Abstract
Objectives. We sought to evaluate the contribution of the New York City Health and Nutrition Examination Survey (NYC-HANES) to local public health surveillance.
Methods. Examination-diagnosed estimates of key health conditions from the 2004 NYC-HANES were compared with the National Health and Nutrition Examination Survey (NHANES) 2003–2004 national estimates. Findings were also compared with self-reported estimates from the Community Health Survey (CHS), an annually conducted local telephone survey.
Results. NYC-HANES estimated that among NYC adults, 25.6% had hypertension, 25.4% had hypercholesterolemia, 12.5% had diabetes, and 25.6% were obese. Compared with US adults, NYC residents had less hypertension and obesity but more herpes simplex 2 and environmental exposures (P < .05). Obesity was higher and hypertension was lower than CHS self-report estimates (P < .05). NYC-HANES and CHS self-reported diabetes estimates were similar (9.7% vs 8.7%).
Conclusions. NYC-HANES and national estimates differed for key chronic, infectious, and environmental indicators, suggesting the need for local data. Examination surveys may provide more accurate information for underreported conditions than local telephone surveys. Community-level health and nutrition examination surveys complement existing data, providing critical information for targeting local interventions.
The principal source of examination-verified health information for the nation is the National Health and Nutrition Examination Survey (NHANES) conducted by the National Center for Health Statistics.1 To objectively assess health indicators, NHANES captures population-level health information through face-to-face interviews, clinical examination, and laboratory testing. These data are critical for national health policy formulation2; however, health estimates are available at the national level only, limiting the local application of these data.
Most local jurisdictions monitor health conditions by surveillance of mortality and reportable diseases and, in some areas, through telephone surveys.3 In New York City (NYC), health estimates are collected through the Community Health Survey (CHS), an annual telephone survey of NYC adults.4 Together with surveillance data, these data guide local public health programs and policies;5 however, they may under- or overestimate the burden of conditions best assessed by a physical examination.
To obtain more-comprehensive local health information, the NYC Department of Health and Mental Hygiene conducted the Health and Nutrition Examination Survey (NYC-HANES), which to our knowledge is the first community-level health and nutrition survey to capture information on key conditions including obesity, diabetes, hypertension, high cholesterol, infectious diseases, and environmental exposures. Although some information on these conditions is available through other routine data sources, NYC-HANES provides objective population-based prevalence estimates. The standardized data-collection methodology also allows for direct comparison with national estimates from NHANES.
In our analyses, we summarize key health indicators obtained from NYC-HANES and compare them to national estimates and local telephone-based estimates.
METHODS
Data Sources
New York City Health and Nutrition Examination Survey.
The 2004 NYC-HANES was a population-based, cross-sectional survey of noninstitutionalized NYC adult residents 20 years or older. We used a 3-stage cluster sampling design to recruit participants from June through December 2004. The survey consisted of a face-to-face computer-assisted interview, a private audio computer-assisted interview, physical examination, and laboratory testing. Pretranslated interviews were conducted in English (76.5%) and Spanish (16.6%). Interviews were also conducted in other languages (6.9%) translated by staff, family members, or a telephone-based translation service. All survey instruments, protocols, equipment, and measurements were standardized to NHANES specifications; all NYC-HANES staff were trained according to the Centers for Disease Control and Prevention protocols used for NHANES.6 Most laboratories that performed testing for NHANES were used for NYC-HANES.7 Detailed information on the study methods has been published elsewhere.8
We identified a total of 3047 adults eligible to participate in NYC-HANES (84% household contact rate); 1999 individuals completed the face-to-face interview and at least 1 comprehensive physical examination measurement (66%). The overall response rate was 55%. Of the 1999 participants, 1812 (91%) provided blood for laboratory testing. A fasting subsample, comprising randomly assigned participants (n = 1482) who fasted from 8 to 23 hours, was used to assess diabetes measures. Participants with a valid fasting plasma glucose level or a self-reported diabetes diagnosis were considered subsample respondents (n = 1350).
Community Health Survey.
The CHS is a series of annual, cross-sectional computer-assisted telephone surveys of NYC residents 18 years or older conducted since 2002. Survey methods are described in detail elsewhere.5 CHS 2004 data are presented when available; where necessary, data collected in 2002 and 2005 are presented and noted. The 2004 cooperation rate was 59% (n = 9585).4 Analyses were limited to adults 20 years and older.
National Health and Nutrition Examination Survey.
NHANES is a routinely conducted population-based, cross-sectional survey of noninstitutionalized US residents 2 months or older. Similar to NYC-HANES, NHANES used a multistage cluster sampling design; survey methods are published elsewhere.9 We analyzed NHANES 2003–2004 data for adults 20 years and older; the response rate was 68.6%.10
Health Indicators
We assessed self-reported estimates and examination-based estimates for key chronic, infectious, and environmental health indicators. Self-reported estimates evaluated for chronic conditions were derived from interview responses. Examination-based estimates used physical measures, sometimes in combination with interview responses. Slight wording differences exist between survey questions and are noted. Sample sizes varied depending on component or item nonresponse.
A finding of self-reported hypertension was derived from participant reports that a health care provider had ever told them that they had high blood pressure. Examination-diagnosed hypertension was defined as an average systolic blood pressure at 140 mm Hg or higher, an average diastolic blood pressure at 90 mm Hg or higher, or taking prescribed antihypertensive medication. Among participants with examination-diagnosed hypertension, those not reporting hypertension were considered unaware of their condition or undiagnosed and those with an average blood pressure below 140/90 mm Hg were considered to have their condition under control.
A finding of self-reported hypercholesterolemia was derived from participant reports that a health care provider had ever told them that they had high cholesterol. We defined examination-diagnosed hypercholesterolemia as a serum total cholesterol at 240 mg/dL or higher or taking prescribed cholesterol lowering medications.11,12 Among participants with examination-diagnosed hypercholesterolemia, those not reporting high cholesterol were considered unaware of their condition or undiagnosed and those with a total cholesterol at lower than 240 mg/dL were considered to have their condition under control.
Self-reported diabetes was derived from participant reports that a health care provider had ever told them that they had diabetes (other than during pregnancy for women). Examination-diagnosed diabetes included self-reported and undiagnosed diabetes. We considered participants without a previous diagnosis but whose fasting plasma glucose level was 126 mg/dL or higher to have undiagnosed diabetes.13 Glycemic control among persons with diabetes was assessed using levels of glycosolated hemoglobin; levels under 7% were considered to be under control.14
We assessed height and weight through interview responses and physical measurement. Body mass index was calculated as weight in kilograms divided by height in meters squared. Participants with a body mass index below 18.5 kg/m2 were underweight, 18.5 to 24.9 kg/m2 were normal weight, 25.0 to 29.9 kg/m2 were overweight and those with a body mass index of 30.0 kg/m2 or more were considered obese.15 Pregnant women were excluded from the NYC-HANES and NHANES analyses; pregnancy status was unavailable in CHS analysis.
Participants provided serum samples, which were analyzed for herpes simplex 2 (HSV-2) antibodies using a type-specific immunodot assay.16 We also tested participants for hepatitis C virus (HCV) antibody using a second-generation enzyme immunoassay. Positive tests were confirmed using Chiron RIBA HCV 3.0 Strip Immunoblot Assay (Chiron Corporation Inc, Emeryville, CA).17 Indeterminate results were excluded from the analysis.
Serum cotinine concentration, a biomarker indicating tobacco smoke exposure, was measured using an isotope-dilution liquid chromatography tandem mass spectrometry method with a detection limit of 0.050 ng/mL.18 The percentage of values over 0.050 ng/mL were estimated for nonsmokers, who were defined as participants with a cotinine concentration at 10 ng/mL or less.
We tested blood metal concentrations using inductively coupled plasma mass spectroscopy methodology.19 Geometric means were estimated for lead, cadmium, and mercury.
Race/Ethnicity
For NYC-HANES and CHS, race/ethnicity was categorized as non-Hispanic White, non-Hispanic Black, Asian, and Hispanic origin. Multiracial respondents selecting a main race were recoded into the selected category. For NHANES, race/ethnicity was classified as non-Hispanic White, non-Hispanic Black, and Mexican American. Participants whose answers did not correspond to these race/ethnicity categories were considered “non-Hispanic Other” across all surveys.
Statistical Analysis
We weighted analyses to account for nonresponse; weights were post-stratified to represent the NYC adult population and then further adjusted to address component- and item-level nonresponse.20 SUDAAN version 9.0 (Research Triangle Institute, Research Triangle Park, NC) was used to obtain standard error estimates by Taylor series linearization. Prevalence estimates were age-adjusted to the 2000 US standard population.21 We calculated crude odds ratios (ORs) to assess associations between race and chronic conditions. The relative standard errors (RSE) and 95% confidence intervals (CIs) were calculated for means and percentages. Estimates with RSEs of 30% or more are noted as unreliable.
We compared NYC-HANES and NHANES examination-based estimates. NYC-HANES self-reported and examination-based estimates were compared with available CHS estimates. The 2-sample t test was used to evaluate differences between survey estimates.22
RESULTS
Population Comparisons
The weighted estimates of demographic characteristics for each survey population are shown in Table 1. NYC-HANES and CHS had similar distributions of gender, age, and race. The NYC-HANES population projection had a larger proportion of adults with less than a high school education than did the CHS projection (27.1 vs 16.5%), more closely reflecting the NYC education distribution according to the 2000 US Census.23 Compared with US adults, NYC adults were slightly younger, had less education, and were more likely to be non-Hispanic Black.
TABLE 1.
Characteristics of Participants 20 Years or Older: NYC-HANES, 2004, CHS, 2004, and NHANES, 2003–2004
| NYC-HANES 2004 (n = 1999) |
CHS 2004 (n = 9066) |
NHANES 2003–2004 (n = 4742) |
||||
| Characteristic | No. | % (95% CI) | No. | % (95% CI) | No. | % (95% CI) |
| Age group, y | ||||||
| 20–39 | 972 | 42.5 (39.2, 45.8) | 3496 | 45.1 (43.8, 46.4) | 1656 | 38.8 (35.9, 41.8) |
| 40–59 | 741 | 35.9 (33.4, 38.6) | 3312 | 34.2 (33, 35.4) | 1336 | 38.5 (36.3, 40.8) |
| ≥ 60 | 286 | 21.6 (18.7. 24.8) | 2258 | 20.7 (19.8, 21.7) | 1750 | 22.7 (20.6, 24.9) |
| Gender | ||||||
| Men | 831 | 46.1 (44.0, 48.2) | 3645 | 46.6 (45.3, 47.9) | 2275 | 47.9 (46.5, 49.4) |
| Women | 1168 | 53.9 (51.8, 56.0) | 5421 | 53.4 (52.1, 54.7) | 2467 | 52.1 (50.6, 53.5) |
| Race/Ethnicity | ||||||
| Non-Hispanic White | 617 | 38.5 (32.5, 44.8) | 3679 | 39.6 (38.5, 40.7) | 2539 | 72.8 (64.7, 79.7) |
| Non-Hispanic Black | 434 | 23.0 (18.3, 28.6) | 2101 | 22.0 (21.1, 22.9) | 948 | 11.4 (8.0, 16.0) |
| Asiana | 260 | 10.8 (8.3, 14.0) | 656 | 9.5 (8.8, 10.3) | NA | NA |
| Hispanica | 655 | 26.1 (21.9, 30.9) | 2353 | 25.4 (24.4, 26.5) | NA | NA |
| Mexican Americanb | NA | NA | NA | NA | 951 | 7.8 (4.5, 13.0) |
| Other Hispanicb | NA | NA | NA | NA | 143 | 3.6 (2.5, 5.1) |
| Non-Hispanic other | 29 | 1.5 (1.0, 2.3) | 277 | 3.5 (3.0, 4.1) | 161 | 4.4 (3.1, 6.1) |
| Education | ||||||
| Less than high school | 572 | 27.1 (23.8, 30.6) | 1483 | 16.5 (15.6, 17.5) | 1402 | 18.5 (16.2, 21.0) |
| High school | 384 | 19.1 (16.9, 21.6) | 2216 | 25.3 (24.2, 26.5) | 1193 | 27.1 (25.0, 29.3) |
| More than high school | 1035 | 53.8 (49.5, 58.1) | 5299 | 58.2 (56.9, 59.4) | 2138 | 54.5 (51.7, 57.2) |
Note. NYC-HANES = New York City Health and Nutrition Examination Survey; CHS = Community Health Survey; NHANES = National Health and Nutrition Examination Survey; CI = confidence interval.
NHANES does not provide estimates for Hispanics or Asians.
NYC-HANES and CHS do not provide estimates for Mexican Americans and other Hispanics.
Chronic Conditions
The examination-based prevalence of key health conditions from NYC-HANES is summarized in Table 2. Approximately one quarter (25.6%) of NYC adults had hypertension. One fourth (25.0%) of hypertensive NYC adults were unaware of their hypertension (undiagnosed), and less than half (43.6%) had their condition under control. Of those who were aware of their hypertension, a significant proportion did not have their condition under control (42.0%; 95% CI = 33.7%, 50.8%). Non-Hispanic Blacks were more likely than were non-Hispanic Whites to have hypertension (OR = 1.5; 95% CI = 1.0, 2.1).
TABLE 2.
Examination-Based Prevalence, Awareness, and Control of Selected Conditions Among Adults 20 Years or Older: NYC-HANES, 2004, and NHANES, 2003–2004
| NYC-HANES 2004 |
NHANES 2003–2004 |
|||
| Health Outcome | No. | % (95% CI) | No. | % (95% CI) |
| Chronic conditions | ||||
| Hypertension | 1975 | 25.6 (23.4, 27.8) | 4401 | 30.6a (28.4, 33.0) |
| Undiagnosedb | 389 | 25.0 (18.9, 32.4) | 1633 | 32.9 (26.4, 40.1) |
| Controlledb | 389 | 43.6 (37.6, 49.9) | 1633 | 36.5 (29.7, 43.8) |
| Hypercholesterolemia | 1768 | 25.4 (22.9, 28.1) | 4475 | 27.3 (26.0, 28.7) |
| Undiagnosedc | 359 | 37.2 (31.3, 43.5) | 1329 | 40.5 (35.1, 46.3) |
| Controlledc | 359 | 29.6 (25.1, 34.4) | 1329 | 27.9 (24.6, 31.6) |
| Diabetes | 1336 | 12.5 (10.3, 15.1 | 1977 | 10.4 (8.7, 12.3) |
| Undiagnosedd | 131 | 27.9 (19.2, 38.6 | 254 | 29.4 (18.6, 43.3) |
| Controlledd | 130 | 54.6 (42.5, 66.1) | 245 | 55.6 (42.2, 68.2) |
| Weight | ||||
| Underweight (BMI < 18.5 kg/m2) | 1915 | 1.6 (1.2, 2.2) | 4227 | 1.7 (1.4, 2.1) |
| Normal weight (BMI 18.5–24.9 kg/m2) | 1915 | 36.6 (33.6, 39.7) | 4227 | 32.5a (30.0, 35.0) |
| Overweight (BMI 25.0–29.9 kg/m2) | 1915 | 36.1 (33.4, 38.9) | 4227 | 34.3 (32.1, 36.6) |
| Obese (BMI > 30 kg/m2) | 1915 | 25.6 (23.2, 28.3) | 4227 | 31.5a (29.0, 34.2) |
| Infectious diseases | ||||
| HSV-2 | ||||
| Aged ≥ 20 years | 1780 | 27.9 (24.8, 31.2) | NAe | NAe |
| Aged 20–49 years | 1281 | 23.6 (20.4, 27.1) | 2227 | 19.2a (17.1, 21.5) |
| HCV | 1786 | 2.3 (1.5, 3.4) | 4460 | 1.9 (1.3, 2.7) |
| Environmental exposures | ||||
| Cotinine (≥ 0.050 ng/mL, nonsmokersf) | 1330 | 56.7 (53.6, 59.7) | 3285 | 44.9a (38.1, 51.8) |
| Leadg | 1811 | 1.79 (1.73, 1.86) | 4525 | 1.52 (1.45, 1.60) |
| Mercuryg | 1811 | 2.73 (2.58, 2.89) | 4525 | 0.98a (0.86, 1.11) |
| Cadmiumg | 1811 | 0.77 (0.75, 0.80) | 4525 | 0.38a (0.36, 0.40) |
Note. NYC-HANES = New York City Health and Nutrition Examination Survey; NHANES = National Health and Nutrition Examination Survey; CI = confidence interval; BMI = body mass index; HSV-2 = herpes simplex virus 2; HCV = hepatitis C virus. All prevalence estimates were age-adjusted to the 2000 US standard population.
Significantly different from NYC-HANES estimate (P < .05).
Among individuals with examination-based hypertension.
Among individuals with examination-based hypercholesterolemia.
Among individuals with examination-based diabetes.
Not available; tested only for participants aged 14 to 49 years.
Nonsmokers defined as participants with serum cotinine concentrations at or below 10 ng/mL.
Values presented as geometric means, not percentages.
Similar to hypertension, 25.4% of adults had hypercholesterolemia. However, a higher proportion of adults with hypercholesterolemia were undiagnosed (37.2%), and fewer had control of their condition (29.6%). Of those who were aware of their hypercholesterolemia, more than half (59.0%; 95% CI = 51.5%, 66.1%) did not have their condition under control.
Approximately one quarter (25.6%) of NYC adults were obese; 61.8% were either overweight or obese. Non-Hispanic Blacks and Hispanics were more likely to be obese than were non-Hispanic Whites (OR = 1.7; 95% CI = 1.2, 2.4; and OR = 1.6; 95% CI = 1.2, 2.2, respectively), and Asians were less likely to be obese than were non-Hispanic Whites (OR = 0.3; 95% CI = 0.2, 0.5). One in 8 NYC adults (12.5%) had diabetes; 54.6% had well-controlled glycosolated hemoglobin levels. More than one quarter of adults with diabetes (27.9%) were undiagnosed at the time of the survey.
Examination-based estimates for chronic conditions from NYC-HANES were compared with those from NHANES (Table 2). NYC adults had lower levels of hypertension (P < .01) and obesity (P < .01) than did US adults; we found no significant differences in the prevalence of diabetes or hypercholesterolemia. In general, we observed similar race/ethnicity disease patterns in NYC and the United States as a whole (data not shown). However, NYC non-Hispanic Blacks had lower hypertension and obesity rates than did their US counterparts: 32.8% of NYC non-Hispanic Blacks had hypertension compared with 40.4% nationally (P < .01), and 32.4% of NYC non-Hispanic Blacks were obese compared with 43.9% nationally (P < .001). NYC and US non-Hispanic Blacks had similar diabetes and hypercholesterolemia rates (data not shown). Although higher rates of hypertension control were found in NYC adults compared with the national average, this difference was not statistically significant. Control of diabetes and hypercholesterolemia was similar between NYC and the United States as a whole.
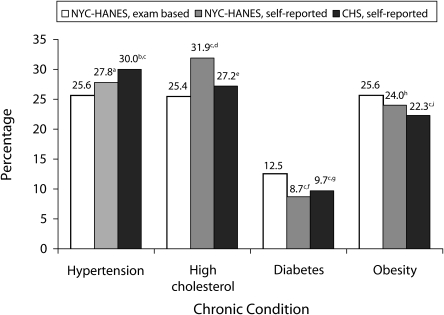
We compared self-reported chronic disease estimates reported in NYC-HANES and CHS (Figure 1). NYC adults interviewed anonymously via telephone were similarly likely to report ever being told they had high blood pressure compared with NYC-HANES face-to-face interviews (30.0 vs 27.8%). Self-reported estimates were lower in CHS than NYC-HANES for elevated cholesterol (27.2 vs 31.9%; P < .05), whereas self-reported obesity estimates were not significantly different (22.3 vs 24.0%; P = .22). Self-reported diabetes was similar between the 2 surveys (9.7% in CHS vs 8.7% in NYC-HANES; P = .39).
FIGURE 1.
Comparison of 2004 NYC-HANES self-reported and examination-based estimates to CHS self-reported 2002, 2004, and 2005 estimates for selected chronic conditions: 2004.
Note. NYC-HANES = New York City Health and Nutrition Examination Survey; CHS = Community Health Survey. All estimates are age-adjusted to the 2000 US Standard population.
aBased on the question, “Have you ever been told by a doctor or other health professional that you had hypertension, also called high blood pressure?”
bBased on the question, “Have you ever been told by a doctor, nurse, or other health professional that you have high blood pressure?” (CHS 2005).
cEstimate was significantly different from NYC-HANES examination-based estimate (P < .05).
dBased on the question, “Have you ever been told by a doctor or other health professional that your blood cholesterol level was high?”
eBased on the question, “Have you ever been told by a doctor or health professional that your blood cholesterol is high?” (CHS 2002).
fBased on the question, “[other than pregnancy], have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?”
gBased on the question, “Have you ever been told by a doctor that you have diabetes?” A woman with a “yes” response was asked, “Was this only when you were pregnant?”
hBased on the questions, “How tall are you without shoes?” and “How much do you weigh without clothes or shoes? [If you are currently pregnant, how much did you weigh before your pregnancy?]”
iBased on the questions, “About how tall are you without shoes?” and “About how much do you weigh without shoes?” If resondent was missing weight but not missing height, respondent was asked, “Do you weigh more than [critical weight for obese]?” and “Do you weigh less than [critical weight for overweight]?” If respondent was missing height but not missing weight, respondent was asked, “Is your height less than [critical height for obese]?” and “Is your height less than [critical height for overweight]?”
Comparing NYC-HANES examination-verified chronic disease estimates to self-reported CHS estimates, we found that CHS participants were significantly more likely to have been told that they had high blood pressure compared with the examination-diagnosed measures of hypertension (P < .001; Figure 1). Additionally, CHS self-reported obesity estimates were significantly lower than the NYC-HANES examination-based estimates (P < .05). The examination-diagnosed diabetes estimate from NYC-HANES was significantly higher than CHS self-reported diabetes (P < .05), reflecting the detection of undiagnosed diabetes (3.8%; 95% CI = 2.6%, 5.4%) in NYC-HANES.
Infectious Diseases
We assessed the prevalence of selected infectious diseases from NYC-HANES (Table 2). The overall prevalence of HSV-2 among all NYC adults was 27.9% (95% CI = 24.8%, 31.2%). Among adults aged 20 to 49 years, the prevalence of HSV-2 among non-Hispanic Blacks was more than 3 times the prevalence among non-Hispanic Whites (40.8 vs 12.5%; P < .001), and women had twice the prevalence of men (31.2 vs 14.4%; P < .001). Of those with HSV-2, most (89.4%) reported never being told that they had herpes. The prevalence of HCV infection among NYC adults was 2.3% (95% CI = 1.5, 3.4); non-Hispanic Blacks had the highest prevalence (4.3%; 95% CI = 2.4%, 7.5%).
Comparing NYC-HANES infectious disease findings to those from NHANES, we found that HSV-2 prevalence among adults aged 20 to 49 years was significantly higher in NYC than nationally (23.6 vs 19.2%; P < .05). Similar racial/ethnic patterns exist in the US and NYC, where non-Hispanic Blacks had higher HSV-2 rates than did non-Hispanic Whites; estimates were not significantly different between NYC and US non-Hispanic Blacks (40.8% vs 47.3%). The prevalence of HCV in NYC was similar to the national estimate.
Environmental Exposures
NYC-HANES estimates of environmental exposure are shown in Table 2. More than half of nonsmokers (56.7%) had cotinine concentrations at 0.050 ng/mL or greater; the cotinine concentrations geometric mean was 0.082 ng/mL. For blood metals, 25.0% of adults had mercury concentrations at 5 μg/L or greater; the geometric mean for mercury concentrations was 2.73 μg/L. Only 8 adults (0.5%; RSE > 30) had lead concentrations above 10 μg/dL. The geometric mean for lead concentrations was 0.79 μg/dL. No adults had cadmium concentrations at 10 μg/L or greater, and the geometric mean was 0.77 μg/L.
Comparing NYC-HANES to NHANES, adult nonsmokers in New York City were significantly more likely to have cotinine concentrations at 0.050 ng/mL or greater than were their US counterparts (56.7 vs 44.9%; P < .001). Mercury concentrations in NYC adults were almost 3 times that of US adults (2.73 μg/L vs 0.98 μg/L; P < .001); cadmium concentrations were twice as high, and lead concentrations were significantly higher than those of US adults (1.79 μg/dL vs 1.52 μg/dL; P < .001).
DISCUSSION
Overview
NYC-HANES provided new information on the health status of NYC adults using the same standardized examination measures employed in NHANES, the nation's most comprehensive examination-based survey. Compared with the US population, NYC adults had comparable levels of diabetes and hypercholesterolemia but lower levels of obesity and hypertension. These data also suggest that compared with US averages, local levels of control may be similar or higher for hypertension and similar for diabetes and hypercholesterolemia. New York City adults had a higher prevalence of HCV and HSV-2 and were exposed to higher concentrations of tobacco smoke and certain metals, particularly mercury, than were their US counterparts. Comparisons of measured and self-reported estimates suggest that self-reported levels of hypertension may be overestimated, whereas self-reported levels of obesity are underestimated.
Chronic Conditions
New York City is one of the largest, most diverse cities in the United States, with a larger percentage of non-Hispanic Blacks and Hispanics and persons living below the poverty level than national averages.24 Nationally, non-Hispanic Blacks and adults in the lower socioeconomic strata have higher rates of hypertension, cholesterol, diabetes, and obesity than either non-Hispanic Whites or adults in the higher socioeconomic strata.10,25–28 In NYC, we also found higher disease prevalence in these groups, each of which are overrepresented in the population; however, we found lower rates of obesity and hypertension among NYC non-Hispanic Blacks compared with US non-Hispanic Blacks, contributing to lower overall prevalence levels in NYC. Findings specific to diabetes and hypercholesterolemia suggest that race-specific rates in NYC are similar to national averages.
The lower rates of hypertension and obesity in NYC are consistent with previous findings that urban non-Hispanic Whites and non-Hispanic Blacks have a lower prevalence of these conditions compared with their rural counterparts.29 Factors influencing disease patterns in NYC and potentially other urban areas may include a higher proportion of immigrants and a greater reliance on public transportation. In NYC, nearly 40% of the adult population is foreign born and 54% of households do not own a car.23,30 Immigrants tend to be less obese and in better health than US-born persons, which may affect chronic diseases rates in NYC.31 Additionally, the lower car-usage rate may result in a more physically active population potentially leading to lower chronic disease rates. This is supported by research linking urban land use to lower obesity rates.32,33 Differing disease patterns in NYC demonstrate the need for local surveys.
Although control levels for hypertension, diabetes, and hypercholesterolemia in NYC were either similar or better than US estimates, a high proportion of NYC adults had chronic conditions that were inadequately controlled. Lack of detection only partially accounts for the levels of inadequate control of chronic disease we observed in NYC adults. Information from NYC-HANES is being used to target interventions to reduce complications, improve quality of life, and reduce premature mortality.34 The effectiveness of such interventions can be assessed through similar follow-up examination surveys.
When self-reported chronic disease estimates were compared with measured estimates, certain discrepancies were found. Hypertension, and to a lesser extent hypercholesterolemia, appear to be overestimated by self-reported estimates of high blood pressure or cholesterol. Participants reporting that they have ever been told they had high blood pressure or high cholesterol may have a history of 1 or more elevated measurements that may not meet the clinical definition for these conditions. Alternatively, there may be a subset of adults who control their condition through lifestyle modifications, such as exercise and healthy eating, and are not captured in the examination-based estimate. Additionally, as demonstrated nationally, a high proportion of diabetes is undiagnosed; self-reported diabetes estimates fail to accurately judge the true disease burden.25 Identifying the extent of underdiagnosis and underreporting is necessary to reduce the burden of chronic disease locally.
Infectious Diseases and Environmental Exposures
Compared with the US population, we found that NYC adults had a higher HSV-2 prevalence, largely influenced by the high rates among non-Hispanic Blacks, who make up a large proportion of the NYC population. Similar to national findings,17 NYC non-Hispanic Blacks tended to have a higher HCV prevalence than did non-Hispanic Whites; however, differences were not statistically significant and the sample size limited more-detailed assessments. Low prevalence diseases like HCV are difficult to characterize in community-level examination surveys in which complexity and cost may result in small samples.
A disturbing finding was the higher exposures to tobacco smoke (among nonsmokers) and certain metals in NYC compared with national levels. Higher NYC levels may in part result from exposure disparities, because racial minorities and those in the lower socioeconomic strata have been shown to have higher concentrations of many environmental contaminants.35 Recent NYC-HANES findings demonstrate that Asians, specifically Chinese-born adults, have increased concentrations of heavy metals.36 The elevated exposure to cotinine, mercury, and cadmium may also reflect other influences of NYC's densely populated urban environment; few studies to date have assessed the effect of urban environments on these exposures. Although the clinical significance of elevated cadmium concentrations is not clear, research has shown health effects from mercury exposure in adults. Specifically, low-level mercury exposure in adults has been demonstrated to cause visual and motor disturbances and to cross the placenta in pregnant women, potentially causing neurodevelopmental problems in children.37–40 The finding that 1.4 million adults in NYC experience mercury concentrations above the New York State reportable concentration underscores the importance of understanding community-level environmental exposure.
Strengths and Limitations
Although examination surveys provide objective indicators of health conditions, telephone surveys are less labor intensive, less expensive, are readily modified for content and sampling strategies (e.g., oversampling), and can be conducted in a timely and routine manner. As such, telephone surveys like CHS are integral for monitoring health trends at the national, regional, and local levels. The advantages of telephone surveys are not overshadowed by their biases (e.g., declining representativeness from decreased response rates, typical noninclusion of cell phone–only households). Telephone and examination surveys can serve as complementary surveillance tools. Reporting biases identified from examination surveys can be applied to routinely measured self-reported estimates to more accurately describe local epidemiology. Ezzati et al.41 demonstrated the usefulness of such an approach by using the relationship between age- and gender-specific estimates of self-reported and measured height and weight to correct annual telephone-based state-level body mass index and obesity measures.
There are limitations in the interpretation of these data. NYC-HANES achieved an overall response rate of 55%, which might have resulted in selection bias. For example, more-affluent adults or persons may have been less receptive to survey recruitment incentives. Alternatively, it is not clear whether persons with health issues such as hypertension or obesity were more or less likely to participate. Survey weights were adjusted to account for factors influencing nonresponse, such as age, borough of residence, and group-level characteristics from the US Census such as the percentage of the population that is non-Hispanic Black, average household size, and median household income. Estimates may also be affected by measurement error; however, standardized quality-assurance procedures helped minimize error. Also, small sample sizes may have limited our ability to detect significant differences between surveys and among demographic variables.
Methodological differences between the surveys may have also affected estimates. Unlike NYC-HANES, NHANES oversamples Mexican Americans, limiting direct comparisons between NYC Hispanics and US Hispanics. Additionally, CHS sample weights were created using population estimates from the 2000 US Census, whereas NYC-HANES utilized the 2004 American Community Survey. Differences in survey estimates may result from these differing methodologies. For example, CHS had a smaller proportion of persons with less than a high school education; because lower educational status is related to many health outcomes, some CHS health estimates may be underestimated. This underestimation is likely most relevant for the comparisons of obesity estimates, where we found that the self-reported CHS estimate is significantly lower than the examination-based NYC-HANES estimate. Standardization to the educational distribution from NYC-HANES resulted in a small increase in the CHS obesity estimate (from 22.3% to 22.7%).
Conclusions
NYC-HANES provides objectively measured estimates of major chronic conditions including hypertension, obesity, hypercholesterolemia, and diabetes. Using standardized examination-based measures, we observed key differences in disease prevalence between NYC and the United States as a whole. Comparisons to self-reported data clarify the extent of under- or overestimation of major chronic health conditions in routine surveillance sources. Examination surveys provide objective health measurement, and telephone surveys provide routine and timely information. Together they provide a comprehensive picture of local public health that can be compared with national estimates. The surveys also provide important baseline information for promoting local health policies that aim to reduce the burden of disease. Continued local- and national-level surveillance using various methodologies is necessary for targeting interventions and monitoring trends.
Acknowledgments
NYC-HANES was partially funded by the National Center for Environmental Health of the Centers for Disease Control and Prevention (grants U50CCJU222455, U50CCU223290, and U59CCU22339202).
We thank Mary Hunyh, Jennifer Norton, Chitra Ramaswamy, and Leena Gupta for their assistance in the analysis of the Community Health Survey data. Additionally we thank Donna Eisenhower and Kevin Konty for their insightful comments. Finally, we thank all the staff from the New York City Department of Health and Mental Hygiene who assisted in the implementation and data collection phases of the survey.
Human Participant Protection
All NYC-HANES protocols and informed consent procedures were reviewed and approved by the New York City Department of Health institutional review board.
References
- 1.National Health and Nutrition Examination Survey, 2005–2006: Overview. Hyattsville, MD: National Center for Health Statistics; [Google Scholar]
- 2.National Center for Health Statistics Programs and Activities. Hyattsville, MD: National Center for Health Statistics; June 2002. DHHS Publication PHS 2002-1200 [Google Scholar]
- 3.Fielding JE, Frieden TR. Local knowledge to enable local action. Am J Prev Med 2004;27(2):183–184 [DOI] [PubMed] [Google Scholar]
- 4. New York City Department of Health and Mental Hygiene. Community health survey 2004 methods. Available at: http://www.nyc.gov/html/doh/html/survey/survey-2004.shtml. Accessed April 4, 2007.
- 5.Mostashari F, Kerker BD, Hajat A, Miller N, Frieden TR. Smoking practices in New York City: the use of a population-based survey to guide policy-making and programming. J Urban Health 2005;82:58–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Center for Health Statistics National Health and Nutrition Examination Survey: survey questionnaires, examination components and laboratory components 1999–2000. Available at: http://www.cdc.gov/nchs/about/major/nhanes/questexam.htm. Last reviewed January 28, 2008. Accessed April 19, 2007
- 7.National Center for Health Statistics NHANES lab methods 2003–2004. Available at: http://www.cdc.gov/nchs/about/major/nhanes/nhanes2003-2004/lab_methods_03_04.htm. Accessed April 7, 2007
- 8.Thorpe LE, Gwynn RC, Mandel-Ricci J, et al. Study design and participation rates of the New York City Health and Nutrition Examination Survey, 2004. Prev Chronic Dis 2006;3(3):A94. [PMC free article] [PubMed] [Google Scholar]
- 9.National Center for Health Statistics Analytic and reporting guidelines: the National Health and Nutrition Examination Survey (NHANES). Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf. Accessed April 19, 2007
- 10.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006;295(13):1549–1555 [DOI] [PubMed] [Google Scholar]
- 11.Ford ES, Mokdad AH, Giles WH, Mensah GA. Serum total cholesterol concentrations and awareness, treatment, and control of hypercholesterolemia among US adults: findings from the National Health and Nutrition Examination Survey, 1999 to 2000. Circulation 2003;107:2185–2189 [DOI] [PubMed] [Google Scholar]
- 12.Carroll MD, Lacher DA, Sorlie PD, et al. Trends in serum lipids and lipoproteins of adults, 1960–2002. JAMA 2005;294(14):1773–1781 [DOI] [PubMed] [Google Scholar]
- 13.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in US adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 1998;21:518–524 [DOI] [PubMed] [Google Scholar]
- 14.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes 2004. JAMA 2004;291(3):335–342 [DOI] [PubMed] [Google Scholar]
- 15.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA 2002;288(14):1723–1727 [DOI] [PubMed] [Google Scholar]
- 16.Lee FK, Coleman RM, Pereira L, Bailey PD, Tatsuno M, Nahmias AJ. Detection of herpes simplex virus type 2-specific antibody with glycoprotein G. J Clin Microbiol 1985;22(4):641–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med 1999;341(8):556–562 [DOI] [PubMed] [Google Scholar]
- 18.Bernert JT, Jr, Turner WE, Pirkle JL, et al. Development and validation of sensitive method for determination of serum cotinine in smokers and nonsmokers by liquid chromatography/atmospheric pressure ionization tandem mass spectrometry. Clin Chem 1997;43:2281–2291 [PubMed] [Google Scholar]
- 19.Palmer C, Lewis M, Jr, Geraghty C, Barbosa F, Jr, Parsons P. Determination of lead, cadmium and mercury in blood for assessment of environmental exposure: a comparison between inductively coupled plasma-mass spectrometry and atomic absorption spectrometry. Spectrochim Acta B At Spectrosc 2006;61:980–990 [Google Scholar]
- 20.Mohadjer L, Montaquila J, Waksberg J. National Health and Nutrition Examination Survey III: Weighting and Examination Methodology. Hyattsville, MD: National Center for Health Statistics; 1996 [Google Scholar]
- 21.Klein RJ, Schoenborn CA. Age Adjustment Using the 2000 Projected US Population. Hyattsville, MD: National Center for Health Statistics; 2001. Healthy People 2010 Statistical Notes, No. 20. Available at: http://www.cdc.gov/nchs/data/statnt/statnt20.pdf. Accessed October 1, 2006 [PubMed] [Google Scholar]
- 22.Daniel WW. Biostatistics: A Foundation for Analysis in the Health Sciences. 6th ed.New York, NY: John Wiley and Sons; 1995 [Google Scholar]
- 23.US Census Bureau. American FactFinder. Census 2000 Summary File 3. Available at: http://factfinder.census.gov. Accessed on April 8, 2006.
- 24.US Census Bureau. American FactFinder. Census 2000 Summary File 1. Available at: http://factfinder.census.gov. Accessed on April 8, 2006.
- 25.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the US population: National Health And Nutrition Examination Survey, 1999–2002. Diabetes Care 2006;29:1263–1268 [DOI] [PubMed] [Google Scholar]
- 26.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA 2003;290(2):199–206 [DOI] [PubMed] [Google Scholar]
- 27.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension 2007;49:69–75 [DOI] [PubMed] [Google Scholar]
- 28.Sharma S, Malarcher AM, Giles WH, Myers G. Racial, ethnic and socioeconomic disparities in the clustering of cardiovascular disease risk factors. Ethn Dis 2004;14(1):43–48 [PubMed] [Google Scholar]
- 29.Mainous AG, III, King DE, Garr DR, Pearson WS. Race, rural residence, and control of diabetes and hypertension. Ann Fam Med 2004;2(6):563–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Census Bureau. Table B08201. Household Size by Vehicles Available–Universe: Households. 2004 American Community Survey. Available at: http://factfinder.census.gov. Accessed April 8, 2006.
- 31.Dey AN, Lucas JW. Physical and Mental Health Characteristics of US- and Foreign-Born Adults: United States, 1998–2003. Hyattsville, MD: National Center for Health Statistics; 2006. Advance Data from Vital and Health Statistics, 369 [PubMed] [Google Scholar]
- 32.Rundle A, Roux AV, Free LM, Miller D, Neckerman KM, Weiss CC. The urban built environment and obesity in New York City: a multilevel analysis. Am J Health Promot 2007;21(suppl 4):326–334 [DOI] [PubMed] [Google Scholar]
- 33.Frank LD, Andresen MA, Schmid TL. Obesity relationships with community design, physical activity, and time spent in cars. Am J Prev Med 2004;27(2):87–96 [DOI] [PubMed] [Google Scholar]
- 34. New York Department of Health and Mental Hygiene. Take Care New York. Available at: http://www.nyc.gov/html/doh/html/tcny/index.shtml. Accessed April 4, 2007.
- 35.Centers for Disease Control and Prevention Third National Report on Human Exposure to Environmental Chemicals. Atlanta, GA: Centers for Disease Control and Prevention; 2006. Available at: http://www.cdc.gov/exposurereport/report.htm. Accessed October 1, 2006 [Google Scholar]
- 36.McKelvey W, Gwynn RC, Jeffery N, et al. A biomonitoring study of lead, cadmium, and mercury in the blood of New York City adults. Environ Health Perspect 2007;115:1435–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ask K, Akesson A, Berglund M, Vahter M. Inorganic mercury and methylmercury in placentas of Swedish women. Environ Health Perspect 2002;110:523–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lebel J, Mergler D, Lucotte M, et al. Evidence of early nervous system dysfunction in Amazonian populations exposed to low-levels of methylmercury. Neurotoxicology 1996;17(1):157–167 [PubMed] [Google Scholar]
- 39.Lebel J, Mergler D, Branches F, et al. Neurotoxic effects of low-level methylmercury contamination in the Amazonian Basin. Environ Res 1998;79:20–32 [DOI] [PubMed] [Google Scholar]
- 40.Vahter M, Akesson A, Lind B, Bjors U, Schutz A, Berglund M. Longitudinal study of methylmercury and inorganic mercury in blood and urine of pregnant and lactating women, as well as in umbilical cord blood. Environ Res 2000;84:186–194 [DOI] [PubMed] [Google Scholar]
- 41.Ezzati M, Martin H, Skjold S, Vander HS, Murray CJ. Trends in national and state-level obesity in the USA after correction for self-report bias: analysis of health surveys. J R Soc Med 2006;99:250–257 [DOI] [PMC free article] [PubMed] [Google Scholar]