Abstract
There is a widespread and growing concern that patents hinder access to life-saving drugs in developing countries.
Recent student movements and legislative initiatives emphasize the potential role that research universities in developed countries could have in ameliorating this “access gap.” These efforts are based on the assumption that universities own patents on a substantial number of drugs and that patents on these drugs are currently filed in developing countries.
I provide empirical evidence regarding these issues and explore the feasibility and desirability of proposals to change university patenting and licensing practices to promote access to medicines in the developing world.
THE PHARMACEUTICAL REVOlution contributed to dramatic reductions in morbidity and mortality from disease in developed countries during the last century. Today, however, as many as 2 billion people in the world—most of them in developing countries—lack access to life-saving drugs.1 Righting this imbalance is among the most important challenges in global public health in this century. One source of the access gap in developing countries is a lack of research on specific diseases within developing countries. Both the public and private sectors devote relatively little research to diseases without markets in developed countries. As a result, relatively few new drugs target diseases specific to developing countries.2 Analysts have also argued that poor health infrastructure, cumbersome drug regulatory procedures, and high tariffs and taxes in developing countries are important obstacles to access.3 A third potential obstacle—my focus—is pharmaceutical patenting in developing countries, which (by restricting generic competition) can raise the prices of drugs and thus hinder access to medicines.
One proposed solution to this last problem targets a perhaps surprising set of actors: research universities and public sector research institutes in developed countries. One of the main advocates of this approach, Universities Allied for Essential Medicines, a student group with over 40 campus chapters (with the slogan “Our Labs, Our Drugs, Our Responsibility”) argues on its Web site:
Many of the world's most important medicines and public health devices are wholly or partly developed in academic laboratories. Their accessibility to those living in poor nations is profoundly affected by the research, licensing and patenting decisions made by universities… . As members of these institutions of higher learning, we believe that universities have an opportunity and a responsibility to improve global access to public health goods—particularly those they have helped develop.4
I explore the feasibility and desirability of proposals to use the power of universities—conferred by ownership of key patents—to help reduce drug prices and promote access in developing countries. I provide and discuss new data, university ownership of key patents, and their propensity to file these patents in developing countries. However, before doing so, it is useful to reflect on the broader institutional and historical context for the current proposals. Drug patents allow their owners to exclude others from using or producing the drug until patent expiration (typically 20 years from the date the patent is filed). By excluding generic competition, patents keep prices high. The typical justification for patent protection is that these temporary high prices are needed to create incentives for firms to invest in research and development. In other words, patents involve tradeoffs: although they create incentives to innovate, they can raise prices and reduce access.
Until the mid-1990s, many developing countries did not allow product patents in pharmaceuticals.5 This generally reflected a conscious policy decision that the benefits from low-cost access to drugs were greater than any potential negative impact that lack of domestic patents would have on the research and development decisions of multinational companies. However, following the World Trade Organization's 1995 Trade-Related Intellectual Property Rights (TRIPs) agreement, all countries were compelled to allow product patents in pharmaceuticals. In the post-TRIPs era, there is widespread concern that, by raising prices, drug patents will reduce access to medicines in developing countries.6
University patenting, too, is a relatively recent development. Throughout much of the 20th century, research universities did not file patents in the biomedical arena, reflecting an ambivalence about limiting access to health research and discoveries.7 This ambivalence faded during the 1970s, and the Bayh–Dole Act of 1980 both removed bureaucratic obstacles to patenting publicly funded research and gave congressional endorsement to the notion that academic patenting and licensing facilitated commercialization of university discoveries. The logic of the Bayh–Dole Act was that, without patents on academic discoveries—often “embryonic” in form and requiring additional development, including clinical trials—firms would lack incentives to develop them to the point where they were commercially useful. Under this theory, patents on academic research—which are then licensed to firms that develop and market the academic technologies—would promote “technology transfer.”8 In the decades following Bayh–Dole, academic patenting and licensing grew dramatically, with the bulk of this growth concentrated in the biomedical arena.9 Academic institutions collect income on licensed patents, including sales-based royalties on products commercialized based on their patents. In the most recent year for which data are available, academic licensing income exceeded 1 billion US dollars.10
These activities have been surrounded by controversies, including debate about whether academic patents in fact are necessary for new product development; whether the presence of patent incentives distorts academic research agendas away from “basic” and toward “applied” research; whether they create conflict of interest in clinical research; and whether academic patents on “research tools” can hinder the progress of scientific research.11,12
The proposals just noted attempt to harness an unintended benefit from academic patenting of biomedical discoveries. By giving universities ownership rights over upstream discoveries, academic patents can give universities the power to compel licensees to not enforce these patents, or any follow-on patents, in developing countries, thus helping to promote access. This movement began in 2001, when, in response to demands from student and health activists, Yale University, the owner of the key patent on an important HIV treatment (stavudine), pressured Bristol-Myers Squibb, the licensee of this patent, to agree not to enforce the patent in South Africa.13 This intervention reportedly led to a 30-fold reduction in the drug's price and a dramatic expansion of HIV treatment programs in South Africa.14
These developments were catalysts for the formation of Universities Allied for Essential Medicines, the campus chapters of which aim to persuade their parent universities to develop patent licensing policies that limit the ability of licensees to enforce academic patents (or related patents held by firms) in developing countries. These proposed licensing terms are generally modeled on the equitable access license developed by legal scholars.15 The movement also led to the introduction of legislation in the US Senate: S. 4040, The Public Research in the Public Interest Act, sponsored by Senator Patrick Leahy (D, VT), which requires that, as a condition for receipt of federal funds, universities include in their licensing agreements clauses limiting the licensees' abilities to enforce academic patents—and the licensees' own patents on drugs with academic patents—against developing-country generic producers. Similar proposals have been endorsed by a range of international bodies, including the Association of American Medical Colleges, the World Health Organization, and the American Association of Arts and Sciences.16
Although this movement is intensifying, there is little empirical information on how large an impact such a strategy would have. Is the Yale case unique, or do academic institutions have ownership rights in a large number of drugs, making this strategy more generally feasible? In addition to citing specific cases in which universities owned key patents, the proposals discussed above are motivated by research showing that academic institutions play an important role in pharmaceutical innovation, drawing on bibliometric data,17 case studies,18,19 and survey evidence.20 However, this previous research on the academic role in pharmaceutical innovation does not explicitly examine the extent to which academic institutions hold patents on the drugs, which is the relevant consideration for proposals to use university ownership of patents to attempt to affect prices and access. Academic research can affect industrial innovation through a range of channels: firms benefit from knowledge obtained through published academic articles and conference presentations, through collaborations with academic scientists, and through hiring trained graduate students. These channels of knowledge and technology transfer are generally not accompanied by patents held by universities. Accordingly, even if universities did significantly contribute to pharmaceutical innovation through these channels, because there are no patents, academic institutions can have little control over the pricing or dissemination of resulting drugs. That is, the broad research on the academic influence on drug development is not directly relevant for considerations of whether universities can help affect access; the salient consideration is whether universities hold patents on their contributions.
For proposals to use university ownership of drug patents to affect drug prices (and access) in developing countries to be feasible, two things would need to be true for such proposals to be reasonable. First, universities would have to own patents on a substantial number of drugs, and second, universities or firms licensing university technologies would have to currently be filing patents in developing countries. If the first statement were false, the proposed interventions would have little effect. If the second were false, the interventions would not be needed. I provide data on these issues.
METHODS
To examine these issues, I began by collecting information on all drugs approved by the US Food and Drug Administration (FDA) between 1988 and 2005 from the FDA's online database,21 which contains data on all FDA-approved drugs, including drug name and ingredient. I focused attention on the 1546 new drug applications approved between 1988 and 2005. “New” drug approvals include not just new molecular entities but also new derivatives of existing molecules, new formulations, new combinations of already approved compounds, and new indications, among other types of drugs. Unfortunately, by focusing on new drug applications, I did exclude numerous biotechnology drugs, which occasionally are filed as biological license agreements rather than new drug applications and thus are not subject to the requirements to list patents in the Orange Book.22 Because the public sector role could be more pronounced for biotechnology drugs, this is a limitation of the current sample. I hope to explore this in future research with other sources of drug patent data.
To facilitate interpretation, I classified these new drug applications by approval year cohorts: 1988 to 1993, 1994 to 1999, and 2000 to 2005. To examine potential differential roles of public sector patents across different types of drugs, I analyzed new molecular entity and other new drug approvals separately. I also determined whether each of the drugs was given “priority review” by the FDA, which is granted when it is “A drug that appears to represent an advance over available therapy.”23 Some have argued that new molecular entities that receive priority review represent higher levels of innovativeness than do other drugs,24 although this has been disputed by others.25 Given the particular importance of HIV/AIDS drugs in the policy debates discussed above and the particular burden of this disease in developing countries,26 I also specifically identified those new drug applications designated by the FDA as “drugs used in the treatment of HIV infections.”27
Information on patents on FDA-approved drugs was collected from the February 2007 edition of the FDA's Orange Book.22 Because the current Orange Book lists only unexpired patents, I supplemented this with legacy data obtained from older editions, dating back to 1988. I collected information on who owned each of these patents from the United States Patent and Trademark Office's Cassis database of bibliographic information from US patents,28 and determined which of these owners were academic institutions, nonprofit research institutes, government laboratories, and hospitals using the Azoulay–Michigan–Sampat concordance.29 I refer to these as “academic” patents in the analyses that follow.
I also examined which of the patents in the Orange Book were also filed in developing countries with data from the Derwent Innovation Index.30 The Derwent database contains information on patent applications in 95 countries. Although the Derwent data may be somewhat noisy, especially for developing countries, it is generally considered the most comprehensive source of data on international patent protection. However, because this data set may miss potential filings, the statistics on international patent filings should be interpreted as lower bounds. I used World Bank classifications based on per capita gross domestic product levels31 to classify 27 of these as low income or lower-middle income (India, Kenya, North Korea, Mongolia, Malawi, Nigeria, Vietnam, Zambia, Zimbabwe, Bulgaria, Brazil, China, Colombia, Cuba, Egypt, Indonesia, Iraq, Iran, Jordan, Sri Lanka, Morocco, Moldova, Peru, Philippines, Thailand, Tunisia, and Ukranian Republic).
RESULTS
Overall Trends
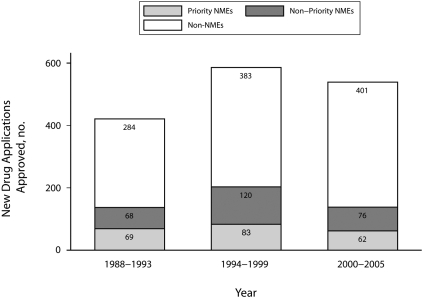
Figure 1 shows the number of new drug approvals over time, distinguishing between new molecular entities that received priority review, new molecular entities that did not receive priority review, and new drug applications that were not new molecular entities. Consistent with previous research,32 the majority of new drug approvals were not new molecular entities. In addition, the share of approvals that were new molecular entitys that received priority review—arguably the most “innovative” drugs—has been decreasing over time, from 16.3% in the 1988 to 1993 cohort, to 14.2% in 1994 to 1999 and to 11.5% in 2000 in 2005.
FIGURE 1.
Approvals over time of priority new molecular entity (NME) drugs, nonpriority NME drugs, and non-NME drugs: 1988–2005.
Drugs With Academic Patents
Overall, 938 (60.7%) of the new drug applications had at least 1 patent. New molecular entities were significantly more likely to have patents than were other new drug applications (79.5% vs 52.3%; P < .01). A drug can be associated with multiple patents, and a patent can cover multiple drugs. In total, the new drug applications in the sample were associated with 1947 patents. Of these, 96 (4.9%) were academic patents.
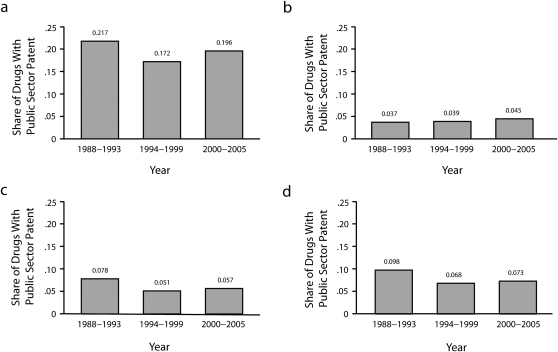
At the new drug application level, 72 (7.7%) of drugs approved over this period had an academic patent. However, new molecular entities were significantly more likely to have an academic patent than were other new drug applications, with 10.3% having at least 1 academic patent compared with 5.9% for non—new molecular entities (P < .001). Additionally, 19.2% of priority new molecular entities had at least 1 academic patent compared with 4.02% of nonpriority new molecular entities (P < .01). Figure 2 shows trends over time and by chemical type. Although no sharp trends over time stand out, note that, consistently throughout this period, about 1 in 5 of the new molecular entities receiving priority approval had an academic patent.
FIGURE 2.
Share of public sector patents, by year, going to (a) priority NME drugs, (b) nonpriority NME drugs, (c) non-NME drugs, and (d) all drugs: 1998–2005.
Note. NME = new molecular entity.
Table 1 lists the 72 drugs with an academic patent, with the new drug application number, drug name, chemical type, approval year, and the academic institution or hospital owning patents on the drug. Several points stand out. First, whereas in most cases, academic institutions held patents bearing on 1 drug application associated with a molecule (typically the new molecular entity), in some cases, the academic patents also extend to follow-on drug applications (as previously discussed, because my unit of analysis was an new drug application, the same trade name or ingredient can appear with multiple new drug applications in this Table 1 [e.g., for different forms or dosages]). For example, for the HIV drug, stavudine, Yale's patent is listed in the Orange Book for the original new molecular entity application (approved in 1994) but also for the subsequent approvals for the oral solution formulation (approved in 1996) and the extended-release formulation (approved in 2002).
TABLE 1.
Drugs Approved by the US Food and Drug Administration With Academic Patents: 1988–2005
| NDA No. | Tradename (Ingredient) | Approval Year | Public Sector Patent Holder |
| 20 212 | Zinecard (dexrazoxane hydrochloride)a | 1995 | New York University |
| 20 412 | Zerit (stavudine)a | 1994 | Yale University |
| 20 413 | Zerit (stavudine) | 1996 | Yale University |
| 21 453 | Zerit XR (stavudine) | 2002 | Yale University |
| 20 819 | Zemplar (paricalcitol)b | 1998 | University of Wisconsin |
| 21 606 | Zemplar (paricalcitol) | 2005 | University of Wisconsin |
| 21 636 | Zegerid (omeprazole; sodium bicarbonate) | 2004 | University of Missouri |
| 21 706 | Zegerid (omeprazole; sodium bicarbonate) | 2004 | University of Missouri |
| 20 597 | Xalatan (latanoprost)a | 1996 | Columbia University |
| 20 961 | Vitravene Preservative Free (fomivirsen sodium)a | 1998 | US Government, HHS |
| 20 569 | Vitrasert (ganciclovir) | 1996 | University of Kentucky |
| 21 119 | Visudyne (verteporfin)a | 2000 | Massachusetts General Hospital |
| 20 154 | Videx (didanosine)a | 1991 | US Government, HHS |
| 20 155 | Videx (didanosine) | 1991 | US Government, HHS |
| 20 156 | Videx (didanosine) | 1991 | US Government, HHS |
| 21 183 | Videx ec (didanosine) | 2000 | US Government, HHS |
| 21 267 | Vfend (voriconazole) | 2002 | University of Kansas |
| 21 602 | Velcade (bortezomib)a | 2003 | US Government, HHS |
| 19 981 | Ultratag (technetium TC-99M red blood cell kit) | 1991 | Associated Universities Inc. |
| 21 752 | Truvada (emtricitabine; tenofovir disoproxil fumarate) | 2004 | Emory University |
| 20 408 | Trusopt (dorzolamide hydrochloride)a | 1994 | University of Florida |
| 21 248 | Trisenox (arsenic trioxide)a | 2000 | Sloan-Kettering |
| 20 505 | Topamax (topiramate)b | 1996 | New England Medical Center |
| 20 844 | Topamax Sprinkle (topiramate) | 1998 | New England Medical Center |
| 20 898 | Thyrogen (thyrotropin alfa)a | 1998 | Sloan-Kettering |
| 20 785 | Thalomid (thalidomide)a | 1998 | Children's Hospital Boston |
| 20 262 | Taxol (paclitaxel)a | 1992 | US Government, HHS |
| 21 055 | Targretin (bexarotene)a | 1999 | SRI International |
| 19 836 | Supprelin (histrelin acetate)a | 1991 | Salk Institute |
| 19 890 | Stadol (butorphanol tartrate) | 1991 | University of Kentucky |
| 20 657 | Sporanox (itraconazole) | 1997 | US Government, HHS |
| 21 106 | Somavert (pegvisomant)a | 2003 | Ohio University |
| 19 608 | Sildaflo (silver sulfadiazine) | 1989 | Research Corporation |
| 21 544 | Seasonale (ethinyl estradiol; levonorgestrel) | 2003 | Medical College of Hampton Roads |
| 21 320 | Plenaxis (abarelix)a | 2003 | Indiana University |
| 20 958 | Pepcid Complete (calcium carbonate; famotidine; magnesium hydroxide) | 2000 | Brigham and Women's Hospital |
| 19 880 | Paraplatin (carboplatin)a | 1989 | Research Corporation (on behalf of Michigan State University) |
| 20 886 | Panretin (alitretinoin)a | 1999 | Salk Institute |
| 19 927 | Nizoral (ketoconazole) | 1990 | University of Tennessee |
| 20 310 | Nizoral A-D (ketoconazole) | 1997 | University of Tennessee |
| 20 326 | Neutrexin (trimetrexate glucuronate)a | 1993 | US Government, HHS |
| 21 487 | Namenda (memantine hydrochloride)b | 2003 | Children's Hospital Boston |
| 20 586 | Meretek UBT kit (with pranactin) (urea, C-13)b | 1996 | Baylor College of Medicine |
| 21 674 | Menostar (estradiol) | 2004 | University of California |
| 21 446 | Lyrica (pregabalin)a | 2004 | Northwestern University |
| 20 845 | Inomax (nitric oxide)a | 1999 | Massachusetts General Hospital |
| 20 199 | Hivid (zalcitabine)a | 1992 | US Government, HHS |
| 20 076 | Habitrol (nicotine) | 1991 | University of California |
| 20 637 | Gliadel (carmustine) | 1996 | Massachusetts Institute of Technology |
| 19 863 | Geref (sermorelin acetate)b | 1990 | Salk Institute |
| 20 443 | Geref (sermorelin acetate) | 1997 | Salk Institute |
| 21 481 | Fuzeon (enfuvirtide)a | 2003 | Duke University |
| 20 038 | Fludara (fludarabine phosphate)a | 1991 | US Government, HHS |
| 20 195 | Fentanyl (fentanyl citrate) | 1993 | University of Utah |
| 20 044 | Exosurf neonatal (cetyl alcohol; colfosceril palmitate; tyloxapol)a | 1990 | University of California |
| 19 677 | Enlon-plus (atropine sulfate; edrophonium chloride) | 1991 | University of California |
| 21 500 | Emtriva (emtricitabine)b | 2003 | Emory University |
| 21 896 | Emtriva (emtricitabine) | 2005 | Emory University |
| 20 193 | Elmiron (pentosan polysulfate sodium)b | 1996 | University of California |
| 21 283 | Diovan (valsartan) | 2001 | Brigham and Women's Hospital |
| 20 869 | Cosopt (dorzolamide hydrochloride; timolol maleate) | 1998 | University of Florida |
| 21 673 | Clolar (clofarabine)a | 2004 | Southern Research Institute |
| 21 197 | Cetrotide (cetrorelix)b | 2000 | Tulane University |
| 19 829 | Ceretec (technetium TC-99M exametazime kit)a | 1988 | University of Missouri |
| 19 785 | Cardiolite (technetium TC-99M sestamibi kit)b | 1990 | Harvard College |
| 20 954 | Busulfex (busulfan) | 1999 | University of Texas |
| 20 404 | Avita (tretinoin) | 1997 | University of California |
| 21 316 | Altoprev (lovastatin) | 2002 | Children's Hospital Boston |
| 21 462 | Alimta (pemetrexed disodium)a | 2004 | Princeton University |
| 19 937 | Adenocard (adenosine)a | 1989 | University of Virginia |
| 20 747 | Actiq (fentanyl citrate) | 1998 | University of Utah |
| 20 162 | Acthrel (corticorelin ovine triflutate)a | 1996 | Salk Institute |
Note. HHS = US Department of Health and Human Services; NDA = new drug application.
New molecular entity that received priority approval.
New molecular entities that did not receive priority approval.
Second, 35 distinct institutions accounted for the “academic” patents on the 72 drugs, and the subset of 39 drugs with academic patents that were new molecular entities were associated with 26 patent holders. That is, ownership of the public sector patents is relatively diffuse.
A third interesting feature of Table 1 is the prominence of HIV/AIDS drugs. Twelve of the 72 drugs with academic patents were HIV/AIDS drugs (about 16.7%), whereas, overall, HIV/AIDS drugs accounted for 47 of the 938 new drug applications with patents approved over the period examined (5.01%). To consider this another way, whereas the share of non–HIV drugs with academic patents was 6.7%, the corresponding share for HIV drugs was 25.5%.
Patent Filings in Developing Countries
The first column of Table 2 shows that, of the 1947 unique patents listed in the Orange Book, 43% (830) were filed in developing countries. Firms were significantly more likely than were academic institutions to file patents in developing countries: 43.9% of nonacademic patents were filed in developing countries, compared with 18.75% of academic patents (P < .01).
TABLE 2.
Share of Drugs Approved by the US Food and Drug Administration Between 1988 and 2005 With Patent Applications in Developing Countries
| Overall |
Filed Pre-1996 |
Filed 1996 or Later |
||||
| Mean (SE) | No. | Mean (SE) | No. | Mean (SE) | No. | |
| All patents | 0.43 (0.01) | 1947 | 0.35 (0.01) | 1414 | 0.64 (0.02) | 533 |
| Academic patents | 0.19 (0.04) | 96 | 0.14 (0.04) | 80 | 0.43 (0.13) | 16 |
| Nonacademic patents | 0.44 (0.01) | 1851 | 0.40 (0.01) | 1334 | 0.64 (0.02) | 517 |
| Nonacademic patents with academic patents on same drug | 0.40 (0.05) | 81 | 0.29 (0.06) | 55 | 0.62 (0.09) | 26 |
| Nonacademic patents without academic patents on same drug | 0.44 (0.01) | 1770 | 0.36 (0.01) | 1279 | 0.65 (0.02) | 491 |
Note. Mean values are percentage of drugs filed in developing countries.
The equitable access license and other policy initiatives discussed previously have a “viral” component, aimed at limiting not only enforcement of academic patents in developing countries, but also the ability of firms to enforce any of their own patents on the same drugs in developing countries. Accordingly, it is also interesting to examine firms' international filing strategies for their own patents on drugs that also have at least 1 academic patent.
Overall, 81 of the firms' 1851 patents were on drugs that also had academic patents. The next 2 rows of Table 2 show the share of firms' patents filed in developing countries in cases in which there was an academic patent on the same drug (row 4), and in cases in which there were not (row 5). Although firms were more likely to file patents in developing countries in cases in which academic institutions did not have patents on the same drugs, the difference was qualitatively small and statistically insignificant (39.5% vs 44.1%; P = .42).
Before the signing of the TRIPs agreement in 1995, product patents on drugs were not allowed in many developing countries. In addition, patent data in developing countries may be more complete in recent years than was previously the case. Accordingly, I also examined pre- and post-1995 filed patents separately in the second and thirds columns of Table 2. For each of the groups, there was a qualitatively and statistically significant (P < .05 for all rows) increase over time in the propensity to file in developing countries. Overall, in the post-1995 cohort of patents, the share of academic drug patents filed in developing countries was 43.8%, compared with 64.4% of nonacademic drug patents. Although the difference was not statistically significant at conventional levels (P = .09), this could reflect the relatively small number of post-1995 academic patents in the sample. Also, as with the overall sample, there was no statistically significant difference between the nonacademic patents on drugs that also had academic patents and other, nonacademic patents (61.5% vs 64.6%; P = .75).
DISCUSSION
The data show that the stavudine case discussed previously is not unique. The overall share of drugs approved between 1988 and 2005 on which universities own patents was relatively low—7.7%—and the share for new molecules was only slightly higher—10.3%. However, universities own patents on nearly 1 in 5 (19.2%) of the drugs that are arguably the most innovative—new molecular entities that received “priority” approval by the FDA; this share has been basically stable since the late 1980s. In addition, universities own key patents on over one quarter of the HIV/AIDS drugs approved since 1988, which is particularly important given the potentially catastrophic impact of this disease in the developing world. The data do not support the arguments of some critics of drug companies33 that the bulk of important pharmaceutical innovation is done by the public sector. However, they do suggest that a nontrivial fraction of marketed drugs, particularly those that may be considered the most novel and clinically useful, emanate from and are patented by universities and hospitals.
The results also suggest that universities, and the firms that commercialize and market drugs with academic patents, currently apply for patents in developing countries. For patents filed after 1995, at least 44% of academic patents, and 62% of firms' patents on drugs with academic patents, were filed in developing countries.
Taken together, these findings suggest that changes to university policies could have important effects and provide evidence of the feasibility of using academic control of key drug patents to promote access in developing countries.
What about the desirability of exercising this control? What are the costs, what are the risks? I believe that the main potential downside risk of exercising this control is that potential licensee firms would balk at these provisions, choosing not to license and develop drugs that they would have done in the absence of restrictions on enforcing patents in developing countries. As discussed previously here, the logic underlying academic patenting and licensing, expressed in the Bayh–Dole Act, is that firms need the promise of monopoly power to have incentives to develop, test, and commercialize university-developed inventions.
By limiting profits from developing countries, would the proposals discussed here hinder incentives to bring academic inventions to market? This seems unlikely, since developing countries represent a trivial proportion of consumption of most pharmaceuticals.34 For drugs against “global” diseases, like cancer, diabetes, cardiovascular disease, and HIV/AIDS, firms rely on markets in developed countries for the bulk of their profits.35 Accordingly, contractual limits on firms' abilities to enforce patents in developing countries would not strongly affect their incentives to commercialize most academic inventions. Paradoxically, the cases in which such limits would be most likely to deter commercialization would be those in which markets in developing countries represent the bulk of potential consumption (i.e., drugs targeted specifically at “neglected” diseases—those without large markets in developed countries). However, very few drugs for neglected diseases are developed and marketed by pharmaceutical companies in the current environment,36,37 and there is relatively little public funding in developed countries for neglected-disease research.38 Neglected diseases are an important, but separate, policy problem.
Even though firms would not rationally walk away from licensing drugs on global diseases if subjected to limits on enforcing patents in developing countries, they may threaten to do so in bargaining over licensing terms. In addition, firms, not universities, likely have stronger bargaining power in licensing negotiations: for most academic inventions, the modal number of licensees expressing interest is zero.38 Given the small number of suitors for most academic patents, individual academic institutions may be unwilling or unable to commit to imposing demands on potential licensees. In this context, top-down requirements from the funders of this research (e.g., those in the Leahy bill) may be necessary to change licensing policies and practices. More generally, the data in the previous sections show that academic ownership of patents on FDA-approved drugs is dispersed across a large number of institutions. It may be difficult, even with strong campus-level activism, to bring about any change at all in the licensing policies of these institutions, or that of other academic institutions that may generate new pharmaceuticals in the future. This too suggests that top-down legislative approaches may be more fruitful in changing academic licensing practices.
Although the results reported in this paper suggest that universities could play a role in enhancing access to drugs, the magnitude of their potential impact remains unclear. Difficulties in obtaining drug consumption data from developing countries make it hard to know the extent to which the university-developed drugs identified here are important for public health in the developing world. Nor is it possible to know the extent to which patents, vis-à-vis other factors, currently inhibit access to these drugs, and thus, the magnitude of the global health impacts that changes in academic licensing policies would have. In-depth (qualitative and quantitative) case study research exploring these issues—oriented around the drugs listed in Table 1—is an important task for future research.
Acknowledgments
This work was funded in part by grants from the Ford Foundation and the Robert Wood Johnson Foundation.
The author thanks Orin Herskowitz, Amy Kapczynski, Arti Rai, Michael Steffen, and Robynn Sturm for thoughtful comments on this research.
End Notes
- 1.HIV-AIDS, Malaria, TB, and Access to Essential Medicines Working Group. “Prescription for Healthy Development: Increasing Access to Medicines.” London: Earthscan, 2005 [Google Scholar]
- 2.Pierre Chirac, Els Torreele, “Global framework on essential health R&D.” Lancet 367, no. 9522 (May 13, 2006): 1560–1561 [DOI] [PubMed] [Google Scholar]
- 3.Amir Attaran, Lee Gillespie-White. “Do Patents for Antiretroviral Drugs Constrain Access to AIDS Treatment in Africa?”. Journal of the American Medical Association. doi: 10.1001/jama.286.15.1886. 286, no. 15 (October 17, 2001): 1886–1892. [DOI] [PubMed] [Google Scholar]
- 4. Universities Allied for Essential Medicines Web site, http://www.essentialmedicine.org (accessed March 1, 2008)
- 5.S. Chaudhuri, The WTO and India's Pharmaceuticals Industry: Patent Protection, TRIPS, and Developing Countries (New York, NY: Oxford University Press, 2005). [Google Scholar]
- 6. Ibid.
- 7.David C. Mowery, Bhaven N Sampat, “University Patents and Patent Policy Debates in the USA, 1925–1980,” Industrial and Corporate Change 10, no. 3 (2001): 781–814 [Google Scholar]
- 8.David Mowery, Richard Nelson, Bhaven Sampat, Arvids Ziedonis, Ivory Tower and Industrial Innovation: University-Industry Technology Transfer Before and After the Bayh-Dole Act (Stanford, CA: Stanford Business Books, 2004). [Google Scholar]
- 9.Pierre Azoulay, Ryan Michigan, Bhaven N Sampat, “The anatomy of medical school patenting,” New England Journal of Medicine 357, no. 20 (November 15, 2007): 2049–2056 [DOI] [PubMed] [Google Scholar]
- 10.AUTM Licensing Survey 2006 (Norwalk, CT: Association of Technology Licensing Managers, 2006). [Google Scholar]
- 11. Mowery, Nelson, Sampat, and Ziedonis, Ivory Tower and Industrial Innovation.
- 12. Azoulay, Michigan, and Sampat, “The anatomy of medical school patenting,” 2049–2056. [DOI] [PubMed]
- 13.D. Lindsey, “Amy and Goliath,” Salon (2001) http://archive.salon.com/news/feature/2001/05/01/aids (accessed March 1, 2008)
- 14.Dave A. Chokshi, “Improving access to medicines in poor countries: the role of universities,” PLoS Medicine 3, no. 6 (June 1, 2006): e136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amy Kapczynski, Samantha Chaifetz, Zachary Katz, Yochai Benkler, “Addressing global health inequities: an open licensing approach for university innovations,” Berkeley Technology Law Journal 20, no. 2 (2005): 1031–1114 [Google Scholar]
- 16.Dave A. Chokshi, Rahul Rajkumar, “Leveraging university research to advance global health,” Journal of the American Medical Association 298, no. 16 (October 24, 2007): 1934–1936 [DOI] [PubMed] [Google Scholar]
- 17.Steven G. McMillan, Francis Narin, David L Deeds, “An analysis of the critical role of public science in innovation: the case of biotechnology,” Research Policy 29, no. 1 (2000): 1–8 [Google Scholar]
- 18.Iain Cockburn, Rebecca Henderson, “Public–private interaction in pharmaceutical research,” Proceedings of the National Academy of Sciences of the U S A 93, no. 23 (November 12, 1996): 12725–12730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. The Benefits of Medical Research and the Role of the NIH (Washington, DC: US Congressional Joint Economic Committee, 2000)
- 20.Wesley M. Cohen, Richard R Nelson, John P Walsh. “Links and impacts: the influence of public research on industrial R&D”. Management Science. 48, no. 1 (2002): 1–23. [Google Scholar]
- 21. US Food and Drug Administration, Drugs@FDA Web site, http://www.accessdata.fda.gov/Scripts/cder/DrugsatFDA (accessed August 7, 2008)
- 22. Approved Drug Products with Therapeutic Equivalence Evaluations, 22nd ed (Washington, DC: US Department of Health and Human Services, 2007) http://www.fda.gov/cder/ob (accessed August 7, 2008)
- 23. US Food and Drug Administration, Drugs@FDA Web site, question 12, http://www.fda.gov/cder/drugsatfda/faq.htm#chemtype_reviewclass (accessed August 7, 2008)
- 24. National Institute for Health Care Management, Changing Patterns of Pharmaceutical Innovation (Washington, DC: National Institute for Health Care Management, 2002)
- 25. PhRMA. “NIHCM's Report on Pharmaceutical Innovation: Fact vs. Fiction,” http://mednet3.who.int/prioritymeds/report/append/8342.pdf (accessed February 20, 2008)
- 26.TC Quinn, “Global burden of the HIV pandemic,” Lancet 348, no. 9020 (1996): 99–106 [DOI] [PubMed] [Google Scholar]
- 27. Food and Drug Administration, “Drugs used in the treatment of HIV infection,” http://www.fda.gov/oashi/aids/virals.html (accessed February 20, 2008)
- 28. United States Patent and Trademark Office's Cassis database of bibliographic information from US patents, http://www.uspto.gov/web/offices/ac/ido/oeip/catalog/products/cassis.htm (accessed September 4, 2008)
- 29. Azoulay, Michigan, and Sampat, “Anatomy of Medical School Patenting,” 2049–2056. [DOI] [PubMed]
- 30. Devwent Innovation Index, http://scientificthomsonreuters.com/products/dii (accessed September 1, 2008)
- 31. The World Bank, “A short history,” http://go.worldbank.org/U9BK7IA1J0 (accessed February 20, 2008)
- 32. See note 15 above.
- 33.Marcia Angell, The Truth About Drug Companies: How They Deceive Us and What to Do About It (New York, NY: Random House, 2004). [Google Scholar]
- 34. Kapczynsi, Chaifetz, Katz, and Benkler, “Addressing global health inequities,” 1031–1114.
- 35.J. Lanjouw, “A patent policy for global diseases,” Innovations 1, no. 1 (2006): 108–114 [Google Scholar]
- 36. See note 2 above.
- 37.Michael Kremer, Rachel Glennerester, Strong Medicine: Creating Incentives for Pharmaceutical Research on Neglected Diseases (Princeton, NJ: Princeton University Press, 2004). [Google Scholar]
- 38.J. Lanjouw, M McLeod, “Pharmaceutical R&D for low-income countries: global trends and participation by Indian firms,” Economic and Political Weekly 40, no. 39 (September 24, 2005): 4232–4242 [Google Scholar]
- 39. Jensen, Thursby, and Thursby, “Disclosure and licensing of university inventions: the best we can do with the sh*t we get to work with,” International Journal of Industrial Organization 21, no. 9 (2003): 1271–1300.