Abstract
BACKGROUND
Currently, histology is used as the endpoint to define success with photodynamic therapy (PDT) in patients with high-grade dysplasia (HGD). Recurrences despite ‘successful’ ablation are common. The role of biomarkers in assessing response to PDT remains undefined. The objectives of the current study were 1) to assess biomarkers in a prospective cohort of patients with HGD/mucosal cancer before and after PDT and 2) to correlate biomarker status after PDT with histology.
METHODS
Patients who underwent PDT for HGD/mucosal cancer were studied prospectively. All patients underwent esophagogastroduodenoscopy, 4-quadrant biopsies every centimeter, endoscopic mucosal resection of visible nodules, and endoscopic ultrasound. Cytology samples were obtained by using standard cytology brushes. Biomarkers were assessed by using fluorescence in situ hybridization (FISH). The biomarkers that were assessed included loss of 9p21 (site of the p16 gene) and 17p13.1 (site of the p53 gene) loci; gains of the 8q24(c-myc), 17q (HER2-neu), and 20q13 loci; and multiple gains. Patients received PDT 48 hours after the administration of sodium porfimer. Demographic and clinical variables were collected prospectively. Patients were followed with endoscopy and repeat cytology for biomarkers. The McNemar test was used to compare biomarker proportions before and after PDT.
RESULTS
Thirty-one patients were studied. The median patient age was 66 years (interquartile range [IQR], 56–73 years), and 28 patients (88%) were men. The mean Barrett segment length was 5 cm (standard error of the mean, 0.5 cm). Post-PDT biomarkers were obtained after a median duration of 9 months (IQR, 3–12 months). There was a statistically significant decrease in the proportion of several biomarkers assessed after PDT. Six patients without HGD after PDT still had positive FISH results for 1 or more biomarkers: of these, 2 patients (33%) developed recurrent HGD.
CONCLUSIONS
In this initial study, histologic downgrading of dysplasia after PDT was associated with the loss of biomarkers that have been associated with progression of neoplasia in Barrett esophagus. Patients with persistently positive biomarkers appeared to be at a higher risk of recurrent HGD. These findings should be confirmed in a larger study.
Keywords: Barrett esophagus, biomarkers, photodynamic therapy, response, esophageal carcinoma
Barrett esophagus (BE) predisposes patients to esophageal adenocarcinoma, a cancer with one of the fastest rising incidence rates over the past decade and a highly lethal malignancy once it is symptomatic.1,2 It is believed that esophageal adenocarcinoma arises as the final step of a postulated sequential change in the metaplastic epithelium, progressing from low-grade dysplasia (LGD), to high-grade dysplasia (HGD), and finally carcinoma. HGD on histologic samples has been used as the most reliable clinical biomarker of potential carcinogenesis, with studies reporting variable rates of progression to esophageal carcinoma (range, 16%–59%).3–5 Interruption of the metaplasia-dysplasia-carcinoma sequence by ablating or resecting this at-risk mucosa has been proposed as a strategy to reduce the incidence of esophageal adenocarcinoma and has served as the rationale for the recommendation of esophagectomy for patients with HGD.
Over the past few years, endoscopic therapy has emerged as an alternative to esophagectomy because of the significant mortality and morbidity associated with esophagectomy.6,7 Multiple modalities, including photodynamic therapy (PDT),8 argon plasma coagulation,9 and multipolar electrocoagulation10 in isolation and in combination with endoscopic mucosal resection (EMR),11,12 have been reported in the treatment of HGD. Variable success rates have been reported (range, 75%–90%). A randomized multicenter trial compared PDT with surveillance and treatment with omeprazole in patients with HGD. After a 24-month follow-up, complete ablation of HGD was noted in 77% of patients versus 39% in the omeprazole group. Thirteen percent of patients who received PDT plus omeprazole progressed to cancer compared with 28% of patients in the omeprazole group.13 This led to U.S. Food and Drug Administration approval for the use of PDT for the treatment of HGD in BE. We recently reported that long-term outcomes (overall mortality and cancer-free survival) were comparable between patients who underwent esophagectomy and patients who received PDT.14
Several genetic alterations have been described in BE. These include loss of cell cycle checkpoint genes, such as p16 and p53. Loss of these genes by allelic loss (deletions or loss of heterozygosity [LOH]), point mutations, or promoter hypermethylation (for p16) have been observed in a substantial number of patients with BE.15,16 Several other genetic alterations that involve gains/amplifications of proto-oncogenes (and growth factors/growth factor receptors) as well as changes in DNA content (as assessed by flow cytometry and image cytometry) also have been described.17,18
Currently, histology is used as the endpoint to define success with ablative therapy in HGD. However, recurrences and/or progression to cancer despite ‘successful’ ablation/resection are common. To our knowledge, predictors of recurrent dysplasia have not been defined. Few studies have assessed genetic alterations after ablation of BE19–21: those studies primarily assessed patients with predominantly nondysplastic BE (a category for which ablation currently is not recommended) rather than HGD (in which endoscopic ablation is gaining acceptance as an alternative to esophagectomy) by using techniques that lack long-term data on successful ablation. Studies also have raised concern about the appearance of cancer-associated biomarkers after ablation (in patients with nondysplastic BE)20: however, data are lacking on the clinical implication of this phenomenon in terms of the recurrence or progression of dysplasia.
The role of biomarkers in defining response to PDT remains unclear. The correlation between histologic response and ‘biomarker response’ also is unknown. We hypothesized that patients who remain positive for genetic alterations despite achieving a histologic response to PDT would be at risk for recurrence of dysplasia. In this study, we used fluorescence in situ hybridization (FISH) to characterize genetic alterations that were present in patients with HGD and mucosal cancer before treatment with PDT and compared them with the alterations observed after PDT. In addition, we assessed the recurrence of dysplasia after initial histologic response and correlated those findings with post-PDT genetic alterations assessed by FISH.
MATERIALS AND METHODS
Patients with HGD and/or mucosal cancer (defined as carcinoma confined to the mucosa without invasion of the submucosa) who were seen in the Barrett Esophagus Unit at St. Mary’s Hospital, Rochester, Minnesota between 2002 and 2006 were included in this study. Inclusion criteria were the presence of HGD/intramucosal carcinoma on biopsies, assessment of biomarkers using FISH before and after PDT, and treatment with PDT. Exclusion criteria included patients with submucosal cancer on EMR (who were referred to surgery) and patients who were unwilling or unable to consent to the study. Clinical, demographic, and endoscopic data were extracted from a prospectively maintained database and included length of Barrett segment, performance of EMR before PDT (including number of EMRs), number of PDT treatments per application, results of post-PDT biopsies (classified as carcinoma, HGD, LGD, or non-dysplastic BE), and biomarkers obtained using FISH before and after PDT.
All patients underwent 4-quadrant biopsies every centimeter of the involved esophagus. All patients had their diagnosis of HGD or mucosal cancer confirmed by 2 experienced gastrointestinal pathologists. Baseline assessments also included endoscopic ultrasound and EMR for any mucosal abnormalities. Computed tomography scans of the chest and upper abdomen were obtained in all patients.
Cytology Specimen Acquisition
Cytology specimens for FISH were obtained during the endoscopy immediately before PDT. During endoscopy, a cytology brush (Hobbs Medical Inc., Stafford Springs, Conn) was swept over the entire Barrett segment after the mucosal surface was sprayed with 10 mL of N-acetyl cysteine (as a mucolytic). Then, the brush was placed in a vial that contained 20 mL of PreservCyt solution (Cytyc Corp., Marlborough, Mass). Endoscopic brushing specimens were processed by washing the brush with 40 mL of 3:1 methanol:glacial acetic acid fixative solution. Cells were sedimented at ×800g for 8 minutes. The supernatant was removed, and the cell pellet was resuspended in 10 mL of 3:1 methanol:glacial acetic acid fixative solution. Next, the cells were sedimented again at ×300g for 8 minutes. All but ≈100 µL of the supernatant was removed. Finally, the cell pellet was resuspended, and the slides were prepared.
Slide Preparation
Approximately 3 µL of the cell pellet suspension were pipetted onto 3 etched 1-cm rings (Gold Seal, Portsmouth, NH), 1 ring for each of the 3 probe sets. Cellularity was assessed with a phase contrast microscope. Additional cell suspension was added to the slide until adequate cellularity was reached (ie, the highest number of cells possible per ring with minimal cell overlap) or until the cell pellet was exhausted. Table 1 describes the biomarkers that were assessed prospectively in this study.
TABLE 1.
Genetic Alterations Assessed Prospectively by Using Fluorescence In Situ Hybridization in Patients With High-grade Dysplasia or Mucosal Cancer in Barrett Esophagus
| Biomarker* | Definition of positive result |
|---|---|
| 9q21/P16 status | Loss |
| 17p 13.1/P53 status | Loss |
| 8q 24/C-MYC status | Gains |
| 17q/HER-2 status | Gains |
| 20q13 status | Gains |
| Cell ploidy | Multiple gains† |
The cutoffs used for 8q (C-MYC), 9p21 (P16), 17q (HER2), and 20q were established by using receiver operating characteristic (ROC) curves. Based on these ROC curves, a specimen was considered positive for P16 loss when ≥11% of cells exhibited hemizygous 9p21 loss, when ≥6% of cells exhibited homozygous 9p21 loss, or when ≥11% of cells exhibited a mixture of hemizygous and homozygous 9p21 loss. Thresholds for categorizing results as positive for the other probes were as follows: ≥14% of cells exhibited p53 loss (cutoff for P53 loss was the average percentage of P53 loss ±3 standard deviation observed in a normal value study) (unpublished observations), or ≥5% of cells demonstrated gains of 8q24, 17q11.2, or 20q13. ‘Multiple gains’ were defined as gains of 2 or more of the 8q24, 17q11.2, or 20q13 loci by using previously defined thresholds.
Defined as gains of 2 or more of the 4 probes in a patient.
Fluorescence In Situ Hybridization
Esophageal brushing cells were harvested, fixed, and placed on a slide as described previously.22 Then, the following fluorescently labeled DNA probes were hybridized to the specimens: 8q24.12–q24.13 (C-MYC), 9p21 (P16), 17p13.1 (p53), 17q11.2–q12 (HER-2), and 20q13.2 (Abbott Molecular Inc., Des Plaines, Ill). Next, the slides were washed and stained with the nuclear counterstain 4′,6-diamidino-2-phenylindole (Abbott Molecular Inc.). Fluorescence microscopy was used with unique band filters that were specific for each of the probe fluorophores to analyze and record all observed signal patterns for 100 (minimum, 50) consecutive, noninflammatory, nonsquamous cells. Enumeration was performed without knowledge of the patient’s clinical or histologic diagnosis. Details regarding the probes and thresholds for positivity are provided in Table 1.
Photodynamic Therapy
Porfimer sodium (Photofrin; Axcan Pharma, Mont-St.-Hilaire, Quebec, Canada) was used as a photosensitizer at a dose of 2 mg/kg. Photofrin was administered intravenously 48 hours before photoradiation, which was performed by using a bare cylindrical diffusing fiber. The cylindrical diffusing fibers were either 2.5 cm or 5.0 cm in length (Fibers Direct, Andover, Mass). The cylindrical diffusing fiber was passed through the accessory channel of the endoscope and placed in the center of the esophageal lumen. The light was delivered from a laser (Lambda Plus [Coherent, Palo Alto, Calif ] or Diomed [Diomed Inc., Andover, Mass]) that produced 630 nm light with an adjusted power output of 400 mW/cm fiber and delivered a total of 200 J/cm fiber energy to the mucosa.
Endoscopic Mucosal Resection
Focal, endoscopically visible lesions underwent EMR for diagnostic purposes to determine histology and to exclude carcinoma. EMR was performed as described previously.23 PDT was delayed for a minimum of 4 weeks if a patient underwent EMR to allow healing of the EMR site(s).
All patients were placed on twice a day proton pump inhibitor therapy after PDT at the standard dose for the pump. Patients were educated carefully regarding PDT and its complications, especially dysphagia and photosensitivity, by physicians, nurse practitioners, and clinical coordinators. Follow-up included endoscopic surveillance with biopsies and EMR, if indicated, every 3 months for 2 years, then every 6 months for 1 or 2 years if HGD was eliminated. If HGD persisted, then patients were followed at 3-month intervals. If LGD was present, then patients were followed every 6 months. If only non-dysplastic Barrett mucosa or normal squamous mucosa was present at 2 years, then patients were followed annually. Post-PDT cytology specimens were acquired at the first endoscopy after PDT (3 months after PDT), and the results were correlated with histology obtained from biopsies that were taken at that visit. Repeat cytology samples were collected during subsequent endoscopy in patients who consented to repeat acquisition of cytology specimens and repeat FISH assays.
Statistical Methods
The associations of baseline characteristics with response outcome were assessed for continuous variables by using the 2-sample t test or the Wilcoxon rank-sum test, depending on the distribution of the baseline variable. The associations for baseline categorical data were assessed by using the chi-square test (or the Fisher exact test when necessary because of small individual cell frequencies). Similarly, the association of response (no vs yes at the time of repeat biomarker assessment) and biomarker status was assessed by using the Fisher exact test. Continuous variables were summarized as the mean (±standard deviation [SD]) or median (interquartile range [IQR]), as warranted. A P value <.05 was considered statistically significant. The difference between the proportions of positive biomarkers (pre-PDT vs post-PDT) was examined only in patients who received PDT by using the McNemar test (exact binomial distribution) at an α level of .05. Data management and statistical analysis were done using SAS software (1989–2002; SAS Institute Inc., Cary, NC).
RESULTS
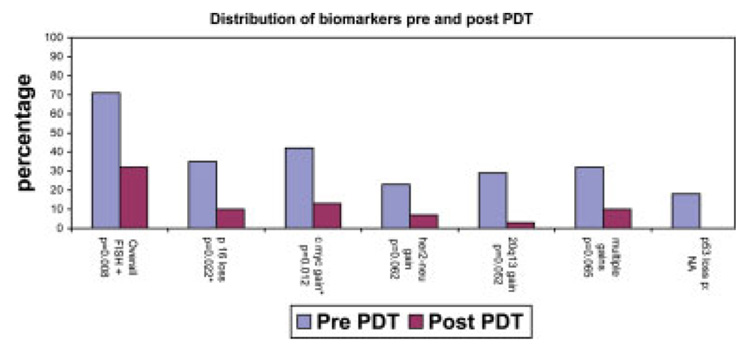
Thirty-one of 71 patients who underwent PDT with biomarker assessment between 2002 and 2005 at the Mayo Clinic, Rochester had biomarkers assessed after PDT. These patients were younger (aged 64.5 years vs 69.7 years; P = .03) than the patients who did not have repeat biomarkers assessed, but they had a comparable sex distribution (88% men vs 95% men, respectively; P = .39), BE segment length (5 cm vs 5.9 cm, respectively; P = .25), and 3-month follow-up histology (chi-square statistic, 1.7; P = .74). The median age of the cohort that had repeat biomarker assessment was 66 years (IQR, 56–73 years). Twenty-eight patients were men (88%). Twenty-five patients (78%) had HGD, and the remaining patients had mucosal cancer. Post-PDT biomarkers were obtained after a median duration of 6 months (IQR, 3–12 months). Twenty-four patients (77%) had no evidence of HGD on biopsies at the time of repeat sampling for biomarkers. The distribution of biomarkers pre-PDT and post-PDT for the entire cohort is illustrated in Figure 1. There was a statistically significant decrease in the proportion of several biomarkers post-PDT. Data on p53 loss were available only on 17 patients because of nonavailability of the probes. Three patients had evidence of p53 loss before PDT, and none had p53 loss after PDT. The median follow-up of patients after PDT was 22 months (IQR, 11.5–25 months).
FIGURE 1.
Comparison of biomarkers that were detected by fluorescence in situ hybridization (FISH) in patients with high-grade dysplasia (HGD)/mucosal cancer before and after photodynamic therapy (PDT) (N = 3). *Statistically significant. NA indicates not available.
The frequency of FISH-positive results in ‘responders’ (with no evidence of dysplasia on surveillance biopsies; n = 21) at the time of repeat biomarker assessment compared with ‘nonresponders’ (n = 10) is shown in Table 2. Despite the elimination of dysplasia, a substantial minority of patients (5%–19%) remained positive by FISH, particularly for gains at oncogene loci (8q24, 17q31.1). Multiple gains were observed in no responders compared with 30% of non-responders. This difference was statistically significant. The association between response status and overall FISH positivity was significant (P = .04) (Table 2).
TABLE 2.
Distribution of Biomarkers in Responders to Photodynamic Therapy at the Time of Repeat Biomarker Assessment (N = 31)
| No. of patients (%) |
|||
|---|---|---|---|
| Biomarker | Responders with no dysplasia, n = 21 | Nonresponders with dysplasia, n = 10 | P* |
| Overall FISH positivity | 4 (19) | 6 (60) | .04 |
| P16 loss | 1 (5) | 2 (20) | .24 |
| P53 loss† | 0 (0) | 0 (0) | — |
| C-MYC gain | 2 (10) | 2 (20) | .58 |
| HER2/neu gain | 1 (5) | 1 (10) | 1.0 |
| 20q13 gain | 0 (0) | 1 (10) | .32 |
| Multiple gains | 0 (0) | 3 (30) | .03 |
FISH indicates fluorescence in situ hybridization.
Fisher exact test.
Data were available on 13 responders and 4 nonresponders.
Follow-up histology of this cohort is described in Figure 2. In the subgroup of patients who had an absence of HGD after PDT (N = 24), 6 patients remained FISH positive for 1 or more biomarkers. Of those 6 patients, 2 patients (33%) developed recurrent HGD. One patient developed HGD 9 months after PDT. He had evidence of amplification at the C-MYC locus on post-PDT FISH assessment. The second patient with recurrent HGD (which was detected 10 months after PDT) had evidence of multiple gains on repeat FISH assessment. Both patients developed recurrent HGD after 2 intervening surveillance endoscopies without HGD, and both were treated endoscopically with EMR and remained free of HGD at the time of the current report. In contrast, none of the 18 patients with negative FISH studies after PDT developed recurrent HGD over a median follow-up of 22 months (IQR, 11.5–25 months).
FIGURE 2.
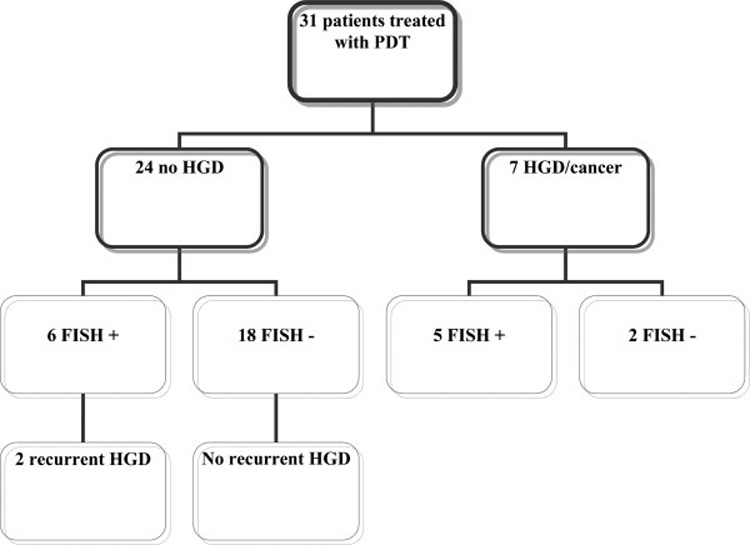
Schematic representation of histologic diagnosis and fluorescence in situ hybridization (FISH) results during follow-up. PDT indicates photodynamic therapy; HGD, high-grade dysplasia; +, positive; −, negative.
DISCUSSION
Barrett esophagus is a major risk factor for esophageal adenocarcinoma. Esophagectomy24 and PDT25 are accepted modalities of treatment for HGD in BE and produce comparable long-term results.14 In this prospective study, we observed a statistically significant decrease in the prevalence of all biomarkers after PDT. A minority of patients with a histologic ‘response’ after PDT continued to show evidence of amplification at proto-oncogene loci: That group of ‘responders’ appeared to be at a higher risk of recurrent HGD on follow-up. The absence of genetic alterations detected by FISH, in addition to histologic response, predicted a ‘durable’ response to PDT.
The specific chromosomal loci examined in this study were chosen based on a previous study in which a panel of FISH probes to these loci was able to distinguish adenocarcinoma and HGD from lesser grades of dysplasia with a sensitivity and specificity of approximately 80%.22 In addition, we selected these probes for their reliable performance characteristics and because of the observation that gain/amplification at proto-oncogene loci may be a more specific indicator of neoplasia than chromosomal losses.22,26
Biomarker studies on squamous islands that appear in BE during prolonged treatment with proton pump inhibitors have reported the presence of increased proliferation and p53 staining.19 Some authors speculated that this may predict increased risk of progression to cancer. They assessed the presence of these biomarkers and cyclooxygenase 2 (COX-2) by using immunohistochemistry (IHC) in residual BE after ablation20 and observed evidence of increased Ki-67 staining, COX-2 staining, and p53 staining at the neosquamocolumnar junction, raising concerns of neoplastic progression after incomplete ablation therapy. That report was limited by the lack of histologic follow-up in patients with positive biomarkers, and the poor accuracy of IHC for the p53 protein compared with gene sequencing. Hage et al. reported the biomarker status of 29 patients (including 8 patients with HGD) after ablative treatment. 21 Markers that were assessed included Ki-67 antigen testing, p53 protein expression by IHC, and chromosome 1 ploidy by in situ hybridization. Those authors observed that, despite the elimination of HGD in 70% of patients, the residual Barrett epithelium continued to show evidence of increased proliferation,. Although the authors speculated that this increased the risk of progression of dysplasia, they did not provide information on recurrence of HGD in these patients on follow-up. Moreover, the significance of increased proliferation in residual epithelium after ablation has yet to be determined.27 In a follow-up study, the authors assessed genetic alterations by using gene sequencing in preablation and postablation biopsy specimens.28 In their cohort, only 3 patients with HGD were included. They observed that the profile of biomarkers was not different before and after ablative therapy despite the absence of HGD on follow-up biopsies. This is in contrast to our finding of a significant change (loss of biomarkers) in post-PDT surveillance cytology specimens. This may be attributable to the larger number of patients with HGD included in our study (3 patients vs 31 patients) or perhaps because of the difference in techniques.
Although, to our knowledge, this is the largest study of its kind to date, the current study has some potential limitations. The possibility cannot be excluded that the lack of HGD on biopsies may reflect sampling error. This sampling error also potentially may affect the correlation of post-PDT biopsies and FISH markers. The fairly long period of follow-up (median, 22 months) without HGD does make this less likely. The proportion of patients with P1615 and P53 loss29,30 in our cohort is lower than that reported in the literature. Compared with gene analysis, dual-probe FISH has moderate sensitivity (68.4%) but high specificity (95.8%).31 However, FISH may be more sensitive in identifying allelic loss, because LOH analyses typically require the presence of ≥70% tumor cells in a sample to be able to detect LOH.31 We also performed FISH on cytology specimens, because the use of tissue sections may lead to sectioning artifacts, which could compromise FISH results. Finally, only 31 patients (44%) underwent repeat FISH assessment after PDT, raising the possibility of selection bias. Patients who did not have repeat biomarker assessments largely had demographic and clinical characteristics that were comparable to those in patients who did have repeat biomarker assessments. Finally, we recognize that, although this may have been the largest study to date in these patients, the sample size of the study was limited, and our results need to be validated in a larger multicenter study.
In conclusion, the absence of HGD after PDT appears to be associated with a significant decrease in the prevalence of biomarkers associated with the progression of neoplasia in BE. Patients with a histologic response (the absence of HGD) who have persistent genetic abnormalities appear to be a higher risk of developing recurrent HGD: This cohort of patients may merit closer and longer surveillance. In contrast, patients who have histologic and ‘genetic’ responses may be at decreased risk of recurrence and may not require further mucosal therapy.
Acknowledgments
Supported by the following grants from the National Institutes of Health; R01 CA111603-01A1, R01CA097048, and R21CA122426-01 (all to Kenneth K. Wang).
Footnotes
A patent has been filed for the fluorescence in situ hybridization (FISH) probe set described in this study. Dr. Kevin C. Halling, Dr. Kenneth K. Wang, Shanon M. Brankley, and the Mayo Clinic have the potential to receive royalties from the sale of this product.
REFERENCES
- 1.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 2.Provenzale D, Schmitt C, Wong JB. Barrett’s esophagus: a new look at surveillance based on emerging estimates of cancer risk. Am J Gastroenterol. 1999;94:2043–2053. doi: 10.1111/j.1572-0241.1999.01276.x. [DOI] [PubMed] [Google Scholar]
- 3.Reid BJ, Levine DS, Longton G, Blount PL, Rabinovitch PS. Predictors of progression to cancer in Barrett’s esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. Am J Gastroenterol. 2000;95:1669–1676. doi: 10.1111/j.1572-0241.2000.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schnell TG, Sontag SJ, Chejfec G, et al. Long-term nonsurgical management of Barrett’s esophagus with high-grade dysplasia. Gastroenterology. 2001;120:1607–1619. doi: 10.1053/gast.2001.25065. [see comment]. [DOI] [PubMed] [Google Scholar]
- 5.Weston AP, Sharma P, Topalovski M, Richards R, Cherian R, Dixon A. Long-term follow-up of Barrett’s high-grade dysplasia. Am J Gastroenterol. 2000;95:1888–1893. doi: 10.1111/j.1572-0241.2000.02234.x. [DOI] [PubMed] [Google Scholar]
- 6.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 7.Orringer MB, Marshall B, Stirling MC. Transhiatal esophagectomy for benign and malignant disease. J Thorac Cardiovasc Surg. 1993;105:265–276. discussion 276–277. [PubMed] [Google Scholar]
- 8.Overholt BF, Panjehpour M, Haydek JM. Photodynamic therapy for Barrett’s esophagus: follow-up in 100 patients. Gastrointest Endosc. 1999;49:1–7. doi: 10.1016/s0016-5107(99)70437-2. [see comment]. [DOI] [PubMed] [Google Scholar]
- 9.Attwood SE, Lewis CJ, Caplin S, Hemming K, Armstrong G. Argon beam plasma coagulation as therapy for high-grade dysplasia in Barrett’s esophagus. Clin Gastroenterol Hepatol. 2003;1:258–263. [see comment]. [PubMed] [Google Scholar]
- 10.Kovacs BJ, Chen YK, Lewis TD, DeGuzman LJ, Thompson KS. Successful reversal of Barrett’s esophagus with multipolar electrocoagulation despite inadequate acid suppression. Gastrointest Endosc. 1999;49:547–553. doi: 10.1016/s0016-5107(99)70380-9. [DOI] [PubMed] [Google Scholar]
- 11.Buttar NS, Wang KK, Lutzke LS, Krishnadath KK, Anderson MA. Combined endoscopic mucosal resection and photodynamic therapy for esophageal neoplasia within Barrett’s esophagus. Gastrointest Endosc. 2001;54:682–688. doi: 10.1067/gien.2001.0003. [DOI] [PubMed] [Google Scholar]
- 12.Pacifico RJ, Wang KK, Wongkeesong LM, Buttar NS, Lutzke LS. Combined endoscopic mucosal resection and photodynamic therapy versus esophagectomy for management of early adenocarcinoma in Barrett’s esophagus. Clin Gastroenterol Hepatol. 2003;1:252–257. [see comment]. [PubMed] [Google Scholar]
- 13.Overholt BF, Lightdale CJ, Wang KK, et al. International, multicenter, partially blinded, randomized study of the efficacy of photodynamic therapy (PDT) using porfimer sodium (POR) for the ablation of high-grade dysplasia (HGD) in Barrett’s esophagus (BE): results of 24-month follow up [abstract] Gastroenterology. 2003;124 4 suppl 1:153. Abstract 151. [Google Scholar]
- 14.Prasad GA, Wang KK, Buttar NS, et al. Long-term survival following endoscopic and surgical treatment of high-grade dysplasia in Barrett’s esophagus. Gastroenterology. 2007;132:1226–1233. doi: 10.1053/j.gastro.2007.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong DJ, Paulson TG, Prevo LJ, et al. p16(INK4a) lesions are common, early abnormalities that undergo clonal expansion in Barrett’s metaplastic epithelium. Cancer Res. 2001;61:8284–8289. [PubMed] [Google Scholar]
- 16.Prevo LJ, Sanchez CA, Galipeau PC, Reid BJ. p53-Mutant clones and field effects in Barrett’s esophagus. Cancer Res. 1999;59:4784–4787. [PubMed] [Google Scholar]
- 17.Walch AK, Zitzelsberger HF, Bruch J, et al. Chromosomal imbalances in Barrett’s adenocarcinoma and the metaplasia-dysplasia-carcinoma sequence. Am J Pathol. 2000;156:555–566. doi: 10.1016/S0002-9440(10)64760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tselepis C, Morris CD, Wakelin D, et al. Upregulation of the oncogene c-myc in Barrett’s adenocarcinoma: induction of c-myc by acidified bile acid in vitro. Gut. 2003;52:174–180. doi: 10.1136/gut.52.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garewal H, Ramsey L, Sharma P, Kraus K, Sampliner R, Fass R. Biomarker studies in reversed Barrett’s esophagus. Am J Gastroenterol. 1999;94:2829–2833. doi: 10.1111/j.1572-0241.1999.1424_d.x. [DOI] [PubMed] [Google Scholar]
- 20.Dvorak K, Ramsey L, Payne CM, et al. Abnormal expression of biomarkers in incompletely ablated Barrett’s esophagus. Ann Surg. 2006;244:1031–1036. doi: 10.1097/01.sla.0000224913.19922.7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hage M, Siersema PD, Vissers KJ, et al. Molecular evaluation of ablative therapy of Barrett’s oesophagus. J Pathol. 2005;205:57–64. doi: 10.1002/path.1685. [DOI] [PubMed] [Google Scholar]
- 22.Brankley SM, Wang KK, Harwood AR, et al. The development of a fluorescence in situ hybridization assay for the detection of dysplasia and adenocarcinoma in Barrett’s esophagus. J Mol Diagn. 2006;8:260–267. doi: 10.2353/jmoldx.2006.050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prasad GA, Wang KK, Lutzke LS, et al. Frozen section analysis of esophageal endoscopic mucosal resection specimens in the real-time management of Barrett’s esophagus. Clin Gastroenterol Hepatol. 2006;4:173–178. doi: 10.1016/j.cgh.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Headrick JR, Nichols FC, 3rd, Miller DL, et al. High-grade esophageal dysplasia: long-term survival and quality of life after esophagectomy. Ann Thorac Surg. 2002;73:1697–1702. doi: 10.1016/s0003-4975(02)03496-3. discussion 1702–1703. [DOI] [PubMed] [Google Scholar]
- 25.Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005;62:488–498. doi: 10.1016/j.gie.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 26.Sokolova IA, Halling KC, Jenkins RB, et al. The development of a multitarget, multicolor fluorescence in situ hybridization assay for the detection of urothelial carcinoma in urine. J Mol Diagn. 2000;2:116–123. doi: 10.1016/S1525-1578(10)60625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnadath KK, Wang KK, Taniguchi K, et al. Persistent genetic abnormalities in Barrett’s esophagus after photodynamic therapy. Gastroenterology. 2000;119:624–630. doi: 10.1053/gast.2000.18012. [DOI] [PubMed] [Google Scholar]
- 28.Hage M, Siersema PD, Vissers KJ, et al. Genomic analysis of Barrett’s esophagus after ablative therapy: persistence of genetic alterations at tumor suppressor loci. Int J Cancer. 2006;118:155–160. doi: 10.1002/ijc.21302. [DOI] [PubMed] [Google Scholar]
- 29.Wu TT, Watanabe T, Heitmiller R, Zahurak M, Forastiere AA, Hamilton SR. Genetic alterations in Barrett esophagus and adenocarcinomas of the esophagus and esophagogastric junction region. Am J Pathol. 1998;153:287–294. doi: 10.1016/S0002-9440(10)65570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neshat K, Sanchez CA, Galipeau PC, et al. p53 mutations in Barrett’s adenocarcinoma and high-grade dysplasia. Gastroenterology. 1994;106:1589–1595. doi: 10.1016/0016-5085(94)90415-4. [DOI] [PubMed] [Google Scholar]
- 31.Wongsurawat VJ, Finley JC, Galipeau PC, et al. Genetic mechanisms of TP53 loss of heterozygosity in Barrett’s esophagus: implications for biomarker validation. Cancer Epidemiol Biomarkers Prev. 2006;15:509–516. doi: 10.1158/1055-9965.EPI-05-0246. [DOI] [PubMed] [Google Scholar]