Abstract
Currently used orthopedic implants composed of titanium have a limited functional lifetime of only 10–15 years. One of the reasons for this persistent problem is the poor prolonged ability of titanium to remain bonded to juxtaposed bone. It has been proposed to modify titanium through anodization to create a novel nanotubular topography in order to improve cytocompatibility properties necessary for the prolonged attachment of orthopedic implants to surrounding bone. Additionally, electrical stimulation has been used in orthopedics to heal bone non-unions and fractures in anatomically difficult to operate sites (such as the spine). In this study, these two approaches were combined as the efficacy of electrical stimulation to promote osteoblast (bone forming cell) density on anodized titanium was investigated. To do this, osteoblast proliferation experiments lasting up to 5 days were conducted as cells were stimulated with constant bipolar pulses at a frequency of 20 Hz and a pulse duration of 0.4 ms each day for 1 hour. The stimulation voltages were 1 V, 5 V, 10 V, and 15 V. Results showed for the first time that under electrical stimulation, osteoblast proliferation on anodized titanium was enhanced at lower voltages compared to what was observed on conventional (nonanodized) titanium. In addition, compared to nonstimulated conventional titanium, osteoblast proliferation was enhanced 72% after 5 days of culture on anodized nanotubular titanium at 15 V of electrical stimulation. Thus, results of this study suggest that coupling the positive influences of electrical stimulation and nanotubular features on anodized titanium may improve osteoblast responses necessary for enhanced orthopedic implant efficacy.
Keywords: titanium, anodization, nanotubular, electrical stimulation, osteoblast, proliferation
Introduction
Titanium is one of the most commonly used implant materials in orthopedics. Although titanium has excellent corrosion resistance and suitable mechanical properties to support physiological loads, its cytocompatibility properties are not sufficient to maintain the implant functionality necessary to heal bone fractures over long periods of time. In fact, conventional titanium-based implants only have functional lifetimes of 10–15 years, making it necessary for younger patients to have at least one revision surgery, and in some cases multiple revision surgeries, before the end of their lives. Specifically, the long-term failure of currently used titanium implants is due to clinical problems such as extensive prolonged fibrous tissue encapsulation, wear debris, infection, stress shielding, etc. Clearly, currently used titanium-based implants fail to satisfy the needs of all patients and improvements are necessary.
The use of nano-structured materials has been proposed to solve some of the aforementioned problems currently associated with orthopedic implants. Nano-structured materials are those materials with at least one dimension less than 100 nm. The main reason for exploring nano-structured materials in orthopedics is that bone itself is a nano-structured tissue. For instance, hydroxyapatite crystals, the main constituent of the inorganic phase of bone, are 2–5 nm thick and collagen type I fibrils, the main constituent of the organic phase of bone, are 0.5 nm in diameter. This implies that bone cells are naturally accustomed to interacting with nanoscale surface features in the body and even synthesize such nano-structured materials.
It has been speculated that when implant surfaces are engineered to mimic the dimensions of the constituent components of bone, a better integration of the implant to surrounding bone can be expected. This is because compared to conventional (or nano-smooth materials) nano-rough materials have more surface area, surface defects, increased numbers of atoms, and altered electron distributions. Collectively, such properties inherent for nano-structured materials change surface reactivity with proteins and subsequently cells compared to conventional materials. Indeed, experimentally, changes in these surface properties on titanium (through anodization which creates novel nanotubular structures) influence the concentration and conformation of adsorbed proteins to alter cellular interactions.
Specifically, Yao and colleagues (2005) observed a 33% increase in osteoblast adhesion on anodized titanium surfaces with respect to conventional titanium. The increase in osteoblast adhesion was correlated with an 18% increase in vitronectin adsorption and a 30% increase in fibronectin adsorption on anodized compared to conventional titanium (Yao et al 2005). Additionally, osteoblasts were more well spread and they were shown to deposit more calcium-containing mineral on anodized nanotubular titanium (a crucial step for the regeneration of bone) compared to conventional titanium. Rodriguez and colleagues (2002) observed greater osteocalcin production by osteoblasts cultured on anodized compared to unanodized titanium surfaces (Rodriguez et al 2002). Lastly, animal experiments recently confirmed a higher interfacial strength between anodized titanium and juxtaposed tissue (Son et al 2003).
Modifying the surfaces of titanium to possess novel nanotubular structures is not the only way to promote bone bonding. The building blocks of living organisms, ions, polar/charged molecules, etc., all create and respond to electrical fields (Kirson et al 2007). For instance, bone has strain and strain rate dependant forward and reverse polarizations that create 10–20 μA currents (Black 1987). In their natural niche, many tissues are exposed to different levels of currents and electrical fields. In fact, electrical stimulation has been used in a number of clinical applications in orthopedics. For example, the proposed methodologies for using electrical stimulation for orthopedic applications varies from anterior and posterior placement of electrodes around a hip implant, to coiling them around with a subcutaneous power source, all the way to placement of an electromagnetic coil at the surgical bed-side. In fact, electrical stimulation has been used clinically for some patients undergoing spinal fusion surgeries, nonunion fractures, and for prior failed joint fusions who suffer from poor bone growth or diseases which cause bone to regrow slowly (Hartig et al 2000; Nelson et al 2003; Aaron et al 2004a).
Some have already combined the benefits of nano-structured materials and electrical stimulation for orthopedic applications and have found exciting results. For example, for in vitro experiments, carbon nanotubes (CNT) were added to poly-lactic acid (PLA) (Supronowicz et al 2001). Although PLA is an insulative material, 20 wt.% addition of CNT transformed PLA into a conductive material. When this composite was exposed to 10 μA of an alternating current for 6 hours a day, a 46% increase in osteoblast proliferation was observed after 2 days and a 307% increase in calcium deposition by osteoblasts was observed after 21 days with respect to nonstimulated samples (Supronowicz et al 2001). Similar results were also observed by Hartig and colleagues (2000) in which the application of a capacitively coupled electrical field increased primary osteoblast-like cell proliferation, alkaline phosphatase activity and extracellular matrix protein synthesis (Hartig et al 2000; Aaron et al 2004a). Khang and colleagues (2008) also combined nano-structured materials and electrical stimulation for cartilage applications by demonstrating enhanced proliferation of chondrocytes (cartilage synthesizing cells) on electrically stimulated CNT/poly-carbonate urethane composites compared to nonstimulated respective substrates.
However, to the best of our knowledge, there has not been any research concerning combining electrical stimulation with one of the most commonly implanted orthopedic biomaterials, titanium, modified to have nano-structured surface features. Due to this reason, the current in vitro study investigated the proliferation of osteoblasts on anodized nanotubular titanium under 4 different voltages for up to 5 days of culture.
Experimental procedures
Specimen preparation
99.2% pure titanium foils (1.6 mm thick) were purchased (Alfa Aesar, Ward Hill, MA) and cut into 1 cm × 1 cm squares. Then, each side of these squares were sonicated (VWR 75D) for 1 hour using a cleaning solution (Branson, IS), followed by 30 minutes of sonication with acetone (Mallinckrodt Chemicals), 70% ethanol and ddH2O. These cleaned samples were termed ‘conventional titanium’ in the present study.
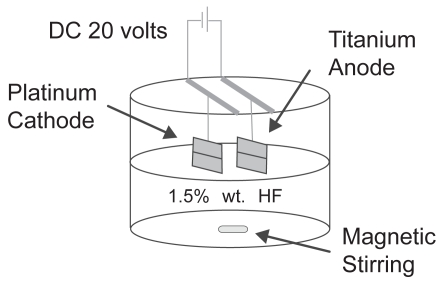
Afterwards, the surface cleaned titanium samples were etched with a 1.5% HF/1.5% HNO3 solution (Mallinckrodt Chemicals) for 5 minutes to remove the oxide layer. This was followed by an anodization process (Figure 1). For anodization, titanium samples were connected to a DC-powered electrochemical cell which had a two electrode configuration. A platinum mesh was used as the cathode and a titanium specimen was used as the anode. These platinum and titanium samples were connected to a DC power supply (3645A DC power supply, Circuit Specialists, Inc.) through copper wires. A constant voltage of 20 V was applied for 10 minutes according to previous studies (Yao et al 2005). The distance between the titanium anode and platinum cathode was kept constant at 1 cm. The electrolyte solution used in this study was 1.5% hydrofluoric acid and the anodization was conducted inside a Teflon beaker. During anodization, the electrolyte solution was constantly stirred with magnetic agitation to reduce the thickness of the double layer at the metal-electrode interface to obtain uniform local current densities on the titanium electrode (Mor and Varghese 2003). After anodization, the specimens were again sonicated with acetone, 70% ethanol and ddH2O, for 30 minutes each. These samples were termed ‘anodized nanotubular titanium’ since, as will be shown, titanium anodized in this manner possesses nanotubes penetrating the titanium surfaces.
Figure 1.
Schematic of the anodization system.
Material characterization
The surfaces were characterized using a LEO 1530 VP FE-4800 Field-Emission Scanning Electron Microscope (SEM). 5 kV accelerating voltages were used to image the specimens. No coatings were used to image the surfaces.
Electrical stimulation
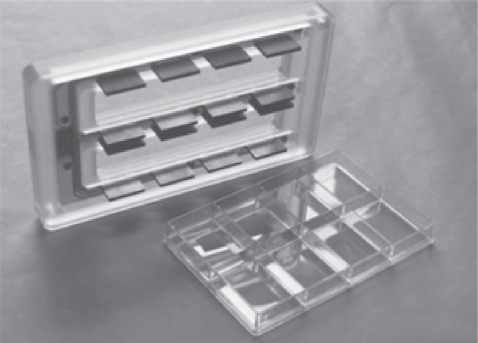
A multi channel electrical stimulator (Ionoptix, Milton, MA) was used to stimulate osteoblasts with electricity. This stimulator generates bipolar pulses at a frequency of 0.01 to 99 Hz, pulse duration of 0.4–24 msec, and voltages of up to ±40 V. There was net charge transport between the electrodes of the electrical stimulation system and the primary field created was an electrical field as opposed to a magnetic field. The stimulator had two components, a voltage generator and a cell culture dish electrode assembly (Figure 2). A voltage generator was connected to the culture dish electrode assembly through a thin ribbon cable, which allowed for a good seal with the cell culture incubator door. Each dish was stimulated separately with a slight time delay, on the order of milliseconds. The resistance (R) of each well was calculated from equation 1:
Figure 2.
Schematic of the 8-well culture dish electrode assembly showing a uniquely manufactured upper lid with graphite electrodes. The electrodes were placed inside the media during electrical stimulation of the cells and the 8-well dish was connected to the banks of the voltage generator.
| (1) |
where d stands for distance between graphite electrodes, A stands for the area of the electrodes in the media, and σ stands for the conductivity of the media. The distance between graphite electrodes was 3.2 cm, the surface area of the electrode in contact with the media was 0.473 cm2 and the conductivity of the media at 36 °C was 0.0146 S/cm, giving a resistance of 463 Ohms for each well. Upon the application of 1 V to the media, 2.16 mA passed through the media. However, the physiological current bone experiences is 10–20 μA, nearly 1000 times lower (Black 1987). In fact, during trial runs, 2.16 mA was found to be lethal for osteoblasts in the present study (data not shown). Therefore, a 75 kΩ potentiometer was connected to the circuit to mimic the physiological current osteoblasts are exposed to during normal activity. Upon the application of 1 V to the system, the current passing through the media was estimated to be 13 μA.
Cell culture
Osteoblasts (population numbers 8 to 15, American Type Culture Collection CEL-11372) were cultured in osteoblast cell culture medium (Dulbecco’s Modified Eagle Medium [DMEM]; Hyclone, Logan, UT) supplemented with 10% fetal bovine serum (FBS; Hyclone) and 1% penicillin-streptomycin (P/S; Hyclone) under standard culture conditions (5% CO2/95% air at 37 °C).
For the cell experiments, all samples were sterilized using a steam autoclave (Amsco Renascence Series, 3021). The osteoblasts in DMEM supplemented with 10% FBS and 1% P/S were seeded onto the specimens at a density of 3500 cell/cm2. Cells were cultured in the same media under standard cell culture conditions for 5 days and cell counts were performed at the 1st, 3rd, and 5th days. The media was changed at the 2nd and 4th days. At the end of the prescribed time period, specimens were gently rinsed in a 1 × PBS buffer solution to remove nonadherent cells. Adherent cells were then fixed with 4% formaldehyde (Fisher Scientific, Pittsburgh, PA) for 10 min and nuclei stained by soaking in a 1% DAPI fluorescent dye (Sigma, St. Louis, MO) for 20 min. Cell densities were counted in situ from five randomly selected, nonintersecting areas on each specimen under a fluorescence microscope (Leica DM 5500B). Numerical data was analyzed using standard analysis of variance (ANOVA). All cell experiments were repeated three times both for conventional and anodized nanotubular titanium samples.
Cell experiments with electrical stimulation
The first electrical stimulation was conducted 8 h after the cells were seeded onto the specimens. The duration of the electrical stimulation was 1 h and the cells were stimulated each day. For all experiments, the pulse duration and frequency were selected based on prior research and kept constant at 0.4 ms and 20 Hz, respectively (Black 1987). The voltages used for these experiments were 1, 5, 10, and 15 V, corresponding to 13, 65, 130, and 195 μA, respectively. No change was observed in pH or temperature of the media after 1 h of electrical stimulation.
Results and discussion
Material characterization
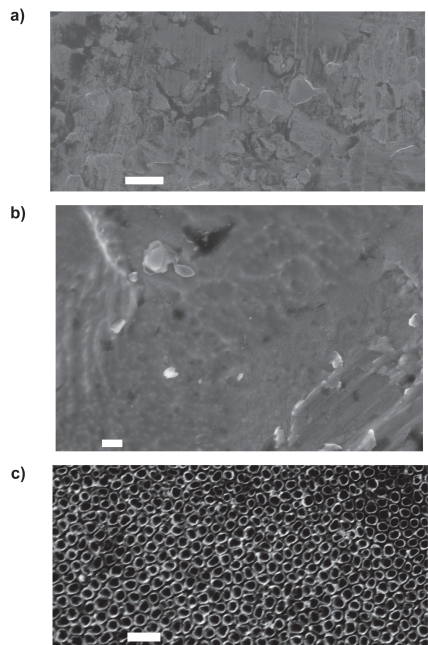
As determined by FESEM, the surfaces of the conventional (unanodized) and anodized nanotubular titanium confirmed that observed in previous studies (Figure 3; Yao et al 2005). Specifically, the anodization process created titania nanotubes on the titanium specimens. The diameters of the titania nanotubes ranged from 40–60 nm. Different researchers have measured different nanotube depths, varying between 200–500 nm depending on the electrolyte concentrations, times, and titanium alloy compositions used (Mor and Varghese 2003; Lee et al 2006). The depths of the nanotubes created in the present study have been estimated at 200–250 nm (Yao et al 2005).
Figure 3.
SEM images showing the surface features of: (a) (low magnification), (b) (high magnification) conventional, and (c) anodized nanotubular titanium.
Notes: Scale bars are: (a, b) 10 μm, and (c) 200 nm.
Osteoblast cell number results
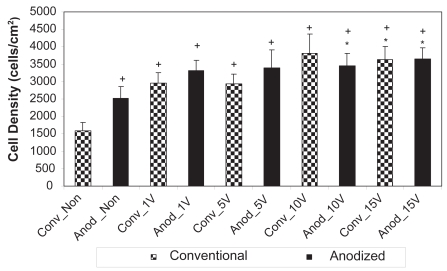
After 1 day of culture, results of the present study confirmed those of other studies that have been completed without electrical stimulation. Specifically, without electrical stimulation, more osteoblasts were counted on anodized nanotubular compared to unanodized titanium after 1 day (Figure 4). Yao and colleagues (2005) first reported higher osteoblast density on anodized nanotubular compared to unanodized titanium after a 4-h adhesion test. Yao and colleagues (2005) continued to report significantly greater alkaline phosphate activity and calcium deposition by osteoblasts cultured on anodized nanotubular compared to unanodized titanium for up to 21 days.
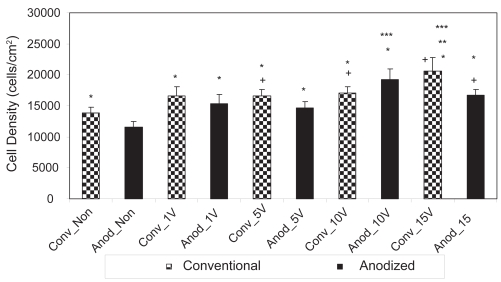
Figure 4.
Osteoblast densities after 1 day on the nonstimulated as well as 1 V, 5 V, 10 V, and 15 V electrically stimulated conventional and anodized nanotubular titanium.
Notes: Values are mean ± SEM; n = 3; *p < 0.05 compared to Anod_Non; +p < 0.05 compared to Conv_Non.
Abbreviations: Anod, anodized nanotubular titanium; Conv, conventional (nonanodized titanium); Non, nonelectrical stimulated.
Interestingly, Karlsson and colleagues (2003) reported similar greater osteoblast adhesion (when not electrically stimulated) on anodized compared to unanodized alumina. The fact that two different metals can be oxidized and promote osteoblast functions leads one to question whether any metal may fit this trend after anodization. Compared to unanodized titanium, Yao and colleagues (2005) explained the observed greater osteoblast adhesion on anodized nanotubular titanium by measuring increased adsorption of vitronectin and fibronectin onto the anodized titanium surfaces.
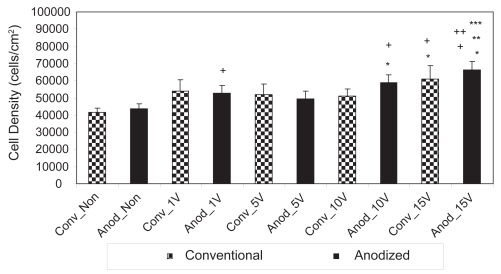
However, in addition to confirming the results of other studies (Yao et al 2005), the present study also provided the first evidence that osteoblasts responded to lower amounts of electrical stimulation when cultured on anodized nano-tubular than unanodized titanium (Figure 4). Specifically, after 1 day of culture, statistically greater numbers of osteoblasts were counted on anodized nanotubular titanium electrically stimulated at 10 V compared to unstimulated anodized materials while the same event did not happen on unanodized titanium until 15 V. After 3 days of culture, statistically greater numbers of osteoblasts were counted on anodized nanotubular titanium stimulated at 10 V compared to 5 V; the same comparison was not statistically different until osteoblasts were stimulated at 15 V on conventional titanium (Figure 5). This same trend of less voltage increasing osteoblast densities on anodized nanotubular compared to unanodized titanium continued after 5 days of culture (Figure 6). Specifically, compared to unstimulated anodized materials, more osteoblasts were counted on anodized nano-tubular titanium stimulated at 10 V while the same levels of osteoblast numbers were not observed until 15 V was reached on conventional, nonanodized titanium after 5 days of culture. Moreover, of any of the substrates of interest to the present study, the largest numbers of osteoblasts were counted on anodized nanotubular titanium electrically stimulated at 15 V after 5 days of culture (Figure 6).
Figure 5.
Osteoblast densities after 3 days on nonstimulated as well as 1 V, 5 V, 10 V, and 15 V electrically stimulated conventional and anodized nanotubular titanium.
Notes: Values are mean ± SEM; n = 3; *p < 0.05 compared to Anod_Non; **p < 0.05 compared to Anod_1 V; ***p < 0.05 compared to Anod_5 V; +p < 0.05 compared to Conv_Non.
Abbreviations: Anod, anodized nanotubular titanium; Conv, conventional (nonanodized titanium); Non, nonelectrical stimulated.
Figure 6.
Osteoblast densities after 5 days on nonstimulated as well as 1 V, 5 V, 10 V, and 15 V stimulated conventional and anodized nanotubular titanium specimens.
Notes: Values are mean ± SEM; n = 3, *p < 0.05 compared to Anod_Non; **p < 0.05 compared to Anod_1 V; ***p < 0.05 compared to Anod_5 V; +p < 0.05 compared to Conv_Non; ++p < 0.05 compared to Conv_10V.
Abbreviations: Anod, anodized nanotubular titanium; Conv, conventional (nonanodized titanium); Non, nonelectrical stimulated.
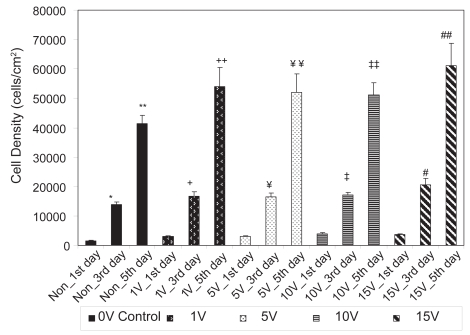
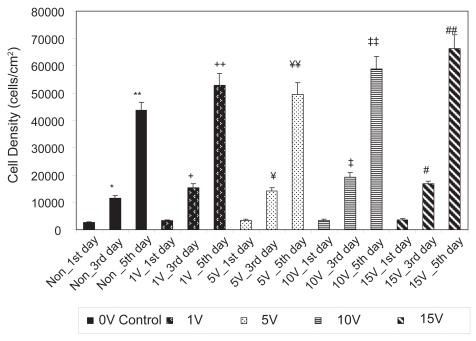
As expected, the results of the present study also demonstrated that osteoblasts grew on both conventional and anodized nanotubular titanium with culture time (from 1 to 3 to 5 days) (Figures 7 and 8). Table 1 lists the percent increases in osteoblast numbers up to 5 days of culture on the substrates of interest to the present study. When the percent increases in osteoblast densities from the 1st to 3rd days of culture are compared, they were found to be higher for conventional titanium compared to anodized nanotubular titanium for all test conditions (excluding the 10 V stimulation, the same voltage consistently highlighted on anodized nanotubular titanium as the one in which osteoblasts performed statistically similar to osteoblasts on conventional titanium at 15 V). Upon comparison of the percent increases in osteoblast densities from the 3rd to 5th days of culture, they were higher for osteoblasts on anodized compared to their conventional counterparts for all voltage conditions. In fact, when percent increases in cell densities from the 1st to 3rd days and from the 3rd to 5th days are compared, osteoblast proliferation was found to decrease with time more on conventional titanium samples than anodized nano-tubular titanium. While the reasons for these trends are not clear at this time, it is important to note that, as mentioned, more osteoblasts were present on anodized nanotubular than conventional titanium after 1 day and it is possible that the combined effects of altered titanium surface properties obtained through anodization and electrical stimulation are responsible for these complex trends.
Figure 7.
Osteoblast densities from 1 to 5 days on conventional (nonanodized) titanium.
Notes: Values are mean ± SEM; n = 3; *p < 0.05 compared to Non_1st day; **p < 0.05 compared to Non_3rd day; +p < 0.05 compared to 1 V_1st day; ++p < 0.05 compared to 1 V_3rd day; ¥p < 0.05 compared to 5 V_1st day; ¥¥p < 0.05 compared to 5 V_3rd day; ‡p < 0.05 compared to 10 V_1st day; ‡‡p < 0.05 compared to 10 V_3rd day; #p < 0.05 compared to 15 V_1st day; and ##p < 0.05 compared to 15 V_3rd day.
Abbreviation: Non, nonelectrical stimulated.
Figure 8.
Osteoblast densities from 1 to 5 days on anodized nanotubular titanium.
Notes: Values are mean ± SEM; n = 3; *p < 0.05 compared to Non_1st day; **p < 0.05 compared to Non_3rd day; +p < 0.05 compared to 1 V_1st day; ++p < 0.05 compared to 1 V_3rd day; ¥p < 0.05 compared to 5 V_1st day; ¥¥p < 0.05 compared to 5 V_3rd day; ‡p < 0.05 compared to 10 V_1st day; ‡‡p < 0.05 compared to 10 V_3rd day; #p < 0.05 compared to 15 V_1st day; and ##p < 0.05 compared to 15 V_3rd day.
Abbreviation: Non, nonelectrical stimulated.
Table 1.
Fold increase in osteoblast density from the 1st to 3rd days and from the 3rd to 5th days on the substrates of interest to the present study. Increased cell proliferation is slower with time and the rate of proliferation slowing is higher for osteoblasts cultured on conventional titanium than anodized nanotubular titanium
| Fold Increase | Conventional 0V | Anodized Nanotubular 0V | Conventional 1V | Anodized Nanotubular 1V | Conventional 5V | Anodized Nanotubular 5V | Conventional 10V | Anodized Nanotubular 10V | Conventional 15V | Anodized Nanotubular 15V |
|---|---|---|---|---|---|---|---|---|---|---|
| From 1st day to 3rd day | 8.76 | 4.57 | 5.64 | 4.63 | 5.66 | 4.31 | 4.49 | 5.57 | 5.69 | 4.59 |
| From 3rd day to 5th day | 2.99 | 3.79 | 3.25 | 3.44 | 3.14 | 3.38 | 2.99 | 3.06 | 2.96 | 3.96 |
Additionally, from these results, one can conclude that electrical stimulation can successfully be used to increase osteoblast proliferation on anodized nanotubular titanium. The data show that the best osteoblast proliferation results were obtained by stimulating with either 10 V or 15 V for each investigated time point regardless of whether titanium was anodized. This indicates for the first time that within the tested voltage range, holding the frequency and pulse duration constant at 20 Hz and 0.4 ms, the optimal voltage window for maximizing osteoblast densities on anodized titanium is 10 V to 15 V. Most importantly, it was shown here that osteoblasts responded to lower voltages on anodized titanium compared to conventional titanium, perhaps because more cells initially adhered to anodized titanium, which would promote more focal contacts and cell–cell contacts allowing for less voltage to electrically stimulate cell growth.
Lastly, although it is still not completely understood, researchers have tried to explain osteoblast proliferation behavior upon electrical stimulation. Both for inductively and capacitively coupled stimulation, bone cell proliferation has been enhanced by increased calmodulin levels, whose concentration was promoted by enhanced calcium levels in the cytosol (Aaron et al 2004b). The calcium concentration in the cytosol was due to voltage gated calcium channels for the case of capacitive coupling, and for inductive coupling, it was due to the release of internal calcium stores (Aaron et al 2004b). The increase in osteoblast proliferation in this study might be due to the increased calcium ions in the cytosol, as well. However, cell signaling is very complex, involving various signal transduction pathways which need to be carefully elucidated in the present system.
Conclusions
To conclude, the optimal voltage window that maximizes osteoblast densities on both conventional and anodized nano-tubular titanium was found to be 10 V to 15 V. Osteoblast density on conventional titanium surfaces was enhanced by 72% by creating anodized nanotubular surface features and electrically stimulating osteoblasts on them at 15 V. Additionally, osteoblasts responded to lower voltages on anodized compared to conventional titanium. For these reasons, the use of an anodized nanotubular titanium topography coupled with electrical stimulation has the potential to enhance the performance of titanium for orthopedic implant applications.
Acknowledgments
The authors would like to thank to the Herman Foundation for funding. The authors report no conflicts of interest in this work.
References
- Aaron RK, Ciombor DM, Simon BJ, et al. Treatment of nonunions with electric and electromagnetic fields. Clin Orthoped Relat Res. 2004a;419:21–9. doi: 10.1097/00003086-200402000-00005. [DOI] [PubMed] [Google Scholar]
- Aaron RK, Boyan BD, Ciombor DM. Stimulation of growth factor synthesis by electric and electromagnetic fields. Clin Orthoped. 2004b;419:30–7. doi: 10.1097/00003086-200402000-00006. [DOI] [PubMed] [Google Scholar]
- Black J. Electrical stimulation. Its role in growth, repair and remodeling of the musculoskeletal system. New York, NY: Praeger Publishers; 1987. [Google Scholar]
- Hartig M, Joos U, Wiesmann HP. Capacitively coupled electric fields accelerate proliferation of osteoblast-like primary cells and increase bone extracellular matrix formation in vitro. Eur Biophys J. 2000;29:499–506. doi: 10.1007/s002490000100. [DOI] [PubMed] [Google Scholar]
- Karlsson M, Palsgard E, Wilshaw PR, et al. Initial in vitro interaction of osteoblasts with nano-porous alumina. Biomaterials. 2003;24:3039–46. doi: 10.1016/s0142-9612(03)00146-7. [DOI] [PubMed] [Google Scholar]
- Khang D, Park GE, Webster TJ. Enhanced chondrocyte densities on carbon nanotube composites: The combined role of nano-surface roughness and electrical stimulation. J Biomed Mater Res Part A. 2008;86A:253–60. doi: 10.1002/jbm.a.31803. [DOI] [PubMed] [Google Scholar]
- Kirson ED, Dbaly V, Tovarys F, et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A. 2007;104:10152–7. doi: 10.1073/pnas.0702916104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WJ, Alhoshan M, Smyrl WH. Titanium dioxide nanotube arrays fabricated by anodizing processes. J Electrochem Soc. 2006;153:B499–505. [Google Scholar]
- Mor GK, Varghese OK. Fabrication of tapered, conical-shaped titania nanotubes. J Mater Res. 2003;18:2588–93. [Google Scholar]
- Nelson FRT, Brighton CT, Ryaby J, et al. Use of physical forces in bone healing. J Am Acad Orthop Surg. 2003;11:344–54. doi: 10.5435/00124635-200309000-00007. [DOI] [PubMed] [Google Scholar]
- Rodriguez R, Kim K, Ong JL. In vitro osteoblast response to anodized titanium and anodized titanium followed by hydrothermal treatment. J Biomed Mater Res A. 2002;65A:352–8. doi: 10.1002/jbm.a.10490. [DOI] [PubMed] [Google Scholar]
- Son W, Zhu X, Shin H, et al. In vivo histological response to anodized and anodized/hydrothermally treated titanium implants. J Biomed Mater Res B Appl Biomater. 2003;66B:520–5. doi: 10.1002/jbm.b.10042. [DOI] [PubMed] [Google Scholar]
- Supronowicz PR, Ajayan PM, Ullmann KR, et al. Novel current-conducting composite substrates for exposing osteoblasts to alternating current stimulation. J Biomed Mater Res. 2001;59:499–506. doi: 10.1002/jbm.10015. [DOI] [PubMed] [Google Scholar]
- Yao C, Perla V, McKenzie J, et al. Anodized Ti and Ti6Al4V possessing nanometer surface features enhances osteoblast adhesion. J iomed Nanotechnol. 2005;1:68–73. [Google Scholar]