our prior deprivation finding, though an increase in baseline RT only among deprived HSs may have prevented the emergence of group differences during smoking cue exposure. Regarding the present data, RT increases when smokers held a lit cigarette may reflect a shift in non-automatic processing resources related to interruption of a well learned behavioral routine (see Tiffany, 1990). Indeed, Tiffany’s cognitive model is unique in its ability to explain equivalent RT increases during smoking cue exposure across the four groups of smokers. The finding that RT and urge ratings were correlated only among the smokers in the highest craving group replicates findings from prior studies (Juliano & Brandon, 1998; Sayette & Hufford, 1994). RT may index drug motivation only in conditions in which there is a powerful craving. That is, when urges are robust, the distraction indexed by RT may reflect to some degree underlying drug motivation. When urges are modest, however, RT increase may be detecting distraction associated with a host of non-motivational activities [e.g., interruption of a smoking behavior, frustration at not being able to smoke, problem solving aimed at coping with the current predicament (see Tiffany, 1990; Sayette, 1999)].
Judgements
Although in the expected direction, AD LIB did not reveal significant effects. This contrasts with Sayette & Hufford (1997). Unlike our initial effort, in which smokers listed first positive and then negative items, participants split the time between positive and negative items as they wished. The prior two-session study also used a within-subject analysis of AD LIB, accounting for individual differences in general responding. Most importantly, the initial study compared scores when smokers were deprived and exposed to cigarettes to a separate session when they were minimally deprived and holding a control cue. In the present study, all smokers completed AD LIB only after smoking cue exposure, perhaps making differences more difficult to obtain.
The SCQ-B generally proved sensitive to our craving manipulations. HSs evaluated positive consequences to be more probable, relative to negative ones than did TCs. There also was a trend suggesting that deprived smokers judged positive consequences to be more probable, relative to negative ones, than did minimally deprived smokers. Craving may distort outcome expectancies, such that positive outcomes appear more likely than negative ones (Marlatt, 1985). These data are consistent with studies linking craving states to information processing biases (Sayette, 1999).
Smoking cue effects were greater for nicotine-deprived than minimally deprived smokers. This was true for both HSs and TCs. On most measures HSs also tended to experience stronger effects than TCs. As expected, deprived HSs showed the greatest response to smoking cues. Only one measure revealed a Group × Deprivation interaction. For composite urge, deprivation effects were greater for HSs than TCs. Otherwise deprivation similarly affected both types of smokers. Both the self-reported urge and RT data also indicate that, compared to control cue exposure, smoking cue exposure increased responding similarly for TCs and HSs. Although TCs experienced weaker craving responses than HSs, both types of smokers appear to be reactive to smoking cues.
A limitation of this study, and any study contrasting HSs and TCs, is that the deprivation conditions were not conceptually equivalent for these two types of smokers: over seven waking hours, HSs would normally smoke more than would TCs. Thus, deprived TCs may have been under less deprivation than deprived HSs. Similarly, for minimally deprived smokers, the brief delay between smoking at study outset and subsequent smoking cue exposure may have elicited a mild urge in HSs, but not TCs, making the minimally deprived conditions slightly different for HSs and TCs. Finally, this study did not include physiological measures of cue reactivity. Such measures have been criticized for failing to change in a consistent pattern (Drummond, Cooper & Glautier, 1990; Tiffany, 1990). Physiological systems serve functions that are independent of craving, and in many cases it is unclear what type of response ought to be related to drug use (Baker & Brandon, 1990; Niaura et al., 1988). Nevertheless, recent findings suggest that inclusion of selected physiological measures conceptually linked to craving would be useful (Sayette et al., 2000).
These findings support use of several measures of craving-related processes. Behavioral choice was sensitive to our manipulations. Magnitude estimation and a composite urge measure relying on both urge rating scales and magnitude estimation may be useful when initial deprivation leads to ceiling effects. This may be critical when studying smokers not attempting cessation (Wertz & Sayette, 2001). Finally, judgement measures permit examination of the cognitive dimension of craving. Not only is it crucial to show that cognitive resources are engaged during craving, but it is useful to examine the nature of these changes (Sayette, 1999). Measures such as SCQ-B and AD LIB hold promise for evaluating how craving alters reasoning processes that may affect smoking. These measures, as well as EEG (Zinser et al., 1999), facial expression analysis (Sayette & Hufford, 1995), neuroimaging (Everitt, 1997), startle reflex (Elash, Tiffany & Vrana, 1995; Hutchison et al., 1999) and measures of implicit and explicit memory (see Sayette, 1999) should improve understanding of the psychological mechanisms underlying craving.
Acknowledgments
This research was supported by a grant from the National Institute on Drug Abuse (R01 DA10605). We thank Raymond Niaura for suggesting the magnitude estimation measure. We also are indebted to Dominic Parrott and Dawn Giuffre and the staff of the Alcohol and Smoking Research Laboratory for their assistance.
References
- Baker TB, Brandon TH. Validity of self-reports in basic research. Behavioral Assessment. 1990;12:33–51. [Google Scholar]
- Baker TB, Morse E, Sherman JE. The motivation to use drugs: a psychobiological analysis of urges. In: Rivers C, editor. The Nebraska Symposium on Motivation: alcohol use and abuse. Lincoln: University of Nebraska Press; 1987. pp. 257–323. [PubMed] [Google Scholar]
- Bickel W, Odum A, Madden G. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Wetter DW, Baker TB. Affect, expectancies, urges, and smoking: do they conform to models of drug motivation and relapse? Experimental and Clinical Psychopharmacology. 1996;4:29–36. [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Cepeda-Bennito A, Tiffany ST. The use of a dual-task procedure for the assessment of cognitive effort associated with cigarette craving. Psychopharmacology. 1996;127:155–163. doi: 10.1007/BF02805989. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Gillespie RA, Baker LH, Kaplan RF. Cognitive changes after alcohol cue exposure. Journal of Consulting and Clinical Psychology. 1987;55:150–155. doi: 10.1037//0022-006x.55.2.150. [DOI] [PubMed] [Google Scholar]
- Copeland AL, Brandon TH, Quinn EP. The Smoking Consequences Questionnaire–Adult: measurement of smoking outcome expectancies of experienced smokers. Psychological Assessment. 1995;7:484–494. [Google Scholar]
- Drummond DC, Cooper T, Glautier SP. Conditioned learning in alcohol dependence: implications for cue exposure treatment. British Journal of Addiction. 1990;85:725–743. doi: 10.1111/j.1360-0443.1990.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Litten RZ, Lowman C, Hunt WA. Craving research: future directions. Addiction. 2000;95:S247–S256. doi: 10.1080/09652140050111816. [DOI] [PubMed] [Google Scholar]
- Elash CA, Tiffany ST, Vrana SR. Manipulation of smoking urges and affect through a brief-imagery procedure: self-report, psychophysiological, and startle probe responses. Experimental and Clinical Psychopharmacology. 1995;3:156–162. [Google Scholar]
- Everitt B. Craving cocaine cues: cognitive neuroscience meets drug addiction research. Trends in Cognitive Sciences. 1997;1:1–2. doi: 10.1016/S1364-6613(97)01009-7. [DOI] [PubMed] [Google Scholar]
- Franken LA, Kroon LY, Hendriks VM. Influence of individual differences in craving and obsessive cocaine thoughts on attentional processes in cocaine abuse patients. Addictive Behaviors. 2000;25:99–102. doi: 10.1016/s0306-4603(98)00112-9. [DOI] [PubMed] [Google Scholar]
- Green BG, Shaffer GS, Gilmore M. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chemical Senses. 1993;18:683–702. [Google Scholar]
- Griffiths R, Rush C, Puhala K. Validation of the multiple-choice procedure for investigating drug reinforcement in humans. Experimental and Clinical Psychopharmacology. 1996;4:97–106. [Google Scholar]
- Griffiths RR, Troisi JR, Silverman K, Mumford GK. Multiple choice procedure: an efficient approach for investigating drug reinforcement in humans. Behavioural Pharmacology. 1993;4:3–13. [PubMed] [Google Scholar]
- Hughes JR. Distinguishing withdrawal relief and direct effects of smoking. Psychopharmacology. 1991;104:409–410. doi: 10.1007/BF02246044. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, Niaura R, Swift R. Smoking cues decreases prepulse inhibition of the startle response and increase subjective craving in humans. Experimental and Clinical Psychopharmacology. 1999;7:250–256. doi: 10.1037//1064-1297.7.3.250. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. Reactivity to instructed smoking availability and environmental cues: evidence with urge and reaction time. Experimental and Clinical Psychopharmacology. 1998;6:45–53. doi: 10.1037//1064-1297.6.1.45. [DOI] [PubMed] [Google Scholar]
- Kerr B. Processing demands during mental operations. Memory and Cognition. 1973;1:401–412. doi: 10.3758/BF03208899. [DOI] [PubMed] [Google Scholar]
- Kunda Z. The case for motivated reasoning. Psychological Bulletin. 1990;108:480–498. doi: 10.1037/0033-2909.108.3.480. [DOI] [PubMed] [Google Scholar]
- Marlatt GA. Cognitive factors in the relapse process. In: Marlatt GA, Gordon JR, editors. Relapse Prevention: maintenance strategies in the treatment of addictive behaviors. New York: Guilford Press; 1985. pp. 128–200. [Google Scholar]
- Niaura RS. Cognitive social learning and related perspectives on drug craving. Addiction. 2000;95:S155–S164. doi: 10.1080/09652140050111726. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedrazza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. Journal of Abnormal Psychology. 1988;97:133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Perkins K, Epstein LE, Grobe J, Fonte C. Tobacco abstinence, smoking cues, and the reinforcing value of smoking. Pharmacology, Biochemistry, and Behavior. 1994;47:107–112. doi: 10.1016/0091-3057(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Perkins K, Grobe J, Fonte C. Influence of acute smoking exposure on the subsequent reinforcing value of smoking. Experimental and Clinical Psychopharmacology. 1997;5:277–285. doi: 10.1037//1064-1297.5.3.277. [DOI] [PubMed] [Google Scholar]
- Rankin H, Hodgson R, Stockwell T. The concept of craving and its measurement. Behaviour Research and Therapy. 1979;17:389–396. doi: 10.1016/0005-7967(79)90010-x. [DOI] [PubMed] [Google Scholar]
- Sayette MA. Cognitive theory and research. In: Leonard K, Blane H, editors. Psychological Theories of Drinking and Alcoholism. 2. New York: Guilford Press; 1999. pp. 247–291. [Google Scholar]
- Sayette MA, Hufford MR. Effects of cue exposure and deprivation on cognitive resources in smokers. Journal of Abnormal Psychology. 1994;103:812–818. doi: 10.1037//0021-843x.103.4.812. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Hufford MR. Urge and affect: a facial coding analysis of smokers. Experimental and Clinical Psychopharmacology. 1995;3:417–423. doi: 10.1037/1064-1297.11.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Hufford MR. Effects of smoking urge on generation of smoking-related information. Journal of Applied Social Psychology. 1997;27:1395–1405. [Google Scholar]
- Sayette MA, Monti PM, Rohsenow DJ, Bird-Gulliver S, Colby S, Sirota A, Niaura RS, Abrams DB. The effects of cue exposure on attention in male alcoholics. Journal of Studies on Alcohol. 1994;55:629–634. doi: 10.15288/jsa.1994.55.629. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Parrott DJ. Effects of olfactory stimuli on urge reduction in smokers. Experimental and Clinical Psychopharmacology. 1999;7:151–159. doi: 10.1037//1064-1297.7.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, ShadeL WG. The measurement of drug craving. Addiction. 2000;95:S189–S210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Fischer L, Zettler-Siegal M, Benowitz N. Nicotine exposure in non-dependent smokers. Archives of General Psychiatry. 1990;47:333–336. doi: 10.1001/archpsyc.1990.01810160033006. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Kassek JD, Gnys M, Zettler-Siegal M. Smoking behavior and smoking history of tobacco chippers. Experimental and Clinical Psychopharmacology. 1994;2:126–142. [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A critique of contemporary urge and craving research: methodological, psychometric, and theoretical issues. Advances in Behaviour Research and Therapy. 1992;14:123–129. [Google Scholar]
- Wertz JM, Sayette MA. The effects of perceived drug use opportunity on self-reported urge. Experimental and Clinical Psychopharmacology. 2001;9:3–13. doi: 10.1037/1064-1297.9.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinser MC, Fiore MC, Davidson RJ, Baker TB. Manipulating smoking motivation: impact on an electrophysiological index of approach motivation. Journal of Abnormal Psychology. 1999;108:240–254. doi: 10.1037//0021-843x.108.2.240. [DOI] [PubMed] [Google Scholar]