Abstract
Objective
Hypoglycemia is associated with a variety of adverse behaviors including fatigue, confusion and social withdrawal. While these clinical symptoms are well characterized, the mechanism of their cause is not understood. Here we investigated how insulin-induced hypoglycemia causes social withdrawal.
Research Design and Methods
Male 8-12-wk-old C57BL/6J mice were injected intraperitoneally (IP) with or without and/or insulin, norepinephrine (NE) and epinephrine (Epi), terbutaline and butoxamine with subsequent measurement of blood glucose, social withdrawal and plasma catecholamines.
Results
Insulin generated (0.75 h post injection) significant hypoglycemia with blood glucose nadirs of 64 ± 4 and 48 ± 5 mg/dl for 0.8 and 1.2 units/kg of insulin, respectively. Insulin (0.8 or 1.2 units/kg) caused near total social withdrawal at 0.75 h with full recovery not occurring until 4 h (0.8 units/kg) or 8 h (1.2 units/kg) post insulin injection. Insulin also caused a marked elevation in plasma catecholamines. Basal 12 h fasting norepinephrine (NE) and epinephrine (Epi) were 287 ± 38 pg/ml and 350 ± 47 pg/ml, respectively. Insulin at 0.8 units/kg increased plasma NE and Epi to 994 ± 73 pg/ml and 1842 ± 473 pg/ml, respectively. Administration of exogenous NE or Epi caused social withdrawal similar in magnitude to insulin. Importantly, administration of the beta-2 adrenergic receptor agonist terbutaline also caused social withdrawal while administration of the beta-2 adrenergic receptor antagonist butoxamine blocked NE-induced social withdrawal. Finally, butoxamine blocked insulin-induced social withdrawal.
Conclusions
These data demonstrate that hypoglycemia-associated social withdrawal is dependent on catecholamines via a beta-2 receptor-mediated pathway.
Keywords: hypoglycemia, insulin treatment, catecholamines, social withdrawal, beta-2 adrenergic receptor
Introduction
Hypoglycemia (defined as blood glucose less than 60 mg/dl) is the most common complication of type 1 diabetes in childhood (Daneman, 2006; Shalitin and Phillip, 2007). It occurs when the administered dose of insulin exceeds the insulin requirement and is especially common in tightly controlled patients (Shalitin and Phillip, 2007). Symptoms of hypoglycemia may be adrenergic in origin due to epinephrine release or related to neuroglycopenia (Service, 1995; Hoffman et al., 1997; Korytkowski et al. 1998; Ste Marie and Palmiter, 2003; Hoffman, 2007). The adrenergic symptoms include: tremor, pallor, rapid heart rate, palpitations and diaphoresis (Binder and Bendtson, 1992; Bolli, 1997; Korytkowski et al., 1998). Neuroglycopenic symptoms range from fatigue, lethargy, headache, drowsiness and behavior change to seizures, unconsciousness and coma (Binder and Bendtson, 1992; Hoffman, 2007). Symptoms of hypoglycemia are classified as mild, moderate or severe (Hoffman, 2007). Mild hypoglycemia is associated with adrenergic symptoms and mild neuroglycopenic symptoms such as headache and behavior change (Frier, 2004; Hoffman, 2007). In addition, mild symptoms are generally recognized by the patient, oneself, and can be adequately treated without the intervention of a second person (Frier, 2004). Moderate and severe cases require second person assistance (Davis et al., 1998; Frier, 2004).
The brain is highly glucose dependent, but it can neither synthesize glucose nor store significant amounts of it (Delamater, 2006; Rao et al., 2006). With the more frequent use of intensive therapies for T1D, symptomatic hypoglycemia has increased in incidence with more than 17% of individuals noting a hypoglycemic episode during a years treatment time (Feingold, 1991). Severe hypoglycemia, particularly that presenting with seizure or coma, may result in permanent impairment especially in children less than five years of age (Cryer, 2008). In addition, repeated episodes of hypoglycemia can negatively impact brain development and learning (Cryer, 2008). Even isolated acute episodes of mild hypoglycemia can transiently impair attention, mentation and memory (Northam et al., 2001).
T1D is, also, linked to an increase in mental health and mood difficulties including anxiety (McAulay et al., 2006), depression (Hislop et al., 2008) and social withdrawal (Delamater, 2006). Withdrawn children are anxious, lonely, fail to exhibit age-appropriate interpersonal problem-solving skills and are deficient in social skills and social relationships (Silverstein et al., 2005). In 2000, the International Society of Pediatric and Adolescent Diabetes (ISPAD) Consensus Guidelines stated that “Psychosocial factors are the most important influences affecting the care and management of diabetes” and these recommendations were reiterated in the 2006/2007 ISPAD guidelines (Delamater, 2007). Unfortunately, very little is known about how T1D causes neurocognitive, psychosocial and behavioral difficulties and how they are regulated in the body either chronically or acutely. Therefore, we sought to investigate the acute mechanism by which insulin-induced hypoglycemia causes the adverse behavior of social withdrawal using a mouse model.
Materials and Methods
Materials
All reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO) except for Humalin R (insulin), which was purchased from Eli Lilly (Indianapolis, IN).
Animals
All animal care and use was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council). C57BL/6J mice were bred in-house from mice purchased from The Jackson Laboratory. Mice were group housed (4-8) in standard shoebox cages (17.15 × 28 cm) in a temperature (23°C) and humidity (45–55%) controlled environment with a 12-h/12-h dark-light cycle (0800 h to 2000 h). Mice were fed pelleted food (NIH 5K52; LabDiet; Purina Mills) and water ad libitum. Male 8- to 12-wk-old animals were used for all experiments. Animals were administered Epi, NE, and insulin at the indicated concentrations via IP injection. Butoxamine and terbutaline were administered IP at 5 mg/kg/mouse.
Blood glucose
Blood was collected from the tail blood as we described previously (Hartman et al., 2004). Briefly, blood glucose levels were measured using a One Touch Ultra glucometer (Johnson & Johnson) per the manufacturer's instructions. In brief, mice were placed in a very shallow shoebox sized container (17.15 × 28 × 4 cm) such that the tail was exposed. The tip of the tail was then secured against the top of the container, snipped and blood drawn. Blood glucose was measured on the same mice utilized in the social withdrawal experiments.
Social withdrawal
Social withdrawal was measured as described (Hartman et al., 2004). In brief, juvenile and adult mice were individually housed for 18 h prior to experimentation. A novel 3- to 4-week-old conspecific juvenile mouse (challenge mouse) was then confined to a 7.62 × 7.62 cm wire mesh enclosure (with a perforated steel top and bottom) which was placed in the corner of the home cage of the adult mouse (test mouse) for 5 min immediately prior to and at the indicated times after treatment (n=3∼4). A novel juvenile was supplied for each interaction at every time point. Interaction (nose contact) between test and challenge mouse was video-recorded. Time spent by the test mouse in exploratory behavior was determined from video records. To control for mouse-to-mouse variability in baseline activity and to allow comparison of relative changes in exploration levels, a pre-exposure (0 h) measurement was used as an internal control for each mouse. Results are expressed as percentage of baseline measurement and shown as means ± SEM. For all behavior experiments, mice were fasted for 12 h then pre-injected IP (where indicated) with the described agonist, antagonist or saline 0.5 h prior to IP insulin or IP saline administration, Unrestricted access to food was provided 0.75 h after agonist or insulin administration. Social exploration was measured at the time points indicated with the clock starting after insulin delivery. In experiments without insulin, the starting point was after agonist or saline delivery. All experiments were performed under red light, during the dark cycle 1 h into darkness.
Movement
Movement was measured in a four arm, black, Plexiglas cross maze (arms = 27.5 cm in length × 8cm in width × 10 cm wall height: central platform = 8 cm × 8 cm) by methods previously described (Ragozzino, 1998). In brief, mice were placed on the center platform at the times indicated. Movement, as assessed by arm entries, was recorded over a 5 min period (from video records). The mouse was required to have all four legs in the arm for an arm entry to have occurred.
Plasma catecholamine analysis
After the indicated treatments, mice were anesthetized with sodium ketamine hydrochloride:xylazine hydrochloride (80 mg/ml:12 mg/ml, ketamine:xylazine) at 1.5 ml/kg body weight and blood removed from the left ventricle. Blood was collected into chilled heparinized centrifuge tubes and spun at 9300 × G for 8 min. Plasma was aspirated and stored at -80° C. Catecholamines were determined from plasma by reverse-phase high performance liquid chromatography (HPLC). Solid phase extraction was with aluminum oxide (Bioanalytical Systems, West Lafayette, IN) and elution was in 0.2 N perchloric acid. Dihydroxybenzylamine was used as an internal standard to determine extraction efficiency. Electrochemical detection (ESA, Chelmsford, MA) utilized a 150 × 2 mm C18 (3 μm) Hypersil column (Keystone Scientific, Bellfonte, PA) fitted with a 2 mm C18 (3 μm) Hypersil javelin guard column (Keystone Scientific). Mobile phase (pH = 3.0) was 75 mM NaH2PO4, 1.7 mM 1-ocatanesulfonic acid, 25 μM Na2EDTA, 7% (vol/vol) acetonitrile, and 0.1% (vol/vol) triethylamine. The interassay coefficient of variation was less than 3%.
Statistical Analysis
Data are presented as mean ± SEM and were analyzed by two- or three-way ANOVA depending on the experimental design with repeated measurements in the time factor as applicable. Post hoc comparisons of individual group means were carried out with the Tukey test (SAS Institute, Cary, NC). Statistical significance was denoted at P < 0.05.
Results
Insulin induced hypoglycemia is associated with social withdrawal
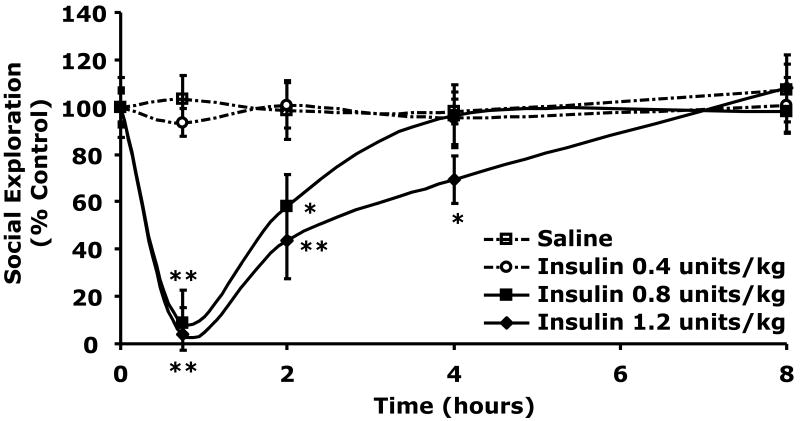
Table 1 demonstrates that when C57BL/6J mice were withheld food for 12 h blood glucose ranged from 117 ± 6 to 131 ± 11 mg/dl When mice were injected IP with insulin blood glucose fell. Blood glucose 0.75 h after 0.4, 0.8 or 1.2 units/kg of insulin was 99 ± 14 mg/dl (p = 0.029), 64 ± 4 mg/dl (p = 0.001) or 48 ± 5 mg/dl (p = 0.0008), respectively compared to control (151 ± 13 mg/dl). Food was made accessible to the mice 0.75 h post injection. At 8 h post insulin injection and 7.25 h post return to unrestricted food access, blood glucose ranged from 189 ± 17 to 200 ± 6 mg/dl in insulin treated and control animals. Fig.1 shows the impact of insulin administration on social exploration. At 0.75 h after IP insulin injection, social withdrawal was nearly complete in mice treated with 0.8 and 1.2 units/kg insulin demonstrating a 91 ± 11% (p = 0.0001) and 96 ± 5% (p = 0.0001) loss in social exploration. Insulin delivered at 0.4 units/kg did not impact social exploration. In addition, recovery from insulin-induced social withdrawal took 4 and 8 h to recovery from after 0.8 and 1.2 units/kg insulin, respectively. Finally, arm entries into a plus maze were examined to assess mouse mobility after administration of 0.8 units/kg insulin. As with social withdrawal, 0.75 h after insulin injection, arm entries in insulin-treated mice were reduced {44 ± 10 vs 14 ± 4 (p = 0.038)}. After 3 h (for arm entries), insulin-treated mice had fully recovered. Taken together these findings indicate that insulin-induced hypoglycemia is associated with social withdrawal and loss of movement.
Table 1. Blood Glucose (mg/dl) After Insulin Injection.
| Treatment | 0 h | 0.75 h | 8 h |
|---|---|---|---|
| Saline | 130 ± 11 | 151 ± 13 | 196 ± 4 |
| Insulin 0.4 units/kg | 131 ± 11 | 99 ± 14 * | 190 ± 19 |
| Insulin 0.8 units/kg | 117 ± 6 | 64 ± 4 * | 200 ± 6 |
| Insulin 1.2 units/kg | 124 ± 18 | 48 ± 5 * | 189 ± 17 |
Fig.1. Insulin induces social withdrawal.
After a 12 h fast, C57BL/6J mice were administered either insulin (Insulin) or saline control (Saline) IP as indicated. Social exploration was measured at 0, 0.75, 2, 4, and 8 h after insulin delivery. Unrestricted access to food was provided after the 0.75 time point. Results are expressed as percentages of the baseline measurement, means ± SEM; n=3, *P <0.05, **P <0.001 Insulin vs. Saline.
Catecholamines cause social withdrawal
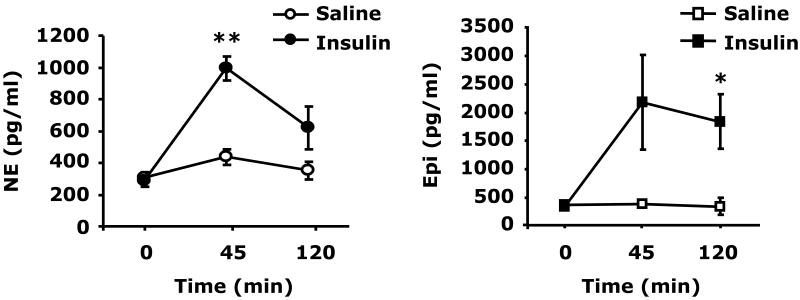
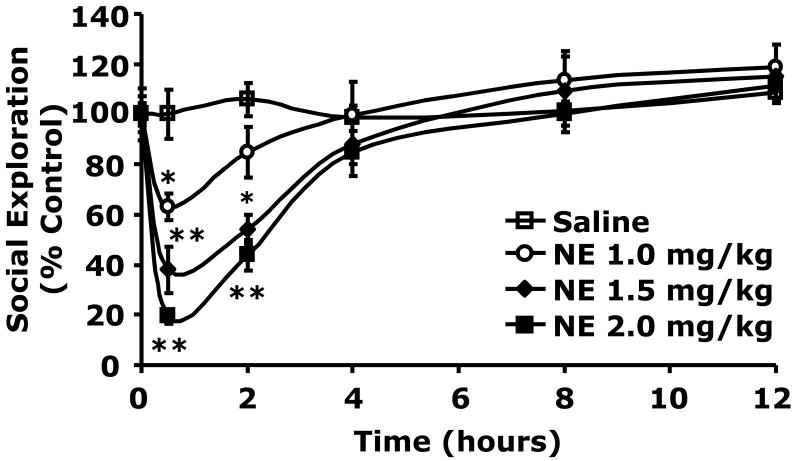
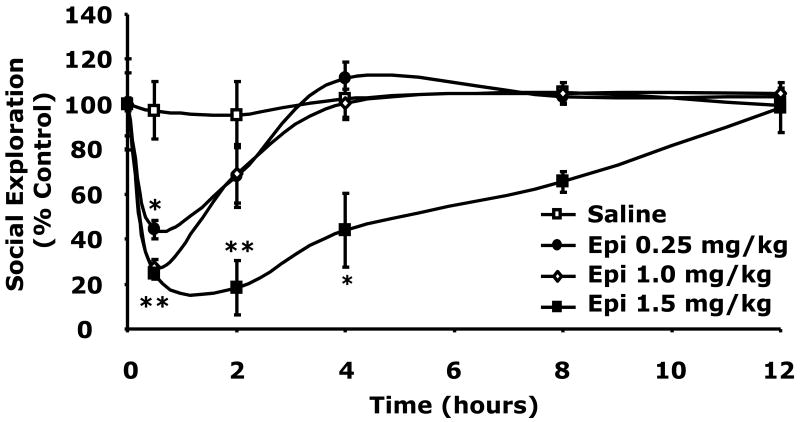
Fig.2 demonstrates that 0.8 units/kg insulin IP induced a marked elevation in plasma NE and Epi. At 0.75 h after insulin, NE was increased compared to control, 994 ± 73 pg/ml vs 439 ± 50 pg/ml (p = 0.001). At 120 min after insulin, NE returned to near control levels, 994 ± 73 pg/ml vs 351 ± 54 pg/ml (p = 0.052). After insulin (0.75 h), Epi increased to 2184 ± 833 pg/ml vs 390 ± 11 pg/ml (p = 0.089) and was significantly elevated at 120 min post insulin, 1842 ± 472 pg/ml vs 351 ± 144 pg/ml (p = 0.01). To determine the impact of catecholamines on social withdrawal, social exploration was examined. Fig.2B shows that when NE was administered IP at 1.0, 1.5 or 2.0 mg/kg social exploration was significantly curtailed 0.5 h after injection {63 ± 5% (p = 0.009), 38 ± 9% (p < 0.001) or 19 ± 3% (p < 0.001), respectively}. Recovery from NE-induced social withdrawal occurred at 2 h for NE at 1.0 mg/kg and at 4 h for NE at 1.5 and 2 mg/kg. Fig.2C demonstrates that Epi was a more potent inducer of social withdrawal. At 0.25, 1.0 and 1.5 mg/kg, Epi caused social exploration to fall to 44 ± 4% (p = 0.0002), 27 ± 3% (p < 0.0001) and 24 ± 2% (p < 0.0001) of control, respectively, 0.5 h after administration. Recovery occurred in 2, 4 and 12 h after 0.25, 1.0 and 1.5 mg/kg Epi, respectively. Taken together these findings indicate that catecholamines cause social withdrawal.
Fig.2. Catecholamines cause social withdrawal.
(A) After a 12 h fast, C57BL/6J mice were administered either insulin (Insulin) or saline control (Saline) at 0.8 units/kg insulin IP as indicated. Plasma catecholamines were measured by HPLC at 0, 45 and 120 min post insulin injection. Results are expressed as mean ± SEM; n = 3, *P <0.01, **P <0.001 Insulin vs. Saline. (B) Mice were administered NE (IP) at the concentrations indicated. Social exploration was measured at 0, 0.5, 2, 4, 8 and 12 h after NE delivery. Results are expressed as percentages of the baseline measurement, means ± SEM; n=4. *P<0.01, **P < 0.0001, NE vs. Saline. (C) Mice were administered Epi (IP) at the concentrations indicated. Social exploration was measured at 0, 0.5, 2, 4, 8 and 12 h after Epi delivery. Results are expressed as percentages of the baseline measurement, means ± SEM; n=4, *P<0.01, **P < 0.0001, Epi vs. Saline.
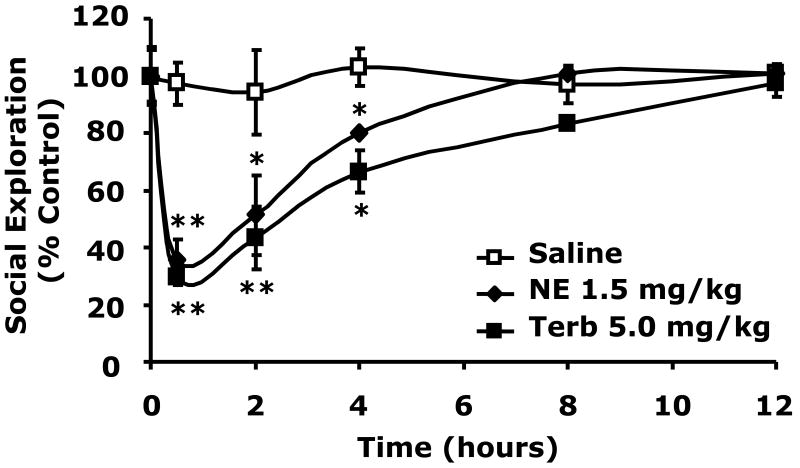
Beta-2 adrenergic receptor stimulation causes social withdrawal which beta-2 adrenergic receptor antagonism prevents
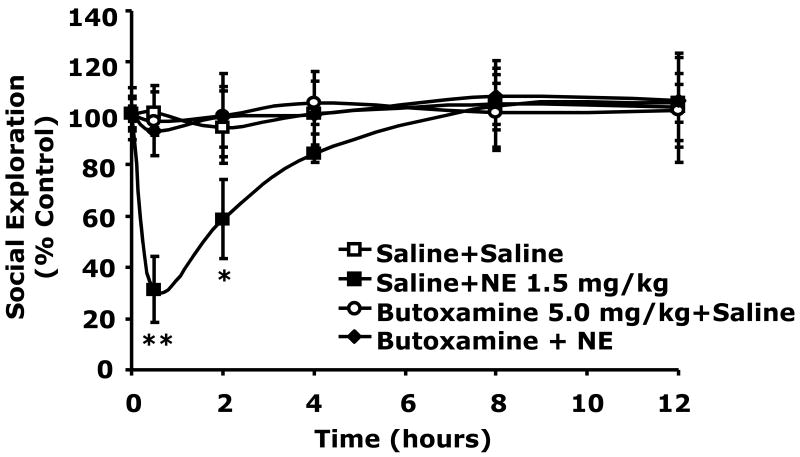
To determine if catecholamine dependent social withdrawal was mediated by the beta-2 adrenergic receptor, beta-2 agonism was performed using the beta-2 agonist terbutaline (Podojil et al., 2004; Ito et al., 2006; Thaker et al., 2006). Fig.3A shows that when terbutaline was administered IP at 5.0 mg/kg social withdrawal occurred similar to that seen with 1.5 mg/kg NE (30 ± 3% vs. 35 ± 7% at 0.5 h), (43 ± 11% vs. 51 ± 14% at 2 h), (67 ± 7% vs. 80 ± 1% at 4 h). Importantly, when the beta-2 antagonist butoxamine (Kaan et al., 1996; Junker et al., 2002) was administered IP at 5.0 mg/kg to mice just prior to NE injection (1.5 mg/kg), NE-dependent social withdrawal was completely blocked. Taken together these findings indicate that catecholamine-dependent social withdrawal is mediated by the beta-2 adrenergic receptor.
Fig.3. Beta-2 adrenergic receptor stimulation causes social withdrawal which beta-2 adrenergic receptor antagonism prevents.
(A) C57BL/6J mice were IP administered NE, terbutaline (Terb) or saline control (Saline) at the concentrations indicated. Social exploration was measured at 0, 0.5, 2, 4, 8 and 12 h after injection. Results are expressed as percentages of the baseline measurement, means ± SEM; n=3, *P<0.01, **P < 0.0001, NE or Terb vs. Saline. (B) Mice were IP administered NE, butoxamine (Butoxamine) or Saline at the concentrations indicated. Social exploration was measured at 0, 0.5, 2, 4, 8 and 12 h after injection. Results are expressed as percentages of the baseline measurement, means ± SEM; n=3, *P < 0.05, **P < 0.0001, NE vs. butoxamine + NE.
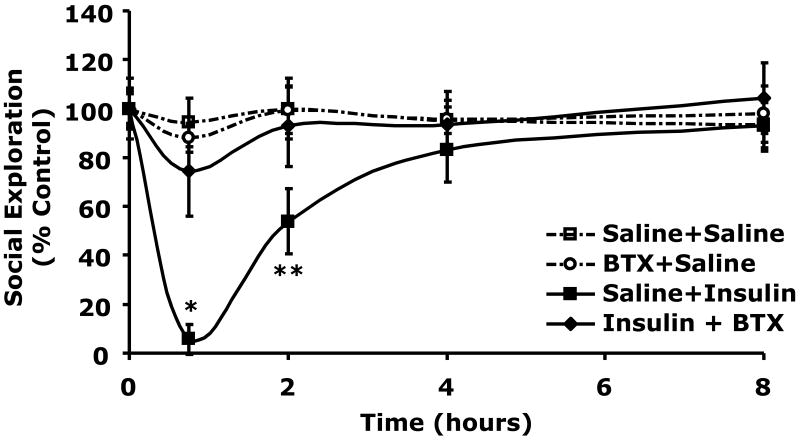
Butoxamine blocks insulin-induced social withdrawal
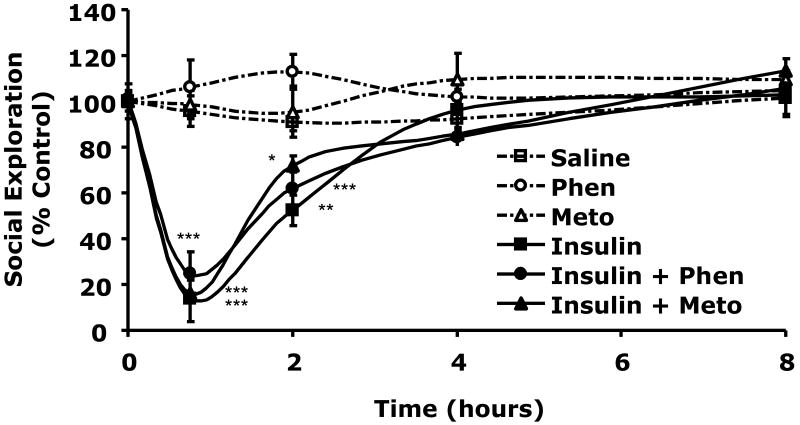
Table 2 demonstrates that when C57BL/6J mice were withheld food for 12 h blood glucose ranged from 137 ± 15 to 149 ± 17 mg/dl. When mice were injected IP with insulin (0.8 units/kg) or insulin (0.8 units/kg) + butoxamine (5 mg/kg) blood glucose fell to 67 ± 4 mg/dl (p < 0.0001) or 78 ± 6 mg/dl (p = 0.0004), respectively compared to control (150 ± 10 mg/dl). At 8 h post insulin injection and 7.25 h post return to unrestricted food access, blood glucose ranged from 202 ± 13 to 231 ± 7 mg/dl in insulin treated and control animals. Butoxamine does not alter the hypoglycemic response to insulin. Fig.4A shows the impact of butoxamine administration on social exploration. At 0.75 h after IP insulin injection, social withdrawal was nearly complete demonstrating a 96 ± 6% (p = 0.0007) loss in social exploration. Importantly, butoxamine completely blocked the effect of insulin-induced hypoglycemia on social withdrawal while the pan-alpha blocker phentoloamine and beta-1 specific antagonist metoprolol did not (Fig.4B). Taken together these findings indicate that insulin-induced hypoglycemia-dependent social withdrawal is mediated by the beta-2 adrenergic receptor.
Table 2. Blood Glucose (mg/dl) After Insulin And Butoxamine Injection.
| Treatment | 0 h | 0.75 h | 8 h |
|---|---|---|---|
| Saline | 149 ± 17 | 150 ± 10 | 226 ± 17 |
| Butoxamine | 139 ± 7 | 151 ± 14 | 202 ± 13 |
| Insulin | 137 ± 15 | 67 ± 4 * | 224 ± 11 |
| Ins + BTX# | 142 ± 7 | 78 ± 6 * | 231 ± 7 |
Ins = insulin, BTX = butoxamine
Fig.4. Butoxamine blocks insulin-induced social withdrawal.
A, After a 12 h fast, C57BL/6J mice were pretreated with or without butoxamine (BTX) (5 mg/kg, IP) as indicated. Mice were then administered insulin (Ins) (0.8 units/kg, IP or saline control (Saline) IP as indicated. Social exploration was measured at 0, 0.75, 2, 4, and 8 h after insulin delivery. Unrestricted access to food was provided after the 0.75 time point. Results are expressed as percentages of the baseline measurement, means ± SEM; n=3, *P < 0.0001, **P = 0.0007 Insulin vs Insulin + butoxamine. B, Like in A, C57BL/6J mice were pretreated with either phentolamine (Phen) (1 mg/kg, IP) or metoprolol (Meto) (10 mg/kg, IP), as indicated. Mice were then administered insulin (0.8 units/kg) or saline control IP. Social exploration was measured as in A. Results are expressed as percentages of the baseline measurement means ± SEM; n=4-6, *p=0.0126 **p=0.0018 ***p<0.0001 Saline vs. Insulin +/- Phen or Meto.
Discussion
We have previously shown that in mouse models of T1D and type 2 diabetes (T2D) social withdrawal induced by innate immune activation is exaggerated and prolonged (Lin et al., 2007). In diabetic mice administered the toll-like receptor 4 (TLR-4) agonist lipopolysaccharide (LPS), prolonged immune-activated social withdrawal appeared dependent on hyperglycemia (Lin et al., 2007). This is likely due to the impact of hyperglycemia on macrophages because hyperglycemia augments LPS-induced pro-inflammatory cytokine production by macrophages via a pathway requiring p38 map kinase (Sherry et al., 2007). In general, social withdrawal as part of classical sickness symptoms is caused by innate immune activation (Dantzer, 2004) and is dependent on pro-inflammatory cytokines, especially TNF alpha and IL-1 beta, and their impact in the brain (Dantzer et al., 2008).
As we show and report in the results for Fig.1, insulin-induced hypoglycemia causes social withdrawal and reduced mouse movement. When insulin is administered at 1.2 units/kg, blood glucose nadirs at 48 mg/dl (Table 1) 0.75 h after insulin injection, which corresponds with nearly complete social withdrawal. Interestingly, hypoglycemia-associated social withdrawal took 8 h to fully recovery from indicating a significant behavioral impact of hypoglycemia extending well beyond the acute event. This phenomenon should not be surprising because hypoglycemia triggers a variety of bioactive compounds that raise blood glucose. These include catecholamines, glucagon, growth hormone and cortisol. Metabolically, these agents stimulate glucose production initially through glycogenolysis and then later through gluconeogenesis, decreased muscle glucose storage/oxidation and use of alternative fuels (Hoffman, 2007). Catecholamines, especially, are key to the early glucose rise in T1D because disease-based loss of pancreatic islet cells also disrupts the ability of the pancreas to produce glucagons (Brown et al., 2008).
Insulin-induced hypoglycemia was also associated with decreased mouse movement as measured by arm entries in a cross maze. When examined as a percent (at 0.8 units/kg insulin), loss of social exploration (at 0.75 h) was greater (91%) than loss of movement (68%). Indicating that loss of social exploration may not just be due to a simple loss of activity. In general, severe insulin-induced hypoglycemia lowers brain ATP stores that can take up to 3 h to fully recovery, if the hypoglycemia is serious enough to cause coma as documented by EEG (Agardh and Rosen, 1983). When insulin was used to drop blood glucose in humans with diabetes from ∼180 mg/dl to ∼40 mg/dl in a time span of 1 h, adrenergic symptoms as measured by pulse returned to normal 1 h after the pulse peaked at 1 h post insulin administration. (Deacon et al., 1977). Unfortunately, in both animals and humans, little has been reported regarding recuperation from insulin-induced hypoglycemia and almost nothing is known about recovery from adverse behaviors associated with insulin-induced hypoglycemia. Most work has focused on how to effectively and rapidly restore blood glucose and other metabolic indicators of hypoglycemia and correlating return of these biomarkers to normal as resolution (Pratley and Salsali, 2007). Only in severe coma-inducing hypoglycemia does the brain tend to be examined, but in these studies behavior and behavioral recovery is ignored.
Gold et al (Gold et al., 1995; Gold et al., 1997) has examined and reviewed the non-cognitive impact of insulin-induced hypoglycemia. In non-diabetic participants, they found that hypoglycemia caused mood changes including a reduction in hedonic tone and energetic arousal and an increase in tense arousal. They also noted that tense-tiredness persisted for at least 30 min after restoration of euglycemia (Gold et al., 1993). Tense-tiredness may be of particular relevance to T1D in that it is a mood where fatigue is mixed with nervousness, tension or anxiety and often underlies depression. (Westfall and Westfall, 2005; Lustman and Clouse, 2007). It is important to note that social withdrawal is a component of these behaviors including tense arousal, fatigue and anxiety (Westfall and Westfall, 2005). In addition, there may be a stratification of hypoglycemia-associated behaviors because we found that peak social withdrawal was more severe than peak loss of movement.
Another question Fig.1 poses is whether insulin, itself, not insulin-induced hypoglycemia causes the social withdrawal observed. Gold et al found that the mood disturbances they observed occurred in the insulin-induced hypoglycemia subjects and not those exposed to hyperinsulinemic glucose clamp (Martelli et al., 1995). In addition, we have shown that in a mouse model of T1D insulin does not induce social withdrawal, but appears to improve social exploration in hyperglycemic mice especially if insulin is administered ICV (Lin et al., 2007). We have also shown that in non-diabetic and T2D mice that IGF-I does not impact baseline social exploration (Johnson et al., 2005).
Fig.2A demonstrates that the insulin dose administered was significant enough to up-regulate plasma NE and Epi. These findings indicated that NE or Epi might be responsible for the social withdrawal seen with insulin-induced hypoglycemia. As Fig.2B and C show, NE and Epi both cause social withdrawal. Interestingly, Epi appears to be a more potent inducer of social withdrawal being able to cause social withdrawal at one quarter the dose of NE. In addition, the impact of Epi on social withdrawal was significantly longer lasting when both were administered at 1.5 mg/kg. Fig.3 shows that the beta-2 adrenergic receptor agonist terbutaline induces social withdrawal and that the beta-2 receptor agonist butoxamine completely blocks NE-induced social exploration. Importantly, butoxamine did not raise blood glucose in response to insulin (Table 2) suggesting that insulin-induced social withdrawal is not mediated directly by hypoglycemia but by the impact that hypoglycemia has on catecholamines. Together these findings point to beta-2 adrenergic stimulation as key to catecholamine-dependent social withdrawal. Critically, the beta-2 antagonist butoxamine blocked insulin-induced social withdrawal (Fig.4A) while the pan-alpha blocker, phentolamine, and the beta-1 blocker, metoprolol, did not. (Fig.4B). These findings strongly support our contention than hypoglycemia-associated social withdrawal induced by insulin is dependent on catecholamines via a beta-2 receptor-mediated pathway. While these finding do not exclude glucagon and/or cortisol/corticosterone as modulators of behavior in insulin-induced hypoglycemia, with regard to social withdrawal, catecholamines appear paramount.
A key question is how NE/Epi cause social withdrawal. Both NE and Epi (as well as terbutaline) are rather polar compounds that do not readily enter the CNS (Westfall and Westfall, 2005). In general, Epi may cause restlessness and apprehension but these feelings in humans are usually ascribed to the effect of Epi on the cardiovascular system, skeletal muscle and/or intermediary metabolism (Westfall and Westfall, 2005). NE is less commonly linked to restlessness and apprehension than Epi (Westfall and Westfall, 2005) and, like Epi, NE is rapidly inactivated by the same enzymes that methylate and oxidatively deaminate Epi (Westfall and Westfall, 2005). In our study, the apparent reason Epi is a more potent inducer of social withdrawal than NE is that Epi is a more effective beta-2 adrenergic agonist than NE (Westfall and Westfall, 2005). Support for this contention is that the beta-2 selective adrenergic agonist terbutaline caused social withdrawal and, in general, beta-2 agonists are more likely to induce feelings of restlessness, apprehension, and anxiety (Westfall and Westfall, 2005). Importantly, these behaviors are linked in certain instances to social withdrawal (Westfall and Westfall, 2005). The probable mechanism by which beta-2 adrenergic stimulation causes social withdrawal either due to adrenergic agents or insulin-induced hypoglycemia and subsequent catecholamine up-regulation is through “stress”-induced hypothalamic NE turnover (Weiss et al., 1975; Anisman and Sklar, 1979) because NE turnover induces social withdrawal in immune-based sickness models (Marvel et al., 2004). Finally, what causes glucoprivic triggering of noradrenergic neurons in the ventromedial hypothalamus is not clear (Levin, 2007), but nearly one-third of young adults with T1D experience psychological distress and this distress appears linked to hypoglycemia especially in those attempting tighter glucose control with subcutaneous insulin infusion (Hislop, 2008). Therefore, the importance of understanding the adverse impact of hypoglycemia on behavior is significant.
Acknowledgments
Support: This research was supported by grants from the National Institutes of Health (DK64862 and NS58525 to G.G.F.) and University of Illinois Agricultural Experiment Station (to G.G.F.). This work was supported in part by a grant from the U.S. Department of Homeland Security, Assistance to Firefighters Grants Office, Research and Development Grants (EMW-2006-FP-02459).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agardh CD, Rosen I. Neurophysiological recovery after hypoglycemic coma in the rat: Correlation with cerebral metabolism. J Cereb Blood Flow Metab. 1983;3:78–85. doi: 10.1038/jcbfm.1983.10. [DOI] [PubMed] [Google Scholar]
- Anisman H, Sklar LS. Catecholamine depletion in mice upon reexposure to stress: Mediation of the escape deficits produced by inescapable shock. J Comp Physiol Psychol. 1979;93:610–625. doi: 10.1037/h0077603. [DOI] [PubMed] [Google Scholar]
- Binder C, Bendtson I. Endocrine emergencies hypoglycaemia. Baillieres Clin Endocrinol Metab. 1992;6:23–39. doi: 10.1016/s0950-351x(05)80329-5. [DOI] [PubMed] [Google Scholar]
- Bolli GB. Hypoglycaemia unawareness. Diabetes Metab. 1997;23(Suppl 3):29–35. [PubMed] [Google Scholar]
- Brown RJ, Sinaii N, Rother KI. Too much glucagon, too little insulin: time course of pancreatic islet dysfunction in new-onset type 1 diabetes. Diabetes Care. 2008;31:1403–1404. doi: 10.2337/dc08-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer PE. Hypoglycemia. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo JL, editors. Harrison's principles of internal medicine. McGraw-Hill; 2008. pp. 2305–2309. [Google Scholar]
- Daneman D. Type 1 diabetes. Lancet. 2006;367:847–858. doi: 10.1016/S0140-6736(06)68341-4. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behaviour: A neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EA, Keating B, Byrne GC, Russell M, Jones TW. Impact of improved glycaemic control on rates of hypoglycaemia in insulin dependent diabetes mellitus. Arch Dis Child. 1998;78:111–115. doi: 10.1136/adc.78.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon SP, Karunanayake A, Barnett D. Acebutolol, atenolol, and propranolol and metabolic responses to acute hypoglycaemia in diabetics. Br Med J. 1977;2:1255–1257. doi: 10.1136/bmj.2.6097.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamater AM. ISPAD clinical practice consensus guidelines 2006–2007 psychological care of children and adolescents with diabetes. 2006. [DOI] [PubMed] [Google Scholar]
- Delamater AM. Psychological care of children and adolescents with diabetes. Pediatr Diabetes. 2007;8:340–348. doi: 10.1111/j.1399-5448.2007.00318.x. [DOI] [PubMed] [Google Scholar]
- Feingold KR. Hypoglycemia - a major risk of insulin therapy. West J Med. 1991;154:469–471. [PMC free article] [PubMed] [Google Scholar]
- Frier BM. Morbidity of hypoglycemia in type 1 diabetes. Diabetes Res Clin Pract. 2004;65(Suppl 1):S47–52. doi: 10.1016/j.diabres.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Gold AE, Deary IJ, Frier BM. Hypoglycemia and cognitive function. Diabetes Care. 1993;16:958–959. doi: 10.2337/diacare.16.6.958. [DOI] [PubMed] [Google Scholar]
- Gold AE, MacLeod KM, Frier BM, Deary IJ. Changes in mood during acute hypoglycemia in healthy participants. J Pers Soc Psychol. 1995;68:498–504. doi: 10.1037//0022-3514.68.3.498. [DOI] [PubMed] [Google Scholar]
- Gold AE, Deary IJ, Frier BM. Hypoglycaemia and non-cognitive aspects of psychological function in insulin-dependent (type 1) diabetes mellitus (IDDM) Diabet Med. 1997;14:111–118. doi: 10.1002/(SICI)1096-9136(199702)14:2<111::AID-DIA309>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Hartman ME, O'Connor JC, Godbout JP, Minor KD, Mazzocco VR, Freund GG. Insulin receptor substrate-2-dependent interleukin-4 signaling in macrophages is impaired in two models of type 2 diabetes mellitus. J Biol Chem. 2004;279:28045–28050. doi: 10.1074/jbc.M404368200. [DOI] [PubMed] [Google Scholar]
- Hislop AL, Fegan PG, Schlaeppi MJ, Duck M, Yeap BB. Prevalence and associations of psychological distress in young adults with type 1 diabetes. Diabet Med. 2008;25:91–96. doi: 10.1111/j.1464-5491.2007.02310.x. [DOI] [PubMed] [Google Scholar]
- Hoffman RP, Sinkey CA, Anderson EA. Hypoglycemic symptom variation is related to epinephrine and not peripheral muscle sympathetic nerve response. J Diabetes Complications. 1997;11:15–20. doi: 10.1016/1056-8727(95)00082-8. [DOI] [PubMed] [Google Scholar]
- Hoffman RP. Sympathetic mechanisms of hypoglycemic counterregulation. Curr Diabetes Rev. 2007;3:185–193. doi: 10.2174/157339907781368995. [DOI] [PubMed] [Google Scholar]
- Ito T, Fujimura N, Omote K, Namiki A. A selective beta2-adrenergic agonist, terbutaline, improves sepsis-induced diaphragmatic dysfunction in the rat. Life Sci. 2006;79:905–912. doi: 10.1016/j.lfs.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Johnson DR, O'Connor JC, Dantzer R, Freund GG. Inhibition of vagally mediated immune-to-brain signaling by vanadyl sulfate speeds recovery from sickness. Proc Natl Acad Sci U S A. 2005;102:15184–15189. doi: 10.1073/pnas.0507191102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker V, Becker A, Hühne R, Zembatov M, Ravati A, Culmsee C, Krieglstein J. Stimulation of beta-adrenoceptors activates astrocytes and provides neuroprotection. Eur J Pharmacol. 2002;446:25–36. doi: 10.1016/s0014-2999(02)01814-9. [DOI] [PubMed] [Google Scholar]
- Kaan SK, Mei QB, Cho CH. A mechanistic study of beta-adrenoceptor antagonists on ethanol-induced gastric damage. Eur J Pharmacol. 1996;317:115–122. doi: 10.1016/s0014-2999(96)00705-4. [DOI] [PubMed] [Google Scholar]
- Korytkowski MT, Mokan M, Veneman TF, Mitrakou A, Cryer PE, Gerich JE. Reduced beta-adrenergic sensitivity in patients with type 1 diabetes and hypoglycemia unawareness. Diabetes Care. 1998;21:1939–1943. doi: 10.2337/diacare.21.11.1939. [DOI] [PubMed] [Google Scholar]
- Levin BE. Neuronal glucose sensing: still a physiological orphan? Cell Metab. 2007;6:252–254. doi: 10.1016/j.cmet.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Lin KI, Johnson DR, Freund GG. LPS-dependent suppression of social exploration is augmented in type 1 diabetic mice. Brain Behav Immun. 2007;21:775–782. doi: 10.1016/j.bbi.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Clouse RE. Depression in diabetes: The chicken or the egg? Psychosom Med. 2007;69:297–299. doi: 10.1097/PSY.0b013e318060cc2d. [DOI] [PubMed] [Google Scholar]
- Martelli A, Allavena A, Campart GB, Canonero R, Ghia M, Mattioli F, Mereto E, Robbiano L, Brambilla G. In vitro and in vivo testing of hydralazine genotoxicity. J Pharmacol Exp Ther. 1995;273:113–120. [PubMed] [Google Scholar]
- Marvel FA, Chen CC, Badr N, Gaykema RP, Goehler LE. Reversible inactivation of the dorsal vagal complex blocks lipopolysaccharide-induced social withdrawal and c-fos expression in central autonomic nuclei. Brain Behav Immun. 2004;18:123–134. doi: 10.1016/j.bbi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- McAulay V, Deary IJ, Sommerfield AJ, Matthews G, Frier BM. Effects of acute hypoglycemia on motivation and cognitive interference in people with type 1 diabetes. J Clin Psychopharmacol. 2006;26:143–151. doi: 10.1097/01.jcp.0000203202.41947.6d. [DOI] [PubMed] [Google Scholar]
- Northam EA, Anderson PJ, Jacobs R, Hughes M, Warne GL, Werther GA. Neuropsychological profiles of children with type 1 diabetes 6 years after disease onset. Diabetes Care. 2001;24:1541–1546. doi: 10.2337/diacare.24.9.1541. [DOI] [PubMed] [Google Scholar]
- Podojil JR, Kin NW, Sanders VM. CD86 and beta2-adrenergic receptor signaling pathways, respectively, increase Oct-2 and OCA-B Expression and binding to the 3′-IgH enhancer in B cells. J Biol Chem. 2004;279:23394–23404. doi: 10.1074/jbc.M313096200. [DOI] [PubMed] [Google Scholar]
- Pratley RE, Salsali A. Inhibition of DPP-4: A new therapeutic approach for the treatment of type 2 diabetes. Curr Med Res Opin. 2007;23:919–931. doi: 10.1185/030079906x162746. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Adams S, Kesner RP. Differential involvement of the dorsal anterior cingulate and prelimbic-infralimbic areas of the rodent prefrontal cortex in spatial working memory. Behav Neurosci. 1998;112:293–303. doi: 10.1037//0735-7044.112.2.293. [DOI] [PubMed] [Google Scholar]
- Rao J, Oz G, Seaquist ER. Regulation of cerebral glucose metabolism. Minerva Endocrinol. 2006;31:149–158. [PubMed] [Google Scholar]
- Service FJ. Hypoglycemic disorders. N Engl J Med. 1995;332:1144–1152. doi: 10.1056/NEJM199504273321707. [DOI] [PubMed] [Google Scholar]
- Shalitin S, Phillip M. The role of new technologies in treating children and adolescents with type 1 diabetes mellitus. Pediatr Diabetes. 2007;8(Suppl 6):72–79. doi: 10.1111/j.1399-5448.2007.00279.x. [DOI] [PubMed] [Google Scholar]
- Sherry CL, O'Connor JC, Kramer JM, Freund GG. Augmented lipopolysaccharide-induced TNF-alpha production by peritoneal macrophages in type 2 diabetic mice is dependent on elevated glucose and requires p38 MAPK. J Immunol. 2007;178:663–670. doi: 10.4049/jimmunol.178.2.663. [DOI] [PubMed] [Google Scholar]
- Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, Deeb L, Grey M, Anderson B, Holzmeister LA, Clark N. American Diabetes Association: Care of children and adolescents with type 1 diabetes: A statement of the american diabetes association. Diabetes Care. 2005;28:186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- Ste Marie L, Palmiter RD. Norepinephrine and epinephrine-deficient mice are hyperinsulinemic and have lower blood glucose. Endocrinology. 2003;144:4427–4432. doi: 10.1210/en.2003-0561. [DOI] [PubMed] [Google Scholar]
- Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, Merritt WM, Lin YG, Mangala LS, Kim TJ, Coleman RL, Landen CN, Li Y, Felix E, Sanguino AM, Newman RA, Lloyd M, Gershenson DM, Kundra V, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Glazer HI, Pohorecky LA, Brick J, Miller NE. Effects of chronic exposure to stressors on avoidance-escape behavior and on brain norepinephrine. Psychosom Med. 1975;37:522–534. doi: 10.1097/00006842-197511000-00006. [DOI] [PubMed] [Google Scholar]
- Westfall TC, Westfall DP. Adrenergic agonists and antagonists. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & gilman's the pharmacological basis of therapeutics. McGraw-Hill; 2005. pp. 237–295. [Google Scholar]