Abstract
Streptococcus suis type 2 was evaluated for hemolysin production. Supernatants of S. suis type 2 grown in Todd-Hewitt broth were assayed for hemolytic activity by a photometric assay. Twenty-two additional serotypes of S. suis (1,3 to 22, and 1/2) were evaluated for hemolysin production; nine of them (1/2, 1, 4, 5, 14, 15, 17, 19, and 20) were positive. The effects of temperature, atmosphere, centrifugation, sonication, chemicals, bovine serum albumin, fetal calf serum, and enzymes on S. suis type 2 hemolysin activity were studied. Maximum hemolysis occurred after incubation in RPMI 1640 medium at 40 degrees C in 6% CO2 and after growth in Todd-Hewitt broth at 37 degrees C under anaerobic conditions. Hemolytic activity was absent after the addition of fetal calf serum and decreased after the addition of trypsin or amylase. However, treatment of erythrocytes with amylase or trypsin prior to incubation with supernatant also resulted in a decrease in hemolytic activity. The addition of bovine serum albumin caused increased hemolytic activity. Dipyridyl and EDTA had negligible effects on hemolysis. Hemolytic S. suis type 2 culture supernatant injected intraperitoneally failed to cause death in BALB/c mice. Data from our study indicate that S. suis type 2 hemolysin is a secreted or loosely cell bound, thermolabile molecule whose activity is growth condition dependent.
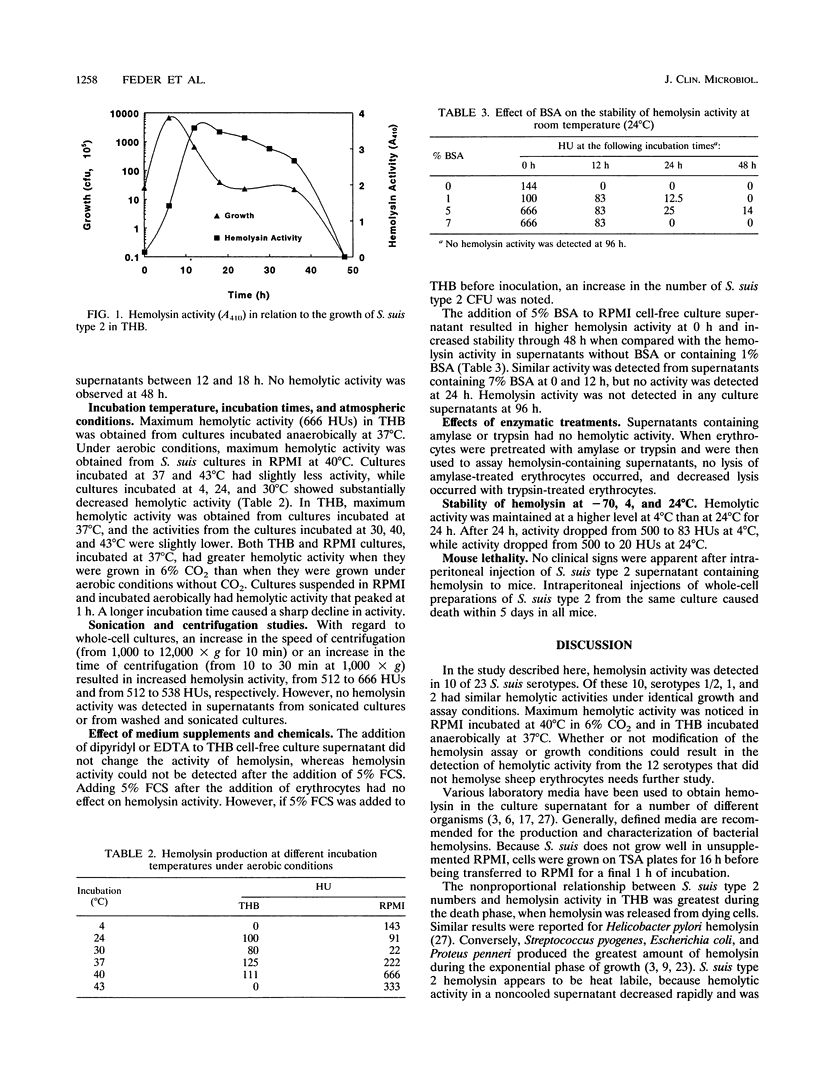
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends J. P., Zanen H. C. Meningitis caused by Streptococcus suis in humans. Rev Infect Dis. 1988 Jan-Feb;10(1):131–137. doi: 10.1093/clinids/10.1.131. [DOI] [PubMed] [Google Scholar]
- Ashdown L. R., Koehler J. M. Production of hemolysin and other extracellular enzymes by clinical isolates of Pseudomonas pseudomallei. J Clin Microbiol. 1990 Oct;28(10):2331–2334. doi: 10.1128/jcm.28.10.2331-2334.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnani P. J., Bhatnagar N., Bhandari S. Production and purification of Escherichia coli hemolysin. Folia Microbiol (Praha) 1988;33(5):393–400. doi: 10.1007/BF02925850. [DOI] [PubMed] [Google Scholar]
- Chengappa M. M., Pace L. W., Williams J. A., Herren C. H., Ascher S. E. Efficacy of tiamulin against experimentally induced Streptococcus suis type-2 infection in swine. J Am Vet Med Assoc. 1990 Dec 1;197(11):1467–1470. [PubMed] [Google Scholar]
- Devenish J., Rosendal S., Bossé J. T. Humoral antibody response and protective immunity in swine following immunization with the 104-kilodalton hemolysin of Actinobacillus pleuropneumoniae. Infect Immun. 1990 Dec;58(12):3829–3832. doi: 10.1128/iai.58.12.3829-3832.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott S. D., Tai J. Y. The type-specific polysaccharides of Streptococcus suis. J Exp Med. 1978 Dec 1;148(6):1699–1704. doi: 10.1084/jem.148.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J., Nicolet J. Immunological properties of Actinobacillus pleuropneumoniae hemolysin I. Vet Microbiol. 1991 Jun;28(1):61–73. doi: 10.1016/0378-1135(91)90099-2. [DOI] [PubMed] [Google Scholar]
- Griffiths B. B., McClain O. The role of iron in the growth and hemolysin (Streptolysin S) production in Streptococcus pyogenes. J Basic Microbiol. 1988;28(7):427–436. doi: 10.1002/jobm.3620280703. [DOI] [PubMed] [Google Scholar]
- Haque A., Sugimoto N., Horiguchi Y., Okabe T., Miyata T., Iwanaga S., Matsuda M. Production, purification, and characterization of botulinolysin, a thiol-activated hemolysin of Clostridium botulinum. Infect Immun. 1992 Jan;60(1):71–78. doi: 10.1128/iai.60.1.71-78.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono I., Aoki T. Nucleotide sequence and expression of an extracellular hemolysin gene of Aeromonas hydrophila. Microb Pathog. 1991 Sep;11(3):189–197. doi: 10.1016/0882-4010(91)90049-g. [DOI] [PubMed] [Google Scholar]
- Holt M. E., Enright M. R., Alexander T. J. Immunisation of pigs with killed cultures of Streptococcus suis type 2. Res Vet Sci. 1990 Jan;48(1):23–27. [PubMed] [Google Scholar]
- Hughes J. H. Physical and chemical methods for enhancing rapid detection of viruses and other agents. Clin Microbiol Rev. 1993 Apr;6(2):150–175. doi: 10.1128/cmr.6.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Todd J., Ma J. N., Veit H. Characterization of a non-hemolytic mutant of Actinobacillus pleuropneumoniae serotype 5: role of the 110 kilodalton hemolysin in virulence and immunoprotection. Microb Pathog. 1991 Apr;10(4):281–296. doi: 10.1016/0882-4010(91)90012-y. [DOI] [PubMed] [Google Scholar]
- Jacques M., Gottschalk M., Foiry B., Higgins R. Ultrastructural study of surface components of Streptococcus suis. J Bacteriol. 1990 Jun;172(6):2833–2838. doi: 10.1128/jb.172.6.2833-2838.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991 Jan;4(1):80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp E. M., van Leengoed L. A. Serotype-related differences in production and type of heat-labile hemolysin and heat-labile cytotoxin of Actinobacillus (Haemophilus) pleuropneumoniae. J Clin Microbiol. 1989 Jun;27(6):1187–1191. doi: 10.1128/jcm.27.6.1187-1191.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y., Haritani M., Mori M., Kishima M., Sugimoto C., Nakazawa M., Yamamoto K. Experimental infections of mice and pigs with Streptococcus suis type 2. J Vet Med Sci. 1991 Dec;53(6):1043–1049. doi: 10.1292/jvms.53.1043. [DOI] [PubMed] [Google Scholar]
- Marchlewicz B. A., Duncan J. L. Properties of a hemolysin produced by group B streptococci. Infect Immun. 1980 Dec;30(3):805–813. doi: 10.1128/iai.30.3.805-813.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall N. E., Ziegler H. K. Role of bacterial hemolysin production in induction of macrophage Ia expression during infection with Listeria monocytogenes. J Immunol. 1991 Oct 1;147(7):2324–2332. [PubMed] [Google Scholar]
- Meyers D. J., Palmer K. C., Bale L. A., Kernacki K., Preston M., Brown T., Berk R. S. In vivo and in vitro toxicity of phospholipase C from Pseudomonas aeruginosa. Toxicon. 1992 Feb;30(2):161–169. doi: 10.1016/0041-0101(92)90469-l. [DOI] [PubMed] [Google Scholar]
- Robertson I. D., Blackmore D. K. Occupational exposure to Streptococcus suis type 2. Epidemiol Infect. 1989 Aug;103(1):157–164. doi: 10.1017/s0950268800030454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozalski A., Kotełko K. Hemolytic activity and invasiveness in strains of Proteus penneri. J Clin Microbiol. 1987 Jun;25(6):1094–1096. doi: 10.1128/jcm.25.6.1094-1096.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. E., Reek F. H., Vecht U., Gielkens A. L., Smits M. A. Repeats in an extracellular protein of weakly pathogenic strains of Streptococcus suis type 2 are absent in pathogenic strains. Infect Immun. 1993 Aug;61(8):3318–3326. doi: 10.1128/iai.61.8.3318-3326.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel J. D. Bacterial etiologic agents in the pathogenesis of urinary tract infection. Med Clin North Am. 1991 Mar;75(2):253–273. doi: 10.1016/s0025-7125(16)30452-7. [DOI] [PubMed] [Google Scholar]
- Vecht U., Wisselink H. J., Jellema M. L., Smith H. E. Identification of two proteins associated with virulence of Streptococcus suis type 2. Infect Immun. 1991 Sep;59(9):3156–3162. doi: 10.1128/iai.59.9.3156-3162.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherall B. L., McDonald P. J., Johnson A. M. Partial characterization of a cell-free hemolytic factor produced by Helicobacter pylori. FEMS Microbiol Immunol. 1992 Feb;4(3):123–128. doi: 10.1111/j.1574-6968.1992.tb04978.x. [DOI] [PubMed] [Google Scholar]


