Abstract
The present study investigated age-related differences in discrimination and reversal learning for olfactory and visual stimuli in 6 mo and 24 mo old rats. Rats were trained to discriminate between two pseudo-randomly selected odors or objects. Once each animal reached a criterion on discrimination trials, the reward contingencies were reversed. Young and aged rats acquired the olfactory and visual discrimination tasks at similar rates. However, on reversal trials, aged rats required significantly more trials to reach the learning criterion on both the olfactory and visual reversal tasks than young rats. The deficit in reversal learning was comparable for odors and objects. Furthermore, the results showed that rats acquired the olfactory task more readily than the visual task. The present study represents the first examination of age-related differences in reversal learning using the same paradigm for odors and objects to facilitate cross-modal comparisons. The results may have important implications for the selection of memory paradigms for future research studies on aging.
Keywords: Aging, Discrimination, Reversal Learning, Olfactory, Visual Object
Introduction
A number of research studies have suggested that problems with remembering and detecting odors may be an early indication of cognitive impairment and dementing diseases in non-demented older adults (Devanand et al., 2002; Djordjevic, Jones-Gotman, De Sousa, & Chertkow, in press; Gilbert & Murphy, 2004b; Graves et al., 1999; Wang et al., 2002; Wilson, Arnold, Schneider, Tang, & Bennett, 2007). Studies have shown older adults to be impaired relative to young adults on a variety olfactory tasks (Doty, Shaman, Kimmelman, & Dann, 1984; Gilbert, Pirogovsky, Ferdon, Brushfield, & Murphy, in press; Gilbert, Pirogovsky, Ferdon, & Murphy, 2006; Larsson & Backman, 1998; Murphy, Bacon, Bondi, & Salmon, 1998; Murphy, Cain, Gilmore, & Skinner, 1991; Murphy et al., 2002; Nordin & Murphy, 1998; Wysocki & Gilbert, 1989). Deficits in odor memory have been reported in healthy older adults at risk for AD based on family history (Schiffman, Graham, Sattely-Miller, Zervakis, & Welsh-Bohmer, 2002) or genetic risk factors (Gilbert & Murphy, 2004a, 2004b; Graves et al., 1999; Murphy et al., 1998). A longitudinal study demonstrated that healthy older anosmics genetically at risk for AD showed a five times greater risk of cognitive decline compared to that of healthy older normosmics without genetic risk factors for AD (Graves et al., 1999). Other studies have reported that that patients with low odor identification scores who were unaware of their olfactory loss were more likely to develop AD by a two year follow up than other patients (Devanand et al., 2002). A number of researchers have emphasized the importance of the use of animal models to examine age-related changes in cognition and behavior (Gallagher & Rapp, 1997; Schoenbaum, Nugent, Saddoris, & Gallagher, 2002a; Solomon, Beal, & Pendlebury, 1988; Woodruff-Pak & Thompson, 1985). However, few studies have examined age-related odor memory impairments in rodents.
As discussed by Kraemer & Apfelbach (2004) the modality of a stimulus to be remembered is critical when determining efficient learning. Studies have shown that rats can readily learn and maintain a high level of performance on odor memory tasks (Alvarez, Wendelken, & Eichenbaum, 2002; Bunsey & Eichenbaum, 1996; Darling & Slotnick, 1994; Fortin, Agster, & Eichenbaum, 2002; Fortin, Wright, & Eichenbaum, 2004; Gilbert & Kesner, 2002, 2003; Kesner, Hunsaker, & Gilbert, 2005; Kesner, Gilbert, & Barua, 2002; Lu, Slotnick, & Silberberg, 1993; Slotnick, Hanford, & Hodos, 2000; Slotnick, Kufera, & Silberberg, 1991; Van Elzakker, O’Reilly, & Rudy, 2003; Wood, Agster, & Eichenbaum, 2004). In addition, it has been shown that acquisition rates and retention of memory for olfactory stimuli may be greater than for visual stimuli in young adult rats (Broadbent, Squire, & Clark, 2007; Rozin & Kalat, 1972). However, it is not clear whether the memory superiority for olfactory stimuli is preserved later in the life of a rat, since selective pressure for olfactory function may not exist following the reproductive lifespan of the animal.
A few studies have reported deficits in olfactory memory in aged rats. Studies have found that olfactory sensitivity was decreased in aged rats relative to young rats (Apfelbach, Russ, & Slotnick, 1991; Kraemer & Apfelbach, 2004). However, differences in olfactory discrimination have not been detected between young and aged animals (Kraemer & Apfelbach, 2004; Luu, Pirogovsky, & Gilbert, in press; Roman, Alescio-Lautier, & Soumireu-Mourat, 1996; Schoenbaum et al., 2002a). On reversal trials when the reward contingencies were reversed, aged rats showed significant impairments compared to young rats (Roman et al., 1996; Schoenbaum et al., 2002a). Therefore, reversal tasks involving olfactory stimuli may be very sensitive to age-related changes in rodents. However, existing studies investigating age-related changes in reversal learning for olfactory stimuli have not made direct comparisons with reversal learning for stimuli from a different sensory modality. As a result, it is not clear whether olfactory reversal learning is any more or less affected by aging than reversal learning for stimuli encoded via other sensory modalities. Thus, it is unclear whether aged rats are a good model for understanding how age-related brain changes might result in impairments in odor memory in older humans.
Studies have reported deficits in reversal learning in aged humans, nonhuman primates, and rodents (Mell et al., 2005; Schoenbaum, Setlow, Saddoris, & Gallagher, 2006; Schoenbaum et al., 2002a; Tsuchida, Kubo, & Kojima, 2002; Voytko, 1999). However, not all studies involving aged primates have reported age-related changes in reversal learning for all types of stimuli (Lai, Moss, Killiany, Rosene, & Herndon, 1995; Rapp, 1990; Tsuchida et al., 2002; Voytko, 1999). Although some studies have demonstrated that rats show age-related impairments on reversal learning tasks involving olfactory stimuli, it has not been clearly demonstrated whether the learning impairment is comparable to stimuli presented in other modalities. Therefore, the present study investigated age-related differences in discrimination and reversal learning for olfactory and visual stimuli in young and aged rats in order to determine whether odor learning is as affected by aging as visual object learning in rats. The same paradigm was used for olfactory stimuli and visual stimuli in order to facilitate cross-modal comparisons. The results of the present experiments offer valuable insight into whether aging has a similar deleterious effect on odor memory in rats and whether aged rats are a suitable animal model for studying the effect of aging on odor memory in older humans. In addition, olfactory based tasks may be very attractive to researchers examining age-related changes in cognition in rats because the tasks are rapidly acquired. Therefore, the results may have important implications for the selection of memory paradigms for future research studies on aging.
Methods
Subjects
Forty Fischer 344/Brown Norway (Harlan Laboratories) male rats approximately 6 months (n = 20) and 24 months (n = 20) of age were used as subjects. The strain is an inbred hybrid offspring of a male Brown Norway rat and a female Fischer 344. Each age group was evenly divided and pseudo-randomly assigned to an olfactory or visual object condition in order to provide cross modal comparisons. Each rat was individually housed in standard plastic tubs located in a colony room. The colony room was maintained on a 12h: 12h light/dark cycle and all testing were conducted during the light phase. All rats had unlimited access to water but were food deprived to 80–90% of their free-feeding weight.
Apparatus
The testing apparatus consisted of a box with a 30 cm × 12 cm floor and four 12 cm high walls. The apparatus was constructed out of wood and all surfaces of the apparatus were painted gray. One removable guillotine door was placed in the center of the box to divide the box into two separate compartments, a start chamber and a choice chamber. The door could be manually opened and closed by the experimenter. One row of three evenly spaced 2 cm diameter and 1.5 cm deep food-wells were drilled into the floor in each chamber. The row of food-wells was positioned 5 cm from the guillotine door and each food-well was separated by 1.5 cm.
Stimuli
Odors
Olfactory stimuli consisted of powdered odorants (cinnamon, cumin, baby powder, or ginger) mixed in sand and presented in clear plastic cups (3 cm diameter and 3 cm high) as described in previous studies (Agster, Fortin, & Eichenbaum, 2002; Bunsey & Eichenbaum, 1996; Dudchenko, Wood, & Eichenbaum, 2000; Dusek & Eichenbaum, 1997; Fortin et al., 2002; Gilbert & Kesner, 2002, 2003; Kesner et al., 2002; Rondi-Reig, Libbey, Eichenbaum, & Tonegawa, 2001; Van Elzakker et al., 2003). Approximately 4–8 g (depending on the particular odor) of the powdered odorant was mixed in 160 g of sand. A food reward, a half piece of Froot Loop cereal, was buried beneath the surface of the sand in order to eliminate any potential visual cues.
Visual Objects
Visual stimuli consisted of small, visually dissimilar objects such as a toy die-cast metal car or a plastic film canister (approximately 4 cm – 6 cm high). The objects were mounted on small flat metal washers (5 cm diameter and 2 mm thick) to stabilize the objects and to completely cover the opening of the food-wells. Selected visual and olfactory stimuli used in the experiment were chosen based on data obtained from prior experiments (Gilbert & Kesner, 2002, 2003). Multiple copies of each object were randomly used during testing to minimize specific olfactory cues associated with a particular object.
Shaping Procedure
During the first week of training, each animal was handled for ~ 0.25 hr daily and allowed to individually explore the test apparatus for 0.25 hr. During the exploration period, the guillotine door was removed from the apparatus and ~ 6 pieces of Froot Loop cereal were distributed across the surface of the apparatus (not in the food-wells). Once the animal was exploring the apparatus consistently, the guillotine door was subsequently raised and lowered to allow the rat to shuttle back and forth between chambers to retrieve a food reward. Each rat assigned to the olfactory task was shaped in the home cage to dig in a clear plastic cup filled with unscented sand to retrieve a food reward. Shaping began by placing a half piece of Froot Loop cereal on the surface of the sand allowing the animal to retrieve the reward. Across subsequent shaping trial presentations, the food reward was buried, partially at first and then deeper in the sand, until the rat was digging in the sand even when the reward was not visible. Once the animal was consistently retrieving the food reward in the home cage, the rat was placed in the apparatus and allowed to shuttle back and forth to retrieve the food reward from the digging cup containing the unscented sand. Digging cups were stabilized so that the rat could not displace the cups or spill the contents. This procedure was followed 12 times each day. Once an animal was digging consistently, the animal was assigned to the olfactory discrimination task.
Rats assigned to the visual object task followed similar shaping procedures; however, each rat was shaped to displace a neutral object rather than to dig in a sand-filled cup in order to receive a food reward. The neutral visual stimulus consisted of a wooden object 2 cm wide and 5 cm tall that was painted white. The object was placed to cover the center-most food-well in the choice chamber of the apparatus. Shaping began by placing a piece of cereal in front of the object on the maze surface. The animal was placed in the start chamber of the apparatus with the guillotine door between the start and choice chambers in the closed position. The animal was allowed to exit the start chamber, retrieve the reward from the choice chamber, and consume the reward with the door in the closed position. Once an animal retrieved the food reward consistently, the food reward was placed in the food well previously covered by the object and the object was positioned on the side of the food well opposite the animal. On each ensuing trial, the object was positioned to cover a larger portion of the food well until the base of the object completely covered the baited food well. This procedure was followed 12 times each day. Once an animal consistently displaced the object when the food well was completely covered, the animal was assigned to the visual object discrimination task.
Olfactory Discrimination Task
Olfactory discrimination was assessed using a two-choice discrimination task described by Kesner et al. (2002). A total of four odors were used in the experiment including cinnamon, ginger, baby powder, and cumin; however, each rat discriminated between only two odors. Odor pairings were determined so that each odor was used equally and all odor pairings were balanced (e.g., cinnamon was used in the same number of pairings as baby powder). For each rat, one odor was randomly assigned as the rewarded odor and the other odor was assigned as the non-rewarded odor. The rat began each trial in the start chamber of the apparatus with the door to the choice chamber closed. The door was opened and the rat was allowed to choose between the two odor cups presented side-by-side in the choice chamber of the apparatus 6.5 cm apart. If the rat dug in the odor cup containing the rewarded odor, the rat received a food reward. However, if the rat dug in the odor cup containing the non-rewarded odor, the rat did not receive a reward and was not allowed to dig in the odor cup containing the rewarded odor. On the next trial, the odor cups containing the two odors were presented in the other side of the apparatus (the start chamber from the previous trial) and the door was opened to allow the animal to choose between the odor cups. Thus on each trial the rat shuttled back and forth between the two chambers so that the experimenter did not need to handle the rat between trials. The position of each odor varied pseudo-randomly on each trial with respect to the left and right position in the choice chamber to eliminate position bias. Each rat received 12 trials per day and was tested until the animal reached a criterion of 9 correct choices out of a sliding block of 10 consecutive trials within the 12 trials. The experimenter recorded the digging response of each rat and the number of trials required to reach the criterion was used as the dependent measure. A 30 s interval was implemented between each trial.
Olfactory Reversal Task
Once each animal reached the learning criterion of 9 correct choices out of a sliding block of 10 consecutive trials, the reward contingencies were reversed on the following day of testing. The previously rewarded odor became the non-rewarded odor and the previously non-rewarded odor became the rewarded odor. Thus, a Froot Loop was buried in the cup containing the rewarded odor and no Froot Loop was buried in the cup containing the non-rewarded odor. Olfactory reversal learning was assessed using the same procedure and criterion as described for the olfactory discrimination task.
Visual Object Discrimination
Visual object discrimination was assessed using the same procedure and criterion as described for the olfactory task; however, two objects were used rather than two odors. A total of four dissimilar objects were used in the experiment. On each trial, the rewarded object was positioned to cover a baited food well containing a food reward, whereas the non-rewarded object covered an adjacent un-baited food well. If the rat displaced the rewarded object, the rat received a food reward. However, if the rat displaced the non-rewarded object, the rat did not receive a reward and was not allowed to displace the rewarded object.
Visual Object Reversal Task
Visual object reversal learning was assessed using the same procedure and criterion as described for the olfactory task.
Results
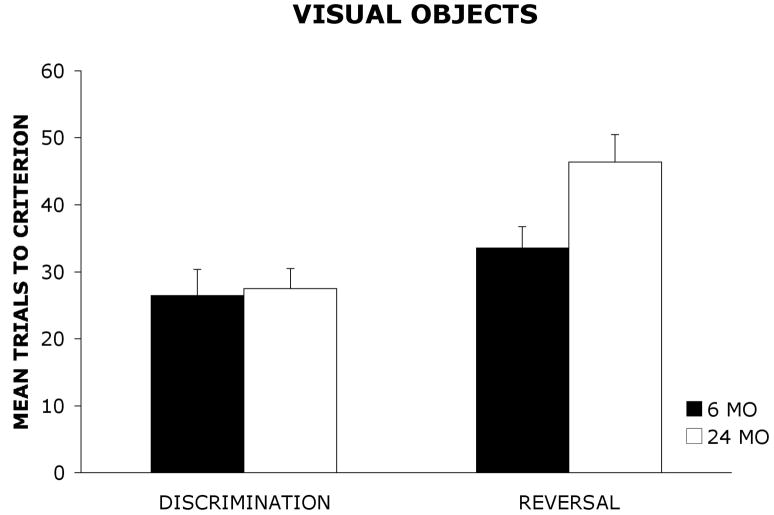
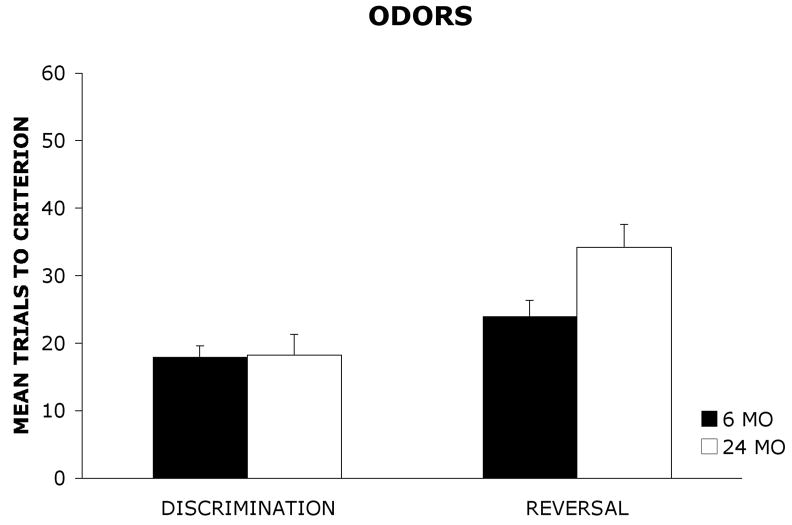
Figure 1 shows the mean (+ standard error) number of trials required by 6 mo and 24 mo old rats to reach the learning criterion on the visual object discrimination and reversal tasks. The mean (+ SE) number of trials required by 6 mo and 24 mo old rats to reach the learning criterion on the olfactory discrimination and reversal tasks are shown in Figure 2.
Figure 1.
Mean trials to criterion (±standard error) for 6 mo and 24 mo old rats on a visual object discrimination and reversal task.
Figure 2.
Mean trials to criterion (±standard error) for 6 mo and 24 mo old rats on an olfactory discrimination and reversal task.
A 2 × 2 × 2 analysis of variance (ANOVA) with age group (6 mo, 24 mo) and stimulus (odors, visual objects) as between-group factors and task (discrimination, reversal) as a within-group factor was used to analyze the data. The dependent variable was the mean number of trials required to reach the learning criterion of 9 correct choices out of a sliding block of 10 consecutive trials. The results revealed a significant main effect of group, F(1, 36) = 5.87, p < .05, indicating that 6 mo old rats outperformed 24 mo old rats. There also was a significant main effect of stimulus, F(1,36) = 15.92, p < .001, suggesting that rats were acquiring the olfactory task more readily than the visual object task. Additionally, there was a significant main effect of task, F(1, 36) = 39.01, p < .001, indicating that rats were acquiring the discrimination task at a faster rate than the reversal task regardless of stimulus modality. Furthermore, the analysis revealed a significant group × task interaction, F(1, 36) = 8.16, p < .01. A Newman-Keuls post hoc comparison test of the group × task interaction revealed that there were no significant differences in the number of trials required by 6 mo and 24 mo old rats to reach the learning criterion on the olfactory or visual object discrimination task. However, on the olfactory reversal task and the visual object reversal task, 24 mo old rats required significantly more trials to reach the learning criterion relative to 6 mo old rats (p < .05). In addition, 24 mo old rats required significantly more trials to reach the learning criterion on the reversal task than the discrimination task (p < .05). However, there were no significant differences in the number of trials required by 6 mo old rats to reach learning criterion on the discrimination and reversal tasks. The analysis did not reveal a significant group × stimulus interaction, F(1, 36) = .11, p = .74, a significant task × stimulus interaction, F(1,36) = .26, p = .61, or a significant task × group × stimulus interaction, F(1, 36) = .06, p = .81.
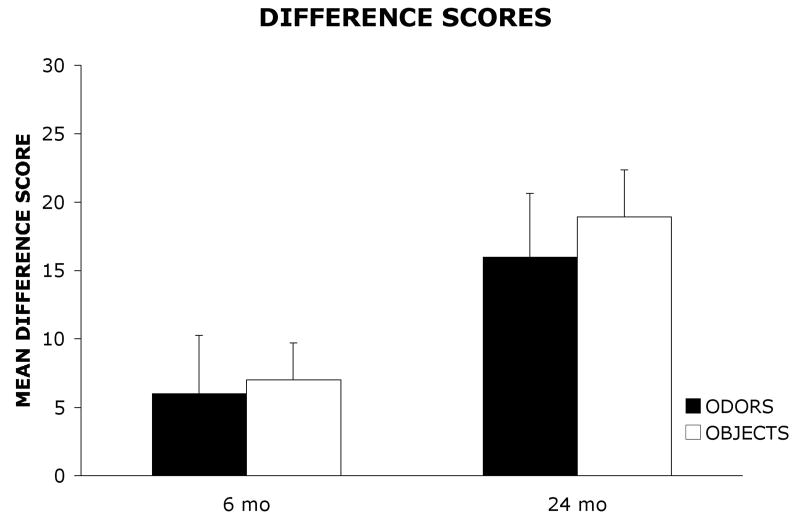
To examine performance differences between the olfactory and visual object conditions, the data were transformed into difference scores for each rat. Difference scores were calculated by subtracting the number of trials to reach the criterion on the discrimination task from the number of trials to reach the criterion on the reversal task. Mean (+ SE) difference scores for 6 mo and 24 mo old rats are shown in Figure 3. A 2 × 2 ANOVA with age group (6 mo, 24 mo) and stimulus (odors, objects) as between-group factors was used to analyze the data. The results revealed a significant main effect of group, F(1, 36) = 8.16, p < .01, indicating that 6 mo old rats showed lower difference scores than 24 mo old rats. However, the results did not reveal a significant main effect of stimulus, F(1, 36) = .26, p = .61, or a significant group × stimulus interaction, F(1,36) = .06, p = .81.
Figure 3.
Mean difference scores (±standard error) for 6 mo and 24 mo old rats on the visual object and olfactory conditions.
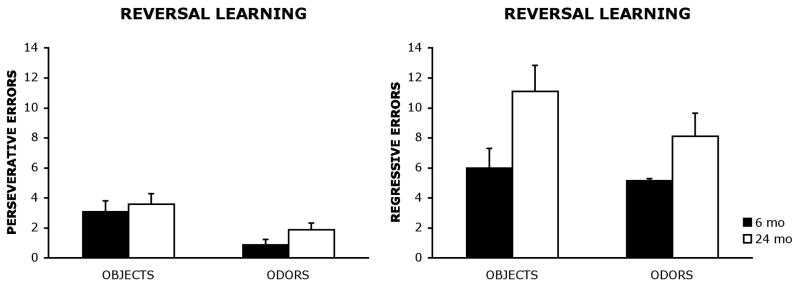
Perseverative and regressive error patterns on the reversal task were analyzed using a criterion described in previous studies (Dias & Aggleton, 2000; Hunt & Aggleton, 1998; Kim & Ragozzino, 2005; Palencia & Ragozzino, 2004; Ragozzino et al., 1999; Ragozzino, Jih, & Tzavos, 2002a; Ragozzino, Ragozzino, Mizumori, & Kesner, 2002b; Ragozzino et al., 2003). These analyses were conducted in order to provide a more fine-grained analysis of error patterns in aged rats. In this task, perseveration occurred during the reversal task when a rat continued to dig in the cup containing the odor previously rewarded on discrimination task trials. Perseveration was operationalized as digging in the incorrect cup on reversal trials for three or more trials in consecutive blocks of 4 trials. Once a rat made less than three errors in a block of four trials the first time, all subsequent errors were counted as regressive errors. As described by Kim and Ragozzino (2005), this provided a measure of the ability to maintain a new choice after initially shifting away from the previously correct choice.
A multivariate analysis of variance (MANOVA) with age group (6 mo, 24 mo) and stimulus (odors, visual objects) as between-group factors and perseverative and regressive errors as dependent measures was used to analyze the data. The results revealed a significant main effect of group for regressive errors, F(1, 36) = 8.03, p < .01, indicating that 24 mo old rats were committing more regressive errors than 6 mo old rats. However, the results did not reveal a significant main effect of group for perseverative errors F(1, 36) = 1.83, p = .18, or a significant group × stimulus interaction for either perseverative, F(1,36) = .21, p = .65, or regressive errors, F(1,36) = .59, p = .45.
Discussion
As mentioned previously, research has shown that the normal aging process in humans may result in significant impairments in odor memory (Gilbert & Murphy, 2004a, 2004b; Gilbert et al., 2006; Murphy et al., 1991; Nordin & Murphy, 1998). Furthermore, research studies have suggested that problems with remembering and detecting odors may be an early indication of cognitive impairment and dementing diseases in non-demented older adults (Devanand et al., 2002; Djordjevic et al., in press; Gilbert & Murphy, 2004b; Graves et al., 1999; Wang et al., 2002; Wilson et al., 2007). Although studies have examined odor learning in rats, it has not been clearly demonstrated whether odor learning is as affected by aging as visual learning in rats. Prior research has shown that rats may possess superior olfactory processing capabilities (Slotnick, 2001); however, it is unclear whether this superiority is preserved across the normal aging process. Therefore, the present study represents the first direct examination of age-related differences in discrimination and reversal learning using the same paradigm for olfactory and visual stimuli to facilitate cross-modal comparisons.
Consistent with past research (Luu et al., in press; Schoenbaum et al., 2002a, 2006), the results of the present study show no significant differences between 6 mo and 24 mo old rats in acquisition of an olfactory or visual discrimination task. This finding suggests that any deficits observed on the present olfactory or visual object reversal tasks are not simply due to an impaired ability to perceive the odors or the visual objects. However, when the reward contingencies were reversed, 24 mo old rats required significantly more trials than 6 mo old rats to reach the learning criterion on both the olfactory and visual object reversal task. In order to examine differences in performance between the odor and visual object conditions, difference scores were computed. The results show that differences scores for 6 mo old rats did not differ significantly on the olfactory or visual object tasks. Similarly, the results show that the differences scores for 24 mo old rats also did not significantly for the olfactory and visual reversal tasks. These findings suggest that 24 mo old rats show comparable impairments in reversal learning for olfactory and visual stimuli. Implications of the results will be discussed in subsequent paragraphs.
Using a criterion described in previous studies (Dias & Aggleton, 2000; Hunt & Aggleton, 1998; Kim & Ragozzino, 2005; Palencia & Ragozzino, 2004; Ragozzino et al., 1999, 2002a, 2002b, 2003) perseverative and regressive errors committed on the olfactory and visual object reversal tasks were examined in order to obtain a more fine-grained analysis of error patterns. The results show that 24 mo old rats committed significantly more regressive errors on both the olfactory and visual object reversal tasks than did 6 mo old rats. However, 6 mo and 24 mo old rats did not significantly differ in the number of perseverative errors committed on the reversal tasks. To date, conflicting reports exist regarding error response patterns observed in aged animals. For example, several studies have reported a tendency for aged animals to perseverate, or continue responding to a previously learned choice pattern, after reward contingencies have been reversed (Bartus, Dean, & Fleming, 1979; Tapp et al., 2003). However, it also has been suggested that response errors committed by aged animals may represent an impaired ability to learn new stimulus-reward associations (Jones & Mishkin, 1972; Lai et al., 1995; Mell et al., 2005), rather than a difficulty inhibiting a previously learned rule or association. Data from the current study supports the latter view, suggesting that 24 mo old rats have a difficult time maintaining a new rule after initially shifting away from previously learned association. Similar findings also have been observed in healthy older adults (Mell et al., 2005). Mell and colleagues (2005), found that aged adults required more trials than young adults to reach criterion on a reversal-learning task. However, there were no significant differences in the number of perseverative errors committed by either group (Mell et al., 2005). It should be noted that task demands and task difficulty vary substantially across the aforementioned studies examining age-related perseverations on reversal tasks. Therefore, perseverations may be minimized on tasks where reversals are more rapidly acquired, such is the case in the present task.
Prior research has shown that young rats tend to show greater acquisition for stimuli presented in the olfactory modality than for stimuli presented in the visual modality (Rozin & Kalat, 1972). Consistent with previous studies (Broadbent et al., 2007; Darling & Slotnick, 1994; Slotnick, 2001; Slotnick et al., 1991), the analyses of the present data show a main effect of stimulus, suggesting that the rats tended to acquire the olfactory task at a faster rate than the visual task. Thus, olfactory-based tasks used to study learning and memory processes in aged rats may be beneficial for behavioral research given that rats may acquire tasks involving olfactory stimuli more readily than visual stimuli. The results may have important implications for the selection of memory paradigms for future research studies on aging.
The normal aging process in humans is associated with a decline in cognitive functioning (Craik & Jennings, 1992). In particular, older adults show impairments on tasks that require the ability to switch cognitive sets (Albert, 1994; Grant & Berg, 1948; Mell et al., 2005; Ridderinkhof, Span, & van der Molen, 2002), such as a reversal-learning task. Impairments in reversal learning documented in old animals have been suggested to stem from age-related changes in the prefrontal cortex (Joly, Deputte, & Verdier, 2006; Mell et al., 2005; Schoenbaum, Nugent, Saddoris, & Setlow, 2002b; Tsuchida et al., 2002). The normal aging process is shown to affect both the structure and function of the prefrontal and orbitofrontal cortices (Cerf-Ducastel & Murphy, 2001, 2003; Craik & Grady, 2002; Morgan, 1987; Murphy, Cerf-Ducastel, Calhoun-Haney, Gilbert, & Ferdon, 2005; Nielson-Bohlman & Knight, 1995; Schoenbaum et al., 2002a; Suzuki et al., 2001; Yousem et al., 1999). Prefrontal cortex dysfunction is suggested to result in a decreased ability to maintain and shift cognitive sets across humans, nonhuman primates, and rats (Anderson, Damasio, Jones, & Tranel, 1991; Bartus et al., 1979; Berg, 1948; Grant & Berg, 1948; Dias, Robbins, & Roberts, 1996, 1997; Huizinga, Dolan, &, van der Molen, 2006; Miller, 2000; Moore, Killiany, Herndon, Rosene, & Moss, 2006; Schoenbaum et al., 2002b).
As discussed by Kim and Ragozzino (2005) the subregions of the prefrontal cortex may contribute differentially to strategy switching during learning tasks. Lesions or temporary inactivations of the rodent prelimbic area do not impair reversal learning of a two-choice discrimination (Birrell & Brown, 2000; Boulougouris, Dalley, & Robbins, 2007; Ragozzino, Detrick, & Kesner, 1999; Ragozzino, Kim, Hassert, Minniti, & Kiang, 2003). However, lesions or pharmacological manipulations of the orbitofrontal region impair reversal learning for olfactory, tactile, or visual cues (Bohn, Giertler, & Hauber, 2003; Boulougouris et al., 2007; Chudasama & Robbins, 2003; Ferry, Lu, & Price, 2000; Izquierdo, Suda, & Murray, 2004; Kim & Ragozzino, 2005; McAlonan & Brown, 2003; Meunier, Bachevalier, & Mishkin, 1997; Rolls, Hornak, Wade, & McGrath, 1994; Schoenbaum et al., 2002a, 2002b). The orbitofrontal cortex may support reversal learning by governing goal-directed behavior or behavior guided by incentive values associated with a stimulus (Saddoris, Gallagher, & Schoenbaum, 2005; Schoenbaum & Roesch, 2005; Schoenbaum & Setlow, 2001; Schoenbaum, Setlow, Nugent, Saddoris, & Gallagher, 2003a; Schoenbaum, Setlow, & Ramus, 2003b; Schoenbaum, Setlow, Saddoris, & Gallagher, 2003c). This type of goal-directed behavior is thought to rely heavily on interconnections between the orbitofrontal cortex and the basolateral amygdala (Saddoris et al., 2005; Schoenbaum, Chiba, & Gallagher, 1998; Schoenbaum et al., 2003b, 2003c). Additionally, the orbitofrontal cortex has been suggested to be particularly important for odor or visual cue reversal learning (Bohn, et al., 2003; Chudasama & Robbins, 2003; Schoenbaum et al., 2002a). Cellular recordings from orbitofrontal cortex neurons in rats identified as “reversal-learning impaired” revealed reductions in neuronal firing when reward contingencies were reversed in an odor discrimination task, whereas neuronal firing patterns in normal controls and aged “reversal-learning unimpaired” rats exhibited a reverse in odor preference (Schoenbaum, et al., 2006). Taken together, the results suggest that age-related impairments in reversal learning in rats may be indicative of a functional decline in the ability to flexibly switch between intra-dimensional sets.
Many previous behavioral studies involving aged rats have used 24 mo old Fisher 344 rats (F344). The present study was conducted using a Fischer 344/Brown Norway (F344/BN) hybrid strain. The F344/BN strain has been shown to live longer than Fischer 344 rats. The 50% survival age for F344/BN male rats is 34 mo, whereas the 50% survival age for F344 male rats is 24 mo (National Institute on Aging). Therefore, the rats in the present study showed significant learning impairments at 24 months of age despite having longer average longevity than other strains of rats used in prior behavioral experiments.
In summary, the results of the present study suggest that odor learning may be as affected by aging as visual learning in rats. As mentioned previously, the present study is the first to use the same paradigm to make direct comparisons between a visual task and an olfactory task utilizing the same paradigm. The results suggest that aged rats may be a suitable animal model for studying the effect of aging on odor memory in older humans. According to Schoenbaum and Setlow (2001), the development of a rodent model for the effects of aging on the medial temporal lobe system has provided considerable information relevant to understanding cognitive decline in normal aging. Achieving a better understanding of the role that the PFC plays in the aging process will greatly depend on the development of an adequate animal model. The present study illustrates the robust effect of aging on reversal learning for stimuli from different sensory modalities. In addition, olfactory based tasks may be very attractive to researchers examining age-related changes in cognition in rats because the tasks are rapidly acquired. Therefore, the results may have important implications for the selection of memory paradigms for future research studies on aging.
Figure 4.
Mean perseverative and regressive errors (±standard error) committed by 6 mo and 24 mo old rats on a visual object and an olfactory reversal task.
Acknowledgments
The research was supported by NIH grant #AG026505 from NIA to Paul E. Gilbert. Trinh Luu was suppported by grant NIH/NIGMS SDSU MARC 5T34GM08303. The authors would like to thank Dr. Claire Murphy for her helpful suggestions on the mansucript. We also thank Caren McDonald, Danielle Fellman, Molly Moreland, Elizabeth Estes, and Chris Moreland for their assistance with data collection.
References
- Agster KL, Fortin NJ, Eichenbaum H. The hippocampus and disambiguation of overlapping sequences. Journal of Neuroscience. 2002;22:5760–5768. doi: 10.1523/JNEUROSCI.22-13-05760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS. Age-related changes in cognitive function. In: Albert ML, Knoefel JE, editors. Clinical Neurology of Aging. New York: Oxford University Press; 1994. pp. 314–328. [Google Scholar]
- Alvarez P, Wendelken L, Eichenbaum H. Hippocampal formation lesions impair performance in an odor-odor association task independently of spatial context. Neurobiology of Learning and Memory. 2002;78:470–476. doi: 10.1006/nlme.2002.4068. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Damasio H, Jones RD, Tranel D. Wisconsin Card Sorting Test performance as a measure of frontal lobe damage. Journal of Clinical and Experimental Neuropsychology. 1991;13:909–922. doi: 10.1080/01688639108405107. [DOI] [PubMed] [Google Scholar]
- Apfelbach R, Russ D, Slotnick BM. Ontogenetic changes in odor sensitivity, olfactory receptor area, and olfactory receptor density in the rat. Chemical Senses. 1991;16:209–218. [Google Scholar]
- Bartus RT, Dean RL, Fleming DL. Aging in the rhesus monkey: Effects on visual discrimination learning and reversal learning. Journal of Gerontology. 1979;34:209–219. doi: 10.1093/geronj/34.2.209. [DOI] [PubMed] [Google Scholar]
- Berg EA. A simple objective test for measuring flexibility in thinking. Journal of General Psychology. 1948;39:15–22. doi: 10.1080/00221309.1948.9918159. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. Journal of Neuroscience. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn I, Giertler C, Hauber W. Orbital prefrontal cortex and guidance of instrumental behaviour in rats under reversal conditions. Behavioural Brain Research. 2003;143:49–56. doi: 10.1016/s0166-4328(03)00008-1. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behavioural Brain Research. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Rats depend on habit memory for discrimination learning and retention. Learning and Memory. 2007;14:145–151. doi: 10.1101/lm.455607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. Conservation of hippocampal memory function in rats and humans. Nature. 1996;379:255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B, Murphy C. fMRI activation in response to odorants orally delivered in aqueous solutions. Chemical Senses. 2001;26:625–37. doi: 10.1093/chemse/26.6.625. [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B, Murphy C. fMRI brain activation in response to odors is reduced in primary olfactory areas of elderly subjects. Brain Research. 2003;986:39–53. doi: 10.1016/s0006-8993(03)03168-8. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to Pavlovian autoshaping and discrimination reversal learning: Further evidence for the functional heterogeneity of the rodent frontal cortex. Journal of Neuroscience. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik FIM, Grady CL. Aging, memory and frontal lobe functioning. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. Oxford, United Kingdom: Oxford University Press; 2002. pp. 528–541. [Google Scholar]
- Craik FIM, Jennings JM. Human memory. In: Craik FIM, Salthouse TA, editors. Handbook of Aging and Cognition. Hillsdale, NJ: Erlbaum; 1992. pp. 51–109. [Google Scholar]
- Darling FM, Slotnick BM. Odor-cued taste avoidance: A simple and efficient method for assessing olfactory detection, discrimination and memory in the rat. Physiology and Behavior. 1994;55:817–822. doi: 10.1016/0031-9384(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Michaels-Marston KS, Liu X, Pelton GH, Padilla M, Marder K, et al. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer’s disease at follow-up. American Journal of Psychiatry. 2002;157:1399–1405. doi: 10.1176/appi.ajp.157.9.1399. [DOI] [PubMed] [Google Scholar]
- Dias R, Aggleton JP. Effects of selective excitotoxic prefrontal lesions on acquisition of nonmatching- and matching-to-place in the T-maze in the rat: Differential involvement of the prelimbic-infralimbic and anterior cingulate cortices in providing behavioral flexibility. European Journal of Neuroscience. 2000;12:4457–4466. doi: 10.1046/j.0953-816x.2000.01323.x. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: Effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behavioral Neuroscience. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Dissociable forms of inhibitory control within the prefrontal cortex with an analog of the Wisconsin Card Sort Test: Restriction to novel situations and independence from “on-line” processing. Journal of Neuroscience. 1997;17:9285–9297. doi: 10.1523/JNEUROSCI.17-23-09285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H. Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiology Aging. doi: 10.1016/j.neurobiolaging.2006.11.014. in press. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94:176–8. doi: 10.1288/00005537-198402000-00004. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Wood ER, Eichenbaum H. Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation. Journal of Neuroscience. 2000;15:2964–2977. doi: 10.1523/JNEUROSCI.20-08-02964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proceedings of the National Academy of Sciences. 1997;94:7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry AT, Lu XC, Price JL. Effects of excitotoxic lesions in the ventral striatopallidal-thalamocortical pathway on odor reversal learning: inability to extinguish an incorrect response. Experimental Brain Research. 2000;131:320–335. doi: 10.1007/s002219900240. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum H. Critical role of the hippocampus in memory for sequences of events. National Neuroscience. 2002;5:458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annual Review of Psychology. 1997;48:339–70. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. Role of the rodent hippocampus in paired-associate learning involving associations between a stimulus and a spatial location. Behavioral Neuroscience. 2002;116:63–71. doi: 10.1037//0735-7044.116.1.63. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. Localization of function within the dorsal hippocampus: the role of the CA3 subregion in paired-associate learning. Behavioral Neuroscience. 2003;117:1385–94. doi: 10.1037/0735-7044.117.6.1385. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Murphy C. Differences between recognition memory and remote memory for olfactory and visual stimuli in nondemented elderly individuals genetically at risk for Alzheimer’s disease. Experimental Gerontology. 2004a;39:433–441. doi: 10.1016/j.exger.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Murphy C. The effect of the ApoE ε4 allele on recognition memory for olfactory and visual stimuli in patients with pathologically confirmed Alzheimer’s disease, probable Alzheimer’s disease, and healthy elderly controls. Journal of Clinical and Experimental Neuropsychology. 2004b;26:779–794. doi: 10.1080/13803390490509439. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Pirogovsky E, Ferdon S, Brushfield A, Murphy C. Differential effects of normal aging on memory for odor-place and object-place associations. Experimental Aging Research. doi: 10.1080/03610730802271914. in press. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Pirogovsky E, Ferdon S, Murphy C. Differential effects of normal aging on source memory for odors and objects. Journal of Gerontology. 2006;61:58–60. doi: 10.1093/geronb/61.1.p58. [DOI] [PubMed] [Google Scholar]
- Grant DA, Berg EA. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a weigl-type card sorting problem. Journal of Experimental Psychology. 1948;34:404–411. doi: 10.1037/h0059831. [DOI] [PubMed] [Google Scholar]
- Graves AB, Bowen JD, Rajaram L, McCormick WC, McCurry SM, Schellenberg GD, et al. Impaired olfaction as a marker for cognitive decline. Neurology. 1999;53:1480–1487. doi: 10.1212/wnl.53.7.1480. [DOI] [PubMed] [Google Scholar]
- Huizinga M, Dolan CV, van der Molen MW. Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia. 2006;44:1–20. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Hunt RP, Aggleton JP. Neurotoxic lesions of the dorsomedial thalamus impair the acquisition but not the performance of delayed matching to place by rats: A deficit in shifting response rules. Journal of Neuroscience. 1998;18:10045–10052. doi: 10.1523/JNEUROSCI.18-23-10045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. Journal of Neuroscience. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly M, Deputte B, Verdier JM. Age effect on olfactory discrimination in a non-human primate, Microcebus murinus. Neurobiology of Aging. 2006;27:1045–1049. doi: 10.1016/j.neurobiolaging.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Mishkin M. Limbic lesions and the problem of stimulus-reinforcement associations. Experimental Neurology. 1972;36:362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE, Barua LA. The role of the hippocampus in memory for the temporal order of a sequence of odors. Behavioral Neuroscience. 2002;116:286–290. doi: 10.1037//0735-7044.116.2.286. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR, Gilbert PE. The role of CA1 in the acquisition of an object-trace-odor paired associate task. Behavioral Neuroscience. 2005;119:781–786. doi: 10.1037/0735-7044.119.3.781. [DOI] [PubMed] [Google Scholar]
- Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiology of Learning and Memory. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer S, Apfelbach R. Olfactory sensitivity, learning and cognition in young adult and aged male wistar rats. Physiological Behavior. 2004;81:435–442. doi: 10.1016/j.physbeh.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Lai ZC, Moss MB, Killiany RL, Rosene DL, Herndon JG. Executive system dysfunction in the aged monkey: Spatial and object reversal learning. Neurobiology of Aging. 1995;16:947–954. doi: 10.1016/0197-4580(95)02014-4. [DOI] [PubMed] [Google Scholar]
- Larsson M, Backman L. Modality memory across the adult life span: Evidence for selective age-related olfactory deficits. Experimental Aging Research. 1998;24:63–82. doi: 10.1080/036107398244364. [DOI] [PubMed] [Google Scholar]
- LaSarge CL, Montgomery KS, Tucker C, Slaton GS, Griffith WH, Setlow B, Bizon JL. Deficits across multiple cognitive domains in a subset of aged Fischer 344 rats. Neurobiology of Aging. 2007;28:928–936. doi: 10.1016/j.neurobiolaging.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Lu XC, Slotnick BM, Silberberg AM. Odor matching and odor memory in the rat. Physiology & Behavior. 1993;53:795–804. doi: 10.1016/0031-9384(93)90191-h. [DOI] [PubMed] [Google Scholar]
- Luu TT, Pirogovsky E, Gilbert PE. Age-related changes in contextual associative learning. Neurobiology of Learning and Memory. doi: 10.1016/j.nlm.2007.09.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behavioural Brain Research. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Mell T, Heekeren HR, Marschner A, Wartenburger I, Villringer A, Reischies FM. Effect of aging on stimulus-reward association learning. Neuropsychologia. 2005;43:554–563. doi: 10.1016/j.neuropsychologia.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M. Effects of orbital frontal and anterior cingulated lesions on object and spatial memory in rhesus monkeys. Neuropsychologia. 1997;35:999–1015. doi: 10.1016/s0028-3932(97)00027-4. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nature Neuroscience Reviews. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. Executive system dysfunction occurs as early as middle-age in the rhesus monkey. Neurobiology of Aging. 2006;27:1484–1493. doi: 10.1016/j.neurobiolaging.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Morgan DG. The dopamine and serotonin systems during aging in humans and rodent brain. A brief review. Progress in Neuro-psychopharmacology and Biological Psychiatry. 1987;11:153–157. doi: 10.1016/0278-5846(87)90053-4. [DOI] [PubMed] [Google Scholar]
- Murphy C, Bacon AW, Bondi MW, Salmon DP. Apolipoprotein E status is associated with odor identification deficits in nondemented older persons. Annals of the New York Academy of Science. 1998;855:744–750. doi: 10.1111/j.1749-6632.1998.tb10654.x. [DOI] [PubMed] [Google Scholar]
- Murphy C, Cain WS, Gilmore MM, Skinner RB. Sensory and semantic factors in recognition memory for odors and graphic stimuli: Elderly versus young persons. American Journal of Psychology. 1991;104:161–192. [PubMed] [Google Scholar]
- Murphy C, Cerf-Ducastel B, Calhoun-Haney R, Gilbert PE, Ferdon S. ERP, fMRI and functional connectivity studies of brain response to odor in normal aging and Alzheimer’s disease. Chemical Senses. 2005;30:170–171. doi: 10.1093/chemse/bjh168. [DOI] [PubMed] [Google Scholar]
- Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. Journal of the American Medical Association. 2002;288:2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- Nielson-Bohlman L, Knight RT. Prefrontal alterations during memory processing in aging. Cerebral Cortex. 1995;5:541–549. doi: 10.1093/cercor/5.6.541. [DOI] [PubMed] [Google Scholar]
- Nordin S, Murphy C. Odor memory in normal aging and Alzheimer’s disease. Annals of the New York Academy of Sciences. 1998;855:686–693. doi: 10.1111/j.1749-6632.1998.tb10646.x. [DOI] [PubMed] [Google Scholar]
- Palencia CA, Ragozzino ME. The influence of NMDA receptors in the dorsomedial striatum on response reversal learning. Neurobiology of Learning and Memory. 2004;82:81–89. doi: 10.1016/j.nlm.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP. Involvement of the prelimbic-infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. Journal of Neuroscience. 1999;19:4585–4594. doi: 10.1523/JNEUROSCI.19-11-04585.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino M, Jih J, Tzavos A. Involvement of the dorsomedial striatum in behavioral flexibility: Role of muscarinic cholinergic receptors. Brain Research. 2002a;953:205–214. doi: 10.1016/s0006-8993(02)03287-0. [DOI] [PubMed] [Google Scholar]
- Ragozzino M, Ragozzino K, Mizumori S, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behavioral Neuroscience. 2002b;116:105–115. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Kim J, Hassert D, Minniti N, Kiang C. The contribution of the rat prelimbic-infralimbic areas to different forms of task switching. Behavioral Neuroscience. 2003;117:1054–1065. doi: 10.1037/0735-7044.117.5.1054. [DOI] [PubMed] [Google Scholar]
- Rapp PR. Visual discrimination and reversal learning in the aged monkey (Macaca mulatta) Behavioral Neuroscience. 1990;104:876–884. doi: 10.1037//0735-7044.104.6.876. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Span MM, van der Molen MW. Perseverative behavior and adaptive control in older adults: Performance monitoring, rule induction, and set shifting. Brain and Cognition. 2002;49:382–401. doi: 10.1006/brcg.2001.1506. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion related learning in patients with social and emotional changes associated with frontal lobe damage. Journal of Neurology, Neurosurgery and Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman FS, AlescioLautier B, Soumireu-Mourat B. Age-related learning and memory deficits in odor-reward association in rats. Neurobiology of Aging. 1996;17:31–40. doi: 10.1016/0197-4580(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Rondi-Reig L, Libbey M, Eichenbaum H, Tonegawa S. CA1-specific N-methyl-D-aspartate receptor knockout mice are deficient in solving a nonspatial transverse patterning task. Proceedings of the National Academy of Sciences. 2001;13:3543–3548. doi: 10.1073/pnas.041620798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozin P, Kalat JW. Learning as a situation specific adaptation. In: Seligman MEP, Hager JL, editors. Biological Boundaries of Learning. New York: Appelton-Dentruy-Crofts; 1972. pp. 66–96. [Google Scholar]
- Saddoris MP, Gallagher M, Schoenbaum G. Rapid associative encoding in basolateral amygdala depends on connections with orbitofrontal cortex. Neuron. 2005;46:321–331. doi: 10.1016/j.neuron.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Natural Neuroscience. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent S, Saddoris MP, Gallagher M. Teaching old rats new tricks: Age-related impairments in olfactory reversal learning. Neurobiology of Aging. 2002a;23:555–64. doi: 10.1016/s0197-4580(01)00343-8. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002b;7:885–90. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B. Integrating orbitofrontal cortex into prefrontal theory: Common processing themes across species and subdivisions. Learning & Memory. 2001;8:134–147. doi: 10.1101/lm.39901. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Nugent SL, Saddoris MP, Gallagher M. Lesions of orbitofrontal cortex and basolateral amygdala complex disrupt acquisition of odor-guided discriminations and reversals. Learning and Memory. 2003a;10:129–140. doi: 10.1101/lm.55203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Ramus SJ. A systems approach to orbitofrontal cortex function: Recordings in rat orbitofrontal cortex reveal interactions with different learning systems. Behavioral Brain Research. 2003b;146:19–29. doi: 10.1016/j.bbr.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003c;39:731–733. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding changes in orbitofrontal cortex in reversal-impaired aged rats. Journal of Neurophysiology. 2006;95:1509–1517. doi: 10.1152/jn.01052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman SS, Graham BG, Sattely-Miller EA, Zervakis J, Welsh-Bohmer K. Taste, smell and neuropsychological performance of individuals at familial risk for Alzheimer’s disease. Neurobiology of Aging. 2002;23:397–404. doi: 10.1016/s0197-4580(01)00337-2. [DOI] [PubMed] [Google Scholar]
- Slotnick B. Animal cognition and the rat olfactory system. Trends in Cognitive Sciences. 2001;5:216–222. doi: 10.1016/s1364-6613(00)01625-9. [DOI] [PubMed] [Google Scholar]
- Slotnick B, Hanford L, Hodos W. Can rats acquire an olfactory learning set? Journal of Experimental Psychology: Animal Behavioral Processes. 2000;26:399–415. doi: 10.1037//0097-7403.26.4.399. [DOI] [PubMed] [Google Scholar]
- Slotnick BM, Kufera A, Silberberg AM. Olfactory learning and odor memory in the rat. Physiology and Behavior. 1991;50:555–561. doi: 10.1016/0031-9384(91)90545-y. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Beal MF, Pendlebury WW. Age-related disruption of classical conditioning: a model systems approach to memory disorders. Neurobiology of Aging. 1988;9:535–46. doi: 10.1016/s0197-4580(88)80110-6. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Critchley HD, Suckling J, Fukuda R, Williams SC, Andrew C, et al. Functional magnetic resonance imaging of odor identification: The effect of aging. Journal of Gerontology. 2001;56:756–760. doi: 10.1093/gerona/56.12.m756. [DOI] [PubMed] [Google Scholar]
- Tapp PD, Siwak CT, Estrada J, Head E, Muggenburg BA, Cotman CW, et al. Size and reversal learning in the beagle dog as a measure of executive function and inhibitory control in aging. Learning and Memory. 2003;10:64–73. doi: 10.1101/lm.54403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida J, Kubo N, Kojima S. Position reversal learning in aged Japanese macaques. Behavioural Brain Research. 2002;129:107–112. doi: 10.1016/s0166-4328(01)00336-9. [DOI] [PubMed] [Google Scholar]
- Van Elzakker M, O’Reilly RC, Rudy JW. Transitivity, flexibility, conjunctive representations, and the hippocampus. I. An empirical analysis. Hippocampus. 2003;13:334–340. doi: 10.1002/hipo.10083. [DOI] [PubMed] [Google Scholar]
- Voytko ML. Impairments in acquisition and reversals of two-choice discrimination by aged rhesus monkeys. Neurobiology of Aging. 1999;20:617–627. doi: 10.1016/s0197-4580(99)00097-4. [DOI] [PubMed] [Google Scholar]
- Wang QS, Tian L, Huang YL, Qin S, He LQ, Zhou JN. Olfactory identification and apolipoprotein E epsilon 4 allele in mild cognitive impairment. Brain Research. 2002;951:77–81. doi: 10.1016/s0006-8993(02)03137-2. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA. The relationship between cerebral Alzheimer’s disease pathology and odour identification in old age. Journal of Neurology Neurosurgery, and Psychiatry. 2007;78:30–35. doi: 10.1136/jnnp.2006.099721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ER, Agster KM, Eichenbaum H. One-trial odor-reward association: a form of event memory not dependent on hippocampal function. Behavioral Neuroscience. 2004;118:26–39. doi: 10.1037/0735-7044.118.3.526. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Thompson RF. Classical conditioning of the eyelid response in rabbits as a model system for the study of brain mechanisms of learning and memory in aging. Experimental Aging Research. 1985;11:109–122. doi: 10.1080/03610738508259290. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Gilbert A. National Geographic Smell Survey: Effects of age are heterogeneous. Annals of the New York Academy of Sciences. 1989;561:12–28. doi: 10.1111/j.1749-6632.1989.tb20966.x. [DOI] [PubMed] [Google Scholar]
- Yousem DM, Maldjian JA, Hummel T, Alsop D, Geckle RJ, Kraut MA, Doty RL. The effect of age on odor-stimulated functional mr imaging. American Journal of Neuroradiology. 1999;20:600–608. [PMC free article] [PubMed] [Google Scholar]