Abstract
The transcription factor Bright up-regulates immunoglobulin heavy chain production from select variable region promoters and requires Bright dimerization, Bruton’s tyrosine kinase (Btk) and the Btk substrate, TFII-I for this activity. Defects in Btk cause X-linked immunodeficiency disease in mice and man. Btk-deficient mice exhibit decreased serum IgM production, B cell developmental blocks, absence of peritoneal B1 cells, and subnormal immune responses against antigens, including phosphorylcholine, which confer protection against Streptococcus pneumoniae. Transgenic mice expressing dominant negative (DN) Bright share similarities with Btk-deficient mice, including decreased serum IgM, poor anti-phosphorylcholine responses, and slightly reduced numbers of mature B cells. Although DN Bright mice developed B1 B cells, these were functionally deficient in immunoglobulin secretion. These data suggest a mechanistic explanation for the abnormal responses to phosphorylcholine observed in Btk-deficient mice, and indicate that Bright functions in a subset of Btk-dependent pathways in vivo, particularly those responses dominated by B1 B cells.
INTRODUCTION
B cell regulator of immunoglobulin heavy chain transcription (Bright) was the first eukaryotic member of the ARID (A+T-rich interacting domain) family of DNA binding proteins described (1,2) but its function in vivo has not been elucidated. ARID-containing proteins are expressed in a wide range of organisms including Drosophila, C. elegans and Xenopus (3) where they have diverse functions, including roles in gene expression, physical development, and cell growth (4). While all contain a similar ARID sequence, only a few of the ARID3 subfamily members (including Bright/ARID3a) bind specific DNA motifs, and gene targets have been identified for only a handful of those (3,4). Bright binds A+T regions flanking the intronic immunoglobulin (Ig) μ heavy chain enhancer as well as to regions 5′ of select VH promoters where it upregulates Ig heavy chain transcription 5- to 7-fold (5,6). The transcriptionally active complex is comprised of a Bright dimer, Bruton’s tyrosine kinase (Btk), and BAP135/TFII-I (7,8) and transcription activation by this complex in vitro depends upon phosphorylation of TFII-I by Btk (6,9). These data implied that Bright functions in a subset of Btk-dependent pathways.
Btk, a Tec family tyrosine kinase, was first identified as the defective gene in X-linked immunodeficient, or xid mice (10,11). Xid and Btk- deficient mice are characterized by blocks in B cell development that result in reduced levels of serum IgM and IgG3 (11,12), increased numbers of immature B cells in the periphery (13), deficient calcium and cell cycle responses in activated B lymphocytes (14,15), absence of peritoneal B1 cells, and failure to respond to immunizations with type II pneumococcal polysaccharide or infection with Streptococcus pneumoniae (16). Although the contributions of Btk to B cell signaling pathways have been clarified (reviewed in (17,18), the mechanisms by which Btk deficiency blocks early B cell development, particularly in Btk deficient humans who typically exhibit earlier and more pronounced blocks in B cell development than occur in mouse models, are unknown (19). Recently, we and other labs (6,20,21) have obtained data suggesting a role for Btk in transcription-mediated processes including pathways that require Bright.
B cells from xid and Btk deficient mice express Bright protein after stimulation with either LPS or CD40L, but do not form stable Bright transcription complexes (22), implying that Bright-mediated transcription is defective in these mice. Indeed, early studies indicated that canonical T15 idiotype responses to phosphorylcholine (PC) were deficient in xid male mice, but were unchanged in female littermates having a functional copy of Btk (16). Anti-PC antibodies result almost exclusively from use of the S107 VH family V1 gene in normal mice. Data from our laboratory demonstrated that both Bright and Btk are required for upregulation of V1 transcription in vitro (6). We therefore hypothesized that impairment of Bright function should lead to defects in V1 gene expression in mice and might explain the defective anti-PC responses observed in xid mice. Furthermore, because Btk also affects B cell development we hypothesized that inhibition of Bright function could impair B cell development.
Bright is expressed in multiple tissues in the mouse embryo, becoming B cell-restricted after birth (23). Similarly, the Drosophila homologue, dri, is expressed throughout embryonic development but is gut-associated in the adult (24). Therefore we speculated that a Bright knockout might have an embryonic lethal phenotype, as was the case for Drosophila and Xenopus homologues (25,26). Therefore, to address the role of Bright in Ig heavy chain expression and B cell development in vivo, dominant negative (DN) Bright transgenic mice were generated that express a double point mutation in the DNA binding domain of Bright. These mutations do not affect interactions of Bright with Btk and TFII-I, but inhibit endogenous Bright activity by allowing formation of inactive Bright dimers that cannot bind DNA (9,27). To eliminate effects due to expression in multiple tissues, the transgene was expressed from the B lineage-specific CD19 promoter. Phenotypic analyses of these mice indicate that Bright contributes directly to the function of peritoneal B1 B cells and normal immune responses against PC. Results indicate that Bright is particularly important for B1 B cell function.
MATERIALS AND METHODS
Generation of transgenic mice
DN Bright was created as previously described (27). The carboxyl terminus of DN Bright was tagged with the myc-His sequence of pcDNA/TO/myc-HisB (Invitrogen). SV40 polyA (amplified from pSP655) and LoxP (ATAACTTCGTATAATGTATGCTATACGAAGTTAT) (28) sequences were subcloned onto the 3′ end of the DN-myc-His construct and the resulting fragment was ligated downstream of the B cell specific 6.3 kb human CD19 promoter (gift of R. Hendricks) (29). Transgenics were generated in FVB/N mice by the OMRF Transgenic Core Facility. Founder mice were identified using primers within Bright (CAGATCCTCTTCTGAGATGAG) and the myc-His tag (CAGATCCTCTTCTGAGATGAG) with PCR conditions of 93°C for 1 min, and 40 cycles of 93°C for 30 sec, 57°C for 30 sec, and 72°C for 45 sec. Homozygous transgenics were bred from heterozygous mice and confirmed by production of 100% transgenic positive progeny (at least 20 pups) with a wild type control. Male and female homozygous mice were analyzed at ages 6 to 13 weeks. In some cases, mice over-expressing wild type Bright from the CD19 promoter (WT-TG) were used (30). C57Bl/6 and BCL-2 Eμ transgenic mice were obtained from Jackson Laboratories. All studies were performed under institutional guidelines for animal use.
Tissue collection, immunization and infection
Sera, spleen, thymus, and bone marrow were harvested as described (30). Peritoneal cells were obtained by lavage with PBS-3% FCS. In some cases, mice were immunized intraperitoneally with PC-KLH or NP-KLH (0.5μg/ml) in Freund’s complete adjuvant (CFA) (Sigma) as described (30). Encapsulated, type 3 Streptococcus pneumoniae, strain WU2 (31) (provided by Dr. Yother, U. of AL, Birmingham) were grown in Todd-Hewitt broth with 0.5% yeast extract to mid-log phase and stored at −80°C in 10% glycerol. Bacterial titers and CFU were determined by growth on blood agar plates prior to intraperitoneal infection.
Flow cytometry and immunohistochemistry
Cell staining and immunohistochemistry were performed as described (30). Analysis used a FacsCalibur with CellQuest Pro software (BD Biosciences). Cell sorting was performed by the Oklahoma Medical Research Foundation Flow Cytometry Core Facility with an advanced MoFlo cell sorter (Cytomation). Fluorescent-labeled antibodies used were: fluorescein isothiocyanate (FITC)-CD19 (ID3), CD21 (7G6), CD4 (RM4-4); R-phycoerythrin (R-PE)-CD8 (53-6.7), CD23 (B3B4), CD5 (53-7.3), CD138 (2-81-2), CD43 (57); allophycocyanin (APC)-CD45R/B220 (RA3-6B2), CD93/C1qRp (AA4.1), peridinin chlorophyll-a protein (PerCP)-CD45R/B220 (RA3-6B2), FITC-IgM, PE-IgD (11–26), biotin- or PE-conjugated anti-CD40 (1C10), CD69 (H1.2F3), CD80 (1G10), CD86 (GL1), I-Aq (KH116) and appropriate rat, mouse, and hamster isotype controls (BD Pharmingen and Southern Biotechnology). APC-streptavidin was used to detect biotin-labeled antibodies. AlexaFluor 546 conjugated goat anti-mouse IgM (Molecular Probes) and rat anti-mouse metallophilic macrophages (Serotec, Raleigh, NC) revealed by AlexaFluor 350 goat anti-rat IgG (Serotec) were used. Tissue sections were viewed by Zeiss LSM510 confocal microscopy and analyzed with Zeiss LSM Image Browser software.
Western blotting and semiquantitative RT-PCR
Single cell suspensions (5 × 104 cells) dissolved in SDS-sample buffer were run on 7.5% SDS-polyacrylamide gels under standard denaturing conditions and transferred to nitrocellulose membranes (22). Blots were incubated with anti-actin (Santa Cruz) or polyclonal rabbit anti-Bright (P. Tucker, U. of Texas, Austin, TX), alkaline-phosphatase conjugated goat anti-rabbit IgG (Southern Biotechnology) and alkaline phosphatase substrate (Bio-Rad). RNA was isolated with TriReagent (MRC), amplified and Southern blotted as described (30). Primers for S107-IgM, actin, DN Bright, Blimp-1 and XBP were described (30,32,33). Primers for membrane and secretory exons of Cμ were: IgM Exon 4 5′-GTGAGCAACTGAACCTGAGGGAGTC-3′; IgM Sec 5′-CAATAGCAGGTGCCGCCTGTGTCAG-3′; and IgM Mem 5′-GGTGACGGTGGTGCTGTAGAAGAG-3′.
In vitro stimulation
Non-B cells were depleted from splenic cell suspensions with anti-Thy-1 and complement and incubated at 2×106 cells per/ml, alone, with 25 μg/ml LPS, or with CD40L-expressing Sf9 or wild type Sf9 control cells as described (22). Cells were pulsed with 1μCi of 3H-thymidine for 6 hours. Peritoneal cells and sorted subpopulations were resuspended (2.5×105 cells/ml) and cultured with or without 20 μg/ml LPS for 3 days. Supernatants were collected for ELISA and RNA was isolated. For activation experiments, B cells were isolated using the B220 enrichment B Cell Isolation kit (Miltenyi Biotech), plated in 6-well plates at 1.0×106 cells/ml and stimulated with LPS (25 μg/ml) for 18 hours. Electrophoretic mobility shift assays (EMSAs) were performed for Bright and octamer binding activity as previously described (22).
ELISAs and ELISPOTS
The clonotyping system-AP kit (Southern Biotechnologies) was used according to the manufacturer’s directions to test for serum isotypes. Standard curves were generated with isotypes of known concentration and Ig levels were quantified using Excel software. Antigen-specific antibodies were detected using PC-BSA or NP-BSA coated plates as described (30). Samples were assessed in duplicate at four or more dilutions and samples were read with an MRX microtiter reader (Dynatech Laboratories). The BD ELISPOT Assay (BD Biosciences) was used. Peritoneal cells were serially diluted onto Multi-Screen*-IP plates (Millipore) precoated with goat anti-mouse Ig and were incubated for three hours at 37°C, followed by goat anti-mouse IgG or IgM directly or indirectly conjugated to horse radish peroxidase. Spots were detected with 3-amino-9-ethyl-carbazole substrate and were counted by the Immunospot Series 1 analyzer with ImmunoSpot 4.0 software (Cellular Technology Ltd.).
RESULTS
Generation of DN Bright transgenic mice
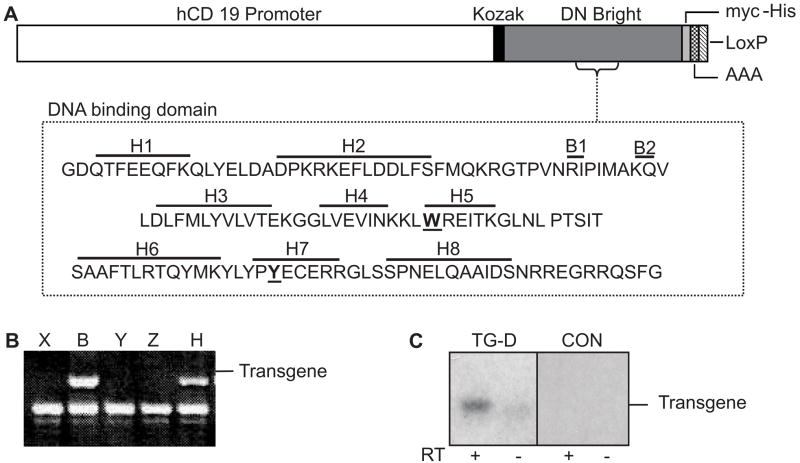
DN Bright (27) expressed from the B cell-specific human CD19 promoter (Figure 1A) was used to generate transgenic mice. The CD19 promoter was chosen because CD19 expression begins at the early pro-B cell stage in mice and continues throughout B cell differentiation with down-regulation occurring in terminally differentiated plasma cells (34). Bright mRNA is first expressed in mouse B lineage cells at the pro-B to pre-B cell stage (23) and is therefore expressed coincidentally in our experiments with DN Bright mRNA early in B cell development, allowing formation of inactive Bright protein dimers to effectively inhibit endogenous Bright function. The tagged carboxyl terminus of the transgene resulted in a slightly larger product that distinguished DN Bright from the endogenous protein allowing us to monitor protein expression. Of 17 founder mice, four generated transgene positive progeny (Figure 1B). Transgene expression levels varied among these lines from very low to moderate as measured by RT-PCR of splenic B cell RNA (Figure 1C). Because we expected robust transgene expression to more effectively inhibit endogenous Bright function, the B and D DN Bright transgenic lines exhibiting moderate DN Bright expression were bred to homozygosity, and the resulting homozygous D and B lines were interbred to produce double transgenic (D/B) heterozygous mice. Both homozygous lines, the double transgenic line and heterozygous lines were analyzed. All animal studies were reviewed and approved by the appropriate institutional review board.
Figure 1. Generation of DN Bright transgenic mice.
A. A schematic diagram depicts the DNA construct used for DN Bright expression from the B cell-specific CD19 promoter with the associated C-terminal his-myc tag, SV40 polyA, and LoxP sequences. Amino acids mutated to alanine (underlined and in bold) in the Bright DNA binding domain are shown. B. DNA from five potential founder mice (denoted X, B, Y, Z and H) was PCR amplified for the Bright transgene. The lower band was an unrelated product confirming the sample DNA integrity. C. A representative experiment of transgene expression from DN Bright positive lines by RT-PCR of splenic RNA with and without reverse transcriptase (RT) is shown for transgenic line D (TG-D) and a negative control (CON). The smudge in the RT− lane was not consistent and was due to probe background.
DN Bright transgenic mice produce decreased levels of serum IgM
To determine whether DN Bright affects Ig production in vivo, as we observed previously in vitro (6), serum IgM, IgG2a, IgG2b, IgG3 and IgA antibody levels were measured in age-matched male mice from three of the transgenic lines (Figure 2). IgM levels were decreased by an average of 50% in the TG mice. IgM levels were decreased in each of the strains and in both heterozygous and homozygous mice. This contrasted with serum IgG2b, IgG3 and IgA levels that were not statistically different between controls and DN Bright mice, irrespective of the level of transgene expression. Unexpectedly, IgG2a levels increased approximately two-fold in homozygous DN Bright mice relative to controls for reasons that remain unclear. Because these mice were on an FVB background, we have been unable to measure IgG1 as previously documented for transgenic mice over-expressing Bright (30). These data clearly indicate that DN Bright expression decreases normal IgM production in vivo.
Figure 2. Serum IgM is reduced in DN Bright TG mice.
Sera obtained from non-immunized 8–12 week old male control (CON) and DN Bright homozygous transgenic (TG) mice were analyzed by ELISA for total IgM (A), IgG2a (B), IgG2b (C), IgG3 (D) and IgA (E). Points indicate values obtained for individual mice. Data are representative of 6 experiments using two to three transgenic lines. Means and standard error bars are indicated. Statistically significant differences are indicated by asterisks (p value = *0.0132, **0.019) as determined by the unpaired two-tailed Student’s t test.
Peripheral lymphocytes develop normally in DN Bright transgenic mice
DN Bright spleens appeared to be slightly smaller than non-transgenic age-matched controls upon visual examination, particularly in one of the three lines; however, total numbers of CD19+ B cells and CD3+ T cells were similar to control mice (Figure 3A). Likewise, splenic architecture appeared normal in tissue sections stained for metallophyllic macrophages and IgM-positive B cells (Figure 3B). Because preliminary examination of the DN Bright transgenic mice suggested that they were slightly smaller than wild type littermates, thymic analyses were routinely performed on each mouse to ensure that any effects we observed were not due to generalized stress. Total numbers of CD4 and CD8 positive T cells were similar in all transgenic and control mice (Figure 3C). Therefore, expression of DN Bright did not noticeably perturb peripheral lymphopoiesis.
Figure 3. Peripheral lymphocytes develop in DN Bright transgenic mice.
A. Representative flow cytometric analyses of whole spleen from a transgenic (TG-D) and control (CON) mouse indicate relative percentages of CD19+ B and CD3+ T cells. B. Spleen sections from non-immunized transgenic and control mice were stained with antibodies to IgM (red) and MOMA-1 (green) to identify IgM+ B cells and metallophyllic macrophages. Data are representative of three mice per group. C. Representative flow cytometric analyses of thymic tissue from transgenic and control mice indicate percentages of CD4 and CD8 single and double positive T cells.
DN Bright transgene expression occurs early in B cell development, but is not maintained in mature B cell subpopulations
Western blots of proteins from DN Bright transgenic tissues showed no DN Bright protein in any tissue of the 3 heterozygous and 2 homozygous lines examined, except in the spleen where it was barely detectable even in homozygous mice (Figure 4A and data not shown). In contrast, robust expression of wild type transgenic protein relative to endogenous Bright was evident in whole spleen cells from transgenic mice over-expressing wild type Bright (WT-TG) (Figure 4A, lane1). The identical CD19 promoter was used for both DN and WT Bright transgenic mice. CD19 expression begins in early B cell development, therefore bone marrow B cell subpopulations served as an initial starting point for our analysis of DN Bright expression. Total bone marrow was isolated and antibodies against B220, IgM, and CD43 were used to distinguish individual B cell subpopulations by flow cytometry. Figure 4B shows a typical analysis of pro-B cells (IgM−B220+CD43+), pre-B (CD43−B220loIgM−), immature (CD43−B220loIgM+), and recirculating (CD43−B220hiIgM+) B cell compartments. No significant differences were observed in relative percentages of bone marrow B lymphocytes between control and DN transgenic mice.
Figure 4. DN Bright protein expression is highest in immature B cell subpopulations.
A. DN Bright protein was not detected in immunoblots (IB) of whole thymus (Thy), heart (Hrt), lung (Lg), kidney (Kd), liver (Lv), or brain (Br) cells from transgenic TG-D mice and was barely detectable in spleen (Spl). Lane 1 contains 10 μg of wild type transgenic (WT-TG) Bright splenic protein demonstrating both transgenic (T) and endogenous (E) Bright. Relative actin levels are indicated below. Data are representative of two experiments from two transgenic lines. B. Representative flow cytometric analyses of bone marrow from DN Bright transgenic (TG-D) and control (CON) mice indicate relative percentages of pro-B (Pro), pre-B (Pre), immature (Imm) and recirculating (Rec) B cells. C. TG-D, CON and WT-TG bone marrow B cell subpopulations were isolated as in (B) and assessed for Bright proteins (left panel). WT-TG spleen (+) was used in lane 1. An independent experiment (right panels) shows corresponding actin levels for Imm and Rec B cells. Data are representative of three experiments. D. Representative flow cytometric analyses of transitional (T1, T2 and T3), marginal zone (MZ) and follicular (FOL) splenic B cells are shown for transgenic and control mice. E. Immunoblots of sorted splenic T1, T2, MZ and FOL B cells from DN Bright (TG-D and TG-B), control (CON) and WT Bright (WT-TG) transgenic lines are shown. Data are representative of 4 experiments. F. DN Bright and actin transcripts were amplified by semi-quantitative RT-PCR from T1 and FOL B cell RNA. Actin levels are quantified below.
To confirm that transgene expression occurred in early B cells, equal numbers of sorted bone marrow B cell subpopulations were analyzed for Bright expression by western blotting (Figure 4C). DN Bright was expressed at low levels in pro-B cells and levels increased (from 2-to 7-fold) in pre-B and immature B cells. Although endogenous Bright was detected at low levels in each of the four subpopulations analyzed (Figure 4C), DN Bright protein expression was not detectable in the mature, recirculating subpopulation of B cells that has migrated back to the bone marrow from the periphery. In contrast, transgenic Bright protein was clearly evident in all bone marrow B cell subsets from WT-TG mice, including the mature, recirculating population (30). These data indicate that DN Bright protein expression begins early in B cell development, but is not maintained at detectable levels at all stages of B cell differentiation.
Because robust levels of DN Bright were detected in immature B cells from bone marrow, but not in the more mature recirculating cells, we determined which splenic B cell subpopulations expressed DN Bright. Four-color analyses were used to identify the transitional T1, T2, T3, marginal zone (MZ), and follicular mature (FOL) B cell subpopulations (35). Figure 4D shows immature B cells (B220+CD93+) analyzed for IgM and CD23 expression to reveal T1, T2, and T3 transitional subpopulations, and the mature MZ and FO cells (B220+CD93−). Western blots using equivalent numbers of sorted cells indicated that only the T1 subpopulation most recently emigrated from the bone marrow exhibited appreciable levels of DN Bright protein. In some cases, very low levels of protein were detected in T2 transitional cells (Figure 4E and not shown). However, none of the heterozygous, homozygous or double transgenic lines examined expressed detectable DN Bright protein in MZ or FOL B cells. In contrast, sorted subpopulations from WT-TG transgenic mice showed robust transgenic protein expression in multiple lines in all splenic B cell subpopulations assessed (30) and (Figure 4E). These data indicate that the absence of detectable DN Bright protein in mature follicular B cells could not be overcome by increasing transgene copy number.
Our earlier findings showed that endogenous Bright was not normally transcribed in most mature splenic B cells, but was abundant in germinal center cells (23). Therefore, we asked if DN Bright transcript levels differed in immature cells that expressed transgenic protein versus mature cells that did not exhibit detectable Bright protein. We reasoned that cells expressing high levels of DN Bright might be eliminated at the T1 to T2 transition such that only cells that had eliminated transgenic protein expression could survive. Therefore, DN transgenic mice crossed ten generations onto the C57Bl/6 background were bred with mice of the same background expressing a BCL2 transgene which acts in an anti-apoptotic fashion to prolong survival of B cells (36). Although total B cell numbers were expanded in the double transgenic mice produced, DN Bright was not detectable in the follicular B cells from these mice by western analyses (not shown). DN Bright mRNA measured by semi-quantitative RT-PCR was present at similar levels in both T1 and mature follicular B cells in the original lines and in the BCL-2 transgenics (Figure 4F and not shown), suggesting that the mature B cells from the DN Bright transgenic mice should be capable of producing DN Bright protein. The reasons for down regulation of DN Bright protein in the mature B cells are unknown, but are likely to occur via a post-transcriptional mechanism.
DN Bright expression decreases total B cell numbers only slightly
Flow cytometric analyses of B cell subpopulations in DN transgenic mice (Figure 4) did not show statistically significant alterations in relative proportions of B cell subpopulations; however, total numbers of B cells were also determined in the DN Bright transgenic versus littermate control mice. Neither total cells nor numbers of B cells within individual bone marrow subpopulations were statistically different among control mice and heterozygous (Tg D+/−) or homozygous (D+/+) DN transgenic mice (Table 1). However, total splenic B cells in TG D+/− mice 7 to 9 weeks old were decreased slightly compared to littermate controls, and both mature follicular and marginal zone B cell subpopulations were significantly reduced compared to controls (Table 1). Homozygous mice (10 to 13 weeks old) did not exhibit differences in total B cell or individual B cell subpopulation numbers compared to age-matched controls (Table 1). To discern if younger mice exhibited greater decreases in peripheral B cells due to DN bright expression, 3 week old mice were examined. While WT transgenic mice of three weeks of age already exhibited increased B cell subpopulations (30), the younger DN transgenic splenic B cell numbers did not show age-dependent decreases due to DN Bright expression (not shown). Data were supported by analyses from two additional heterozygous founder lines, another homozygous line (N=13) and the double transgenic (N=12). Thus, while DN Bright expression did not block maturation of immature splenic B cells into mature B cells, as occurs in xid mice, DN Bright expression reduced total mature B cell numbers in spleens of young heterozygous transgenics.
Table 1.
Lymphocyte subpopulations in heterozygous and homozygous DN transgenics.
| CON (7–9wks) | TG D+/− (7–9 wks) | CON (10–13 wks) | TG D+/+ (10–13wks) | |
|---|---|---|---|---|
| Bone Marrow Cells (per femur) | ||||
| Total (×107) | 2.10 ± 0.34(8) | 2.05 ± 0.31(9) | 1.96 ± 0.22(13) | 1.79 ± 0.13(9) |
| B220+ (×106) | 1.7 ± 0.3 | 2.2 ± 0.2 | 2.8 ± 0.4 | 2.4 ± 0.3 |
| Pro B (×105) | 4.7 ± 0.8 | 5.3 ± 0.6 | 4.4 ± 0.4 | 4.4 ± 0.7 |
| Pre B (×105) | 8.8 ± 2.0 | 11.6 ± 1.9 | 10.3 ± 2.0 | 8.9 ± 1.7 |
| Immature (×105) | 3.1 ± 0.9 | 3.4 ± 0.6 | 3.8 ± 0.7 | 3.3 ± 0.6 |
| Recirculating (×105) | 8.0 ± 2.2 | 6.1 ± 1.3 | 6.7 ± 1.0 | 5.4 ± 1.2 |
| Spleen Cells | ||||
| Total (×107) | 19.7 ± 1.9(6) | 13.6 ± 1.2(9) | 15.7 ± 1.2(11) | 13.6 ± 1.5(11) |
| T1 (×106) | 3.8 ± 0.6 | 3.4 ± 0.5 | 6.0 ± 1.3 | 7.5 ± 1.7 |
| T2 (×106) | 5.2 ± 1.0 | 4.0 ± 0.3 | 9.2 ± 1.6 | 7.8 ± 1.4 |
| T3 (×106) | 3.5 ± 0.9 | 1.9 ± 0.4 | 3.5 ± 0.6 | 3.0 ± 0.5 |
| MZ (×106) | 11.5 ± 1.4 | 7.8 ± 1.1* | 6.6 ± 0.5 | 7.0 ± 0.6 |
| Follicular (×106) | 39.5 ± 4.4 | 28.0 ± 3.2** | 33.1 ± 3.7 | 32.0 ± 3.6 |
Male or female mice of ages indicated were analyzed. Numbers represent the mean ± SEM. Sample size is shown in parentheses after average total cell number for each tissue. Student’s T test,
p = 0.0325;
p = 0.0285.
CD40L and anti-IgM induce normal responses in DN Bright splenic lymphocytes
To determine if DN Bright transgenic B cells exhibited defects in B cell activation, B cells were purified from transgenic and control spleens and were stimulated with F(ab)’2 anti-IgM (not shown) or LPS. Relative levels of surface MHC II, CD69, CD86, CD80 and CD40 were assessed by flow cytometry. DN Bright splenic B cells expressed and upregulated each of these activation markers as effectively as non-transgenic control cells with either stimulus (not shown). In addition, proliferative responses to CD40L were tested. DN Bright TG and control lymphocytes responded similarly to CD40L as measured by 3H-thymidine incorporation (not shown); however, in all cases, cultured DN Bright cells that were not stimulated with CD40L incorporated more 3H-thymidine than unstimulated non-transgenic cells. These data suggest that DN Bright splenic B cells can be activated similarly to wild type B lymphocytes in vitro and indicate that the presence of DN Bright protein at earlier stages did not affect the functional capacity of these cells to respond to external stimuli.
Peritoneal B cells develop in DN Bright transgenic mice, but are functionally deficient
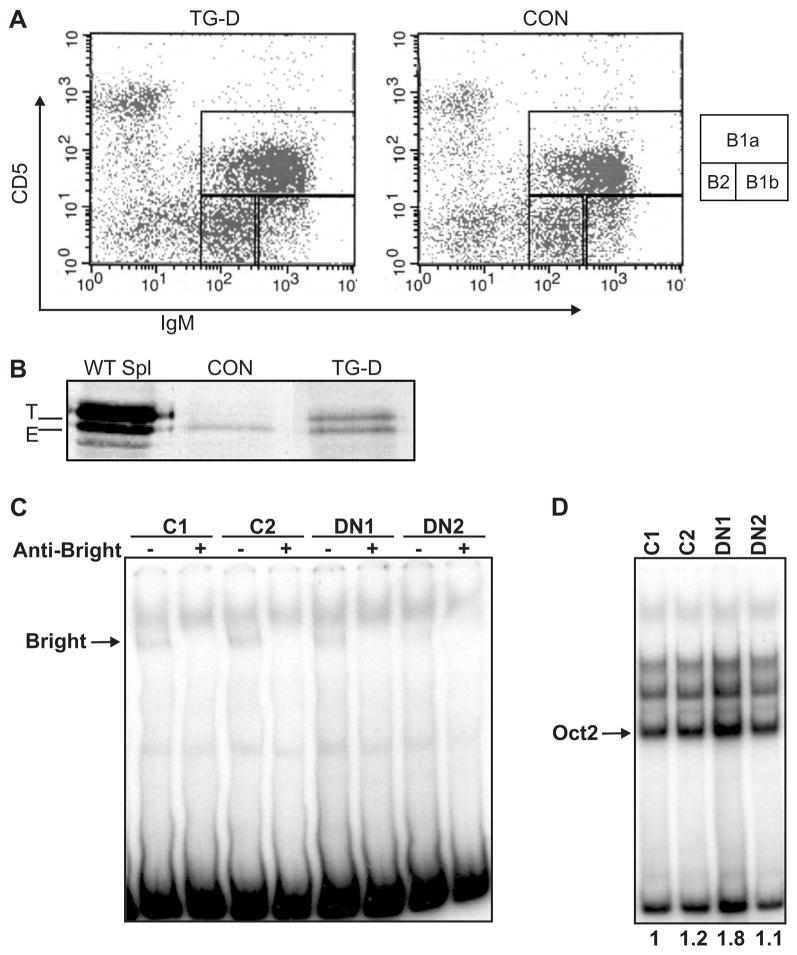
Half of the naturally protective serum IgM produced in mice is secreted by peritoneal B1 B cells (reviewed in (37)). It was therefore possible that reduced serum IgM in the DN Bright transgenic mice was due to defects in peritoneal B cells. Because Btk-deficient mice fail to develop B1a peritoneal B cells, but can produce B1b and B2 peritoneal B cells (38,39), we therefore hypothesized that DN Bright mice would be deficient in B1a cells. The DN Bright mice, however, not only produced both B1a and B1b cells (Figure 5A), but total numbers of peritoneal cavity B cells were significantly increased (p=0.0486, two-tailed Student’s T test) by approximately 30% in 11 TG mice relative to controls (2.6×106 cells versus 2.0×106 cells, respectively). Relative proportions of B1a, B1b and B2 B cells did not differ between transgenics and control mice (Figure 5A). Because mature follicular B cells did not express detectable DN Bright protein, we examined peritoneal cavity B cells for DN Bright protein expression. Total peritoneal cavity cells expressed DN Bright protein (Figure 5B) at levels similar to those seen in bone marrow immature B cells (Figure 4C). These data indicate that expression of DN Bright in peritoneal B cells does not inhibit B1 cell development.
Figure 5. DN Bright functions in peritoneal B1 cells.
A. Representative flow cytometric analyses of peritoneal cells from transgenic (TG-D) and control (CON) mice show similar proportions of B1a, B1b and B2 B cells. B. Western blot of wild type transgenic spleen (WT Spl) and peritoneal cavity cells from CON and DN Bright (TG-D) mice show transgenic (T) and endogenous (E) Bright protein. C. Peritoneal B cell extracts from two control mice (C1 and C2) and two DN Bright transgenic mice (DN1 and DN2) were assessed for Bright binding activity by EMSA with and without anti-Bright antibody. The Bright complex is indicated with an arrow. Data are representative of 5–7 control and transgenic mice assessed. D. The extracts used in (C) were compared for relative protein levels using an EMSA for octamer protein binding. Oct2 levels, as shown by the arrow, were quantified as indicated by the numbers below each lane.
To determine if expression of DN Bright was sufficient to inhibit DNA binding activity of the endogenous Bright protein, EMSAs were performed using nuclear extracts from total peritoneal B cells. Figure 5C shows binding of endogenous Bright to the prototypic Bright binding site (5) from two control mice. Bright binding was substantially inhibited in two thirds of the DN Bright mice tested as shown by sample DN2, but was readily detected in a third of the mice tested, as indicated in sample DN1. However, the DN1 sample also showed nearly two-fold more total protein as demonstrated by octamer binding activity (Figure 5D). Thus, there was clearly inhibition of DNA-binding activity in the peritoneal B cells.
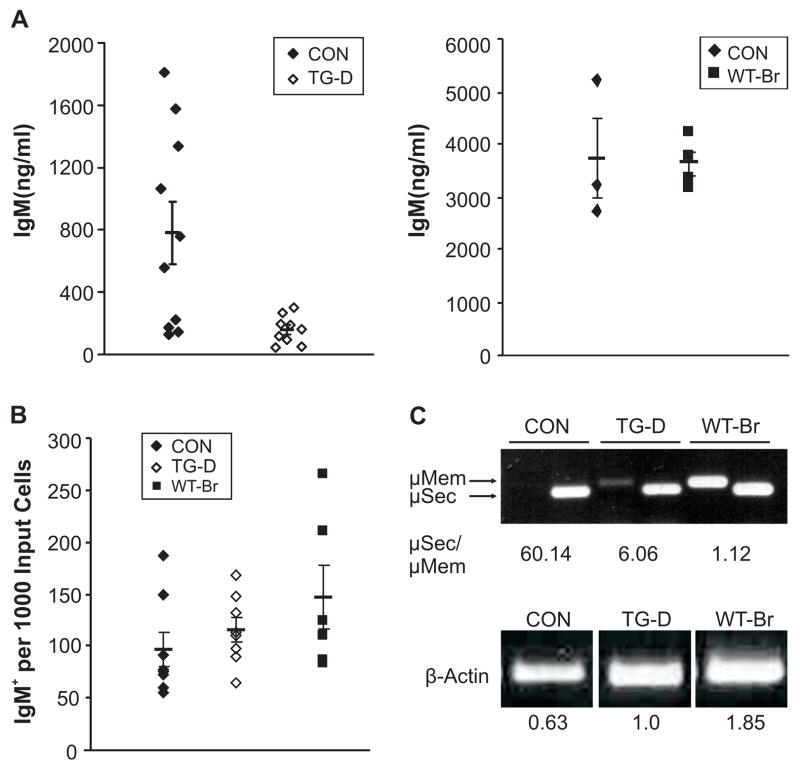
To determine if DN Bright peritoneal B cells were functionally normal, these cells were stimulated in vitro with LPS, and IgM production was measured by ELISA (Figure 6A). Although basal IgM production from unstimulated cells was similar in all mice, IgM production was consistently decreased from DN Bright transgenic peritoneal B cells relative to controls. WT-transgenic peritoneal B cells showed no statistical difference in secretion from normal non-transgenic controls (Figure 6A, right panel), suggesting the decrease in secretion in the DN transgenics is due to the DN protein. Although ELISPOT analyses of total peritoneal B cells indicated that total numbers of DN Bright IgM-producing cells were not decreased, but were slightly increased relative to controls, as were the numbers of WT-Bright IgM-producing cells (Figure 6B). Therefore, the decreased secretion of IgM from DN Bright peritoneal cells in Figure 6A cannot be explained by fewer IgM secreting cells. Semi-quantitative RT-PCR showed no differences in Blimp-1 or XBP mRNAs in the DN Bright transgenic B1 cells compared to control B1 cell RNA (not shown), suggesting that decreased IgM production was not the result of defects in regulation of these transcripts. However, examination of membrane versus secretory IgM by PCR demonstrated that while control B cells expressed 60-fold more secretory IgM than membrane IgM, DN transgenic B cells consistently expressed less secretory versus membrane message (μsec/μmem = 6.1) (Figure 6C). Peritoneal B cells from WT transgenic mice produced approximately equivalent amounts of secretory and membrane μ mRNA, but this may be consistent with a general increase in Ig transcription due to increased levels of Bright protein, particularly since IgM secretion was not inhibited in these cells (Figure 6A). Together, these data suggest that reduced serum IgM in the DN Bright mice is due to decreased IgM production from each cell, rather than decreased numbers of IgM-producing cells. Therefore, we conclude that Bright function in peritoneal B cells is important for maintaining normal IgM production.
Figure 6. DN Bright expression inhibits B1 cell function.
A. Peritoneal B cells from CON and TG-D mice (left panel) or WT-Br mice (right panel) were cultured with 20 μg/ml LPS for 72 hours and secreted IgM was measured by ELISA. Symbols represent individual cultures. Data shown are representative of 2–3 experiments performed. Means and standard error bars are indicated. p=0.0183, Student’s T test for TG-D compared to CON. B. ELISPOT analyses of LPS stimulated peritoneal B cells from CON, TG-D and WT-Br mice show numbers of IgM+ cells obtained per input cell. Standard error bars and means are shown. Data are representative of 3 experiments. C. Peritoneal B cell mRNAs from control (CON), DN and WT transgenic mice were stimulated with LPS as in (A) and assessed by PCR for relative levels of μ membrane (mem) versus secretory (sec) IgM. Ratios were quantified and are listed below. Actin levels for each sample are shown and quantified. Data are representative of samples from 4 to 7 mice each.
Anti-PC and responses to S. pneumoniae infection are attenuated in DN Bright TG mice
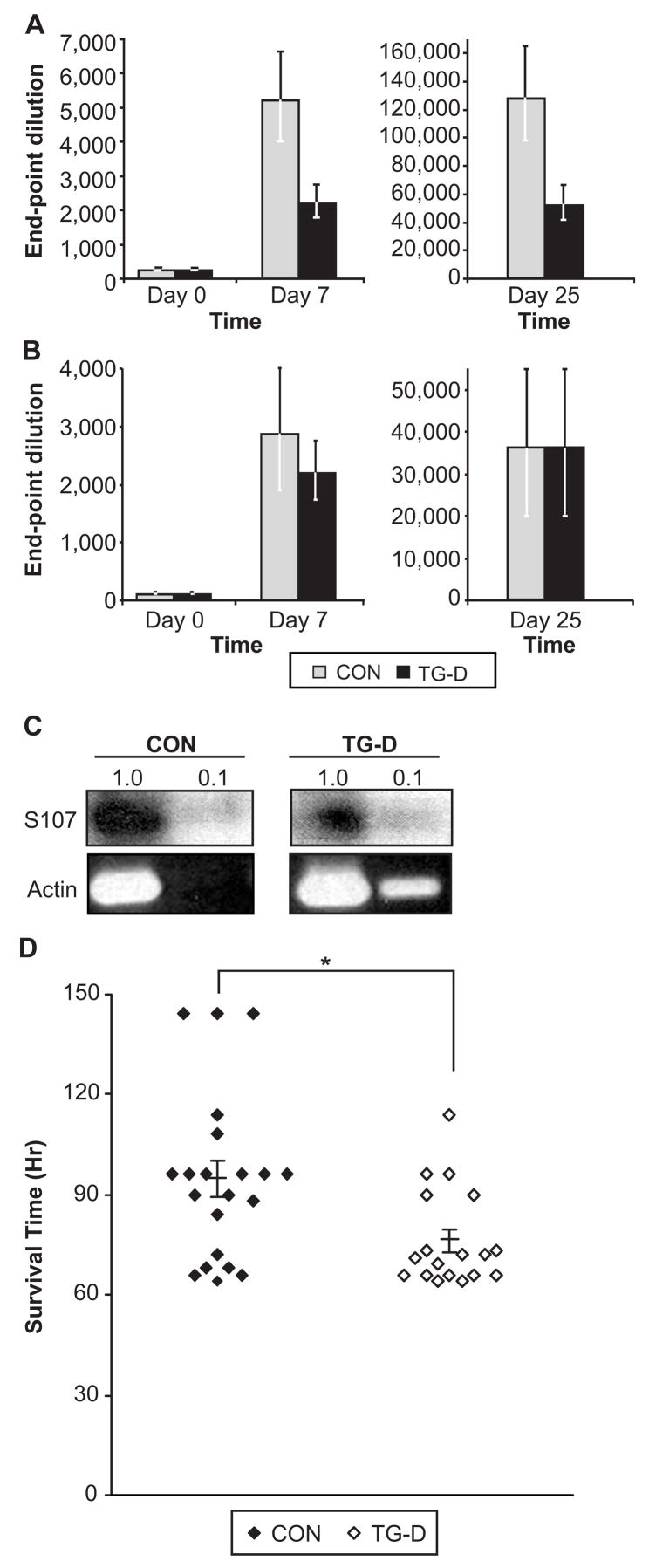
Antibody responses to PC have been shown to be predominantly of the IgM isotype and are primarily generated by B cells using the S107 V1 heavy chain gene (16). We showed that enhanced expression of V1 in vitro depends on Bright function and is inhibited by DN Bright (6). Furthermore, immune responses against PC were shown to be defective in xid mice, while responses to the hapten NP were normal (16). These results suggested the hypothesis that responses using the V1 gene would be inhibited in the DN Bright transgenic mice. In further support of this hypothesis, over-expression of WT-Bright caused more than a 10-fold increase in PC-specific IgM (30). To determine if anti-PC responses were impaired by DN Bright expression, animals were pre-bled and immunized (Day 0) with PC-KLH or with NP-KLH. Sera were collected at several time points after immunization and analyzed for PC- or NP-specific IgM by ELISA. DN Bright mice exhibited significant deficiencies in IgM responses to PC compared to non-transgenic controls at both days 7 and 25 (Figure 7A). In addition to IgM, sera from immunized mice were analyzed at day 25 for PC-specific total Ig to detect isotype switched antibodies. Homozygous DN Bright mice also exhibited significant decreases in PC-specific antibodies relative to those of control mice (Figure 7A). These data were confirmed as representing more than a 2-fold decrease at the μg level when compared to a standard IgM (BH8) anti-PC antibody (graciously provided by Dr. J. Kearney, Birmingham, AL). Heterozygous DN Bright transgenic mice exhibited intermediate responses to PC-KLH relative to control and homozygous DN Bright transgenic mice (not shown). In contrast, control and DN transgenic mice immunized with NP-KLH showed similar levels of NP-specific antibody at both days 7 (IgM) and 25 (Ig) (Figure 7B). Furthermore, mRNA expression from the V1 S107 gene in unimmunized mice, determine by RT-PCR, was decreased (from 33% of the control down to 3.6% for the lowest dilution) relative to levels observed in the same numbers of sorted cells in normal control mice (Figure 7C). These data suggest that DN Bright does not globally impair all immune responses, but selectively inhibits a subset of immune responses including that against PC.
Figure 7. DN Bright expression causes deficient anti-PC responses and increased susceptibility to S. pneumoniae.
Sera from five control (CON) and four transgenic (TG-D) preimmune and post-immunized 8–12 week old male mice were analyzed by ELISA for PC-specific IgM (A). The last positive dilution from triplicate samples was the defined endpoint. At day 7, p= 0.0268 by two-tailed Student’s t test. At day 25, p=0.0483 by one-tailed Student’s t test. B. Mice immunized with NP-KLH were analyzed similarly for anti-NP-specific antibodies. Standard error bars are indicated. C. RNA from CON and TG-D DN Bright follicular B cells was amplified for S107 V1 IgM and actin by RT-PCR. D. CON and TG-D mice were infected with S. pneumoniae and monitored for survival. Symbols represent the hours survived by one mouse. All samples fall within two standard deviations from the mean. Means and standard error bars are indicated. p= 0.019, Mann-Whitney analysis and 0.0079 by Student’s T test.
PC is a component of the cell wall of S. pneumoniae that has been shown to elicit protective responses against this organism (31). To confirm and extend the data showing that DN Bright TG mice were deficient in anti-PC responses, mice were infected intraperitoneally with S. pneumoniae. Mice infected with 10–100 bacterial CFU were monitored over a period of 4 to 7 days and times of death post infection were recorded (Figure 7D). The mean survival time for control mice was 95 hours, while DN Bright transgenic mice died at an average of 76 hours post-infection. These experiments indicate that, DN Bright transgenic mice are more susceptible to infection with S. pneumoniae than normal controls, but they are not as sensitive as Btk-deficient mice where death occurred at an average of 48 hours (31). We conclude that Bright contributes to normal immune responses against this important pathogen.
DISCUSSION
These data represent the first in vivo analyses resulting from Bright inhibition. Transgenic mice expressing DN Bright in B lineage cells developed all B cell subpopulations, suggesting that Bright function is not critical for B cell maturation once CD19+ B lineage commitment has occurred. However, DN Bright protein expression was not maintained beyond the immature B cell stage in the spleen, so no conclusions can be drawn regarding Bright’s role in mature or terminally differentiated splenic B cells. Nonetheless, serum IgM levels were decreased by DN Bright expression, and peritoneal B1 cells both expressed DN Bright protein and exhibited impaired function. Furthermore, our data indicate that Bright function is important for a subset of immune responses that uniquely utilize the S107 family V1gene for antibodies against PC and S. pneumoniae. These data suggest a mechanistic reason for observations made over twenty years ago which indicated that xid mice were specifically deficient in responses against PC and S. pneumoniae (16,31), and show that Bright contributes to these responses. Moreover, these data suggest that Bright function is critical for only a subset of immune responses.
Several similarities exist between DN Bright and Btk-deficient mice. Both lines exhibit reduced levels of serum IgM relative to controls, although IgG3 levels are only reduced in Btk-deficient mice. Normal IgG3 levels in DN Bright mice could be due to incomplete inhibition of Bright function in mature B cells. The majority of serum IgM is produced by peritoneal B cells, an important component of the innate immune response (37,40). In Btk-deficient and xid mice reduced IgM levels correlate with an absence of B1 peritoneal B cells (10-12). In contrast, DN Bright transgenic mice exhibited increased numbers of peritoneal cavity B cells with high expression of DN Bright protein. In other systems, increased cell numbers develop to compensate for functional defects. In support of that hypothesis, IgM secretion was impaired in DN Bright peritoneal B cells, and production of the secretory form of IgM was repressed relative to controls. Btk may also contribute to Bright function in B1 cells as indicated by experiments with mice expressing intermediate levels of Btk and suggesting that Btk plays a role in B1 cell maintenance once they develop (41). Because DN Bright may not have been expressed in the earliest B1 B cell progenitors, we cannot assess whether Bright is also important for B1 lineage development.
CD19−/− mice also display decreased levels of IgM (34). In this case, reduced IgM levels are thought to result from combined effects of decreased total B cell numbers in the bone marrow, spleen, and peritoneal cavity, and a decrease in proliferative responses to LPS, anti-IgM, and IL-4 (42). DN Bright splenic B cells responded normally to LPS, anti-IgM and CD40 ligand stimulation suggesting that activation was not impaired in DN Bright mice. These findings were consistent with the low levels of DN Bright protein exhibited by transgenic follicular B cells. Although endogenous Bright is also down-regulated in follicular B cells (23), WT-TG Bright mice consistently expressed detectable levels of transgenic protein from the identical CD19 promoter (30). DN Bright protein expression was down-regulated in follicular B cells of homozygous DN Bright mice that expressed the highest levels of DN Bright in immature B cells, suggesting that cell survival might depend upon active down-regulation of DN Bright protein. However, constitutive expression of BCL-2 in follicular B cells was not able to overcome the down regulation of DN Bright protein in double transgenic follicular B cells. Therefore, the reasons for post-translational down-regulation of DN Bright in these mice remain obscure.
Btk-deficient mice exhibit defects in the maturation of immature B cells to follicular B cells and this results in production of fewer total B cells (43,44). In these mice, immature transitional T1 B cells accumulate and comprise a higher percentage of B lymphocytes relative to normal controls, while follicular B cell numbers decrease and total marginal B cells remain equivalent to control numbers. Several lines of evidence support the idea that Bright functions in immature B cells. Both endogenous and DN Bright protein are abundantly expressed in those cells. DN Bright protein appears to be down-regulated specifically after the T1 transitional stage, implying that Bright function is important for T1 to T2 transition. Finally, over-expression of wild type Bright in transgenic mice led to a 3- to 5-fold increase in total numbers of T1 cells (30). Others have shown that transitional B cells in Btk-deficient mice do not proliferate normally in vitro in response to anti-IgM signals (43,44), and both proliferation and cell cycle abnormalities have been observed previously in xid mice (15,45). Roles for human Bright in cell cycle regulation have been suggested (46,47). Further experiments will be required to elucidate how Bright functions in immature B cells.
Another similarity between xid and DN Bright transgenic mice is the selective impairment of responses to specific subsets of antigens. Data showing that xid mice do not produce T15 antibodies after immunization with PC-KLH, while normal female littermates generated robust T15 idiotype responses (16) established a stringent requirement for Btk in anti-PC responses. DN Bright transgenic mice also showed significant decreases in PC-specific Ig relative to controls, suggesting that Bright and Btk function in the same molecular pathway in this response. Likewise, both Btk-deficient (31), and DN Bright transgenic mice were more susceptible than control mice to death after infection with the PC-containing bacteria, S. pneumoniae. Although xid B cells can rearrange the S107 V1 locus normally, the resulting B cells do not produce normal levels of anti-PC antibodies (48). In our experiments, B cells from non-immunized DN Bright transgenic mice exhibited reduced mRNA transcripts using the S107 V1 heavy chain gene relative to normal controls, consistent with our in vitro data indicating the importance of Bright for transcription of the V1 gene. It is not clear in either xid or DN Bright transgenic B cells whether reductions in V1 expression are due to reduced numbers of V1 expressing cells, or to impaired Ig production from this specific B cell population. Nonetheless, these data indicate that Bright and Btk are both critical for responses involving the S107 V1 gene.
One interesting possibility raised by these data is that all Ig heavy chain promoters are not regulated identically. Responses to antigens other than PC, such as NP-KLH, were not impaired in DN Bright mice or in xid mice (49). While DNA binding motifs for Bright exist within the 5′ flanking regions of the V1 gene, other V region genes are not associated with Bright binding sites (50,51). Precedence exists for differential requirements for the transcription factor OcaB in regulation of subsets of light chain genes (52), but differential regulation of heavy chain genes in vivo has not been suggested. Alternatively, other data suggest that B cells expressing subsets of heavy chain genes are uniquely targeted toward distinct B cell subpopulations (53). Existence of independent B lineages that specifically express the V1 gene and require Bright might explain our observations. A small subpopulation (4–10%) of immature bone marrow B cells was identified which preferentially expressed the S107 gene in Balb/c mice (54). However, it is not clear that these cells represent an independent lineage of B cells. Elegant studies using xid mice expressing an anti-PC heavy chain transgene resulted in severely impaired peripheral B cell development, suggesting that the PC-specific B cells were deleted or failed to exit the bone marrow properly (49), while TNP-specific B cells developed normally on the xid background. Our data suggest that the defects observed in those mice could have resulted from impaired Btk/Bright dependent regulation of the B cell receptor. It is not clear whether Bright functions solely through regulation of the Ig locus or through other gene targets, which is certainly the case in non-B cells. Bright is expressed in multiple embryonic tissues in the mouse (23), and regulates non-B cell pathways in Xenopus and Drosophila (25,26). Further experiments will be required to distinguish among these possibilities.
DN Bright associates with Btk in vitro, but does not bind DNA (27). Therefore, it was a formal possibility that high levels of DN Bright protein could simply sequester Btk and inhibit its function, producing another form of Btk deficiency in the DN Bright transgenic mice. This cannot explain the phenotype of the DN Bright transgenic mice because it differed substantially from that of xid mice. Preliminary data from Bright knockout mice also suggest that they differ phenotypically from xid mice (Webb and Tucker unpublished results). Moreover, data from mice expressing even higher levels of wild type Bright than were observed in the DN Bright transgenic mice were autoimmune rather than immunodeficient (30), indicating that DN Bright did not exert its effect simply by sequestering Btk. It will ultimately be important to determine if Ig secretion and B cell differentiation of conventional B2 cells is affected by Bright inhibition as it is in B1 cells. However, those studies await additional model systems.
Acknowledgments
This work was supported by NIH AI44215.
The authors thank K. Chumbley for technical assistance, Drs. J. Yother and D. Briles, and J. Kearney for advice and reagents, Ms. M. Flynn for manuscript preparation and Drs. J. Knight and P. Wilson for critically reading the manuscript.
Footnotes
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the United States National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
References
- 1.Webb CF, Das C, Coffman RL, Tucker PW. Induction of immunoglobulin μ mRNA in a B cell transfectant stimulated with interleukin-5 and a T-dependent antigen. J Immunol. 1989;143:3934–3939. [PubMed] [Google Scholar]
- 2.Herrscher RF, Kaplan MH, Lelsz DL, Das C, Scheuermann R, Tucker PW. The immunoglobulin heavy-chain matrix-associating regions are bound by Bright: a B cell-specific trans-activator that describes a new DNA-binding protein family. Genes Dev. 1995;9:3067–3082. doi: 10.1101/gad.9.24.3067. [DOI] [PubMed] [Google Scholar]
- 3.Wilsker D, Patsialou A, Dallas PB, Moran E. ARID proteins: a diverse family of DNA binding proteins implicated in the control of cell growth, differentiation, and development. Cell Growth Differ. 2002;13:95–106. [PubMed] [Google Scholar]
- 4.Kortschak RD, Tucker PW, Saint R. ARID proteins come in from the desert. Trends Biochem Sci. 2000;25:294–299. doi: 10.1016/s0968-0004(00)01597-8. [DOI] [PubMed] [Google Scholar]
- 5.Webb CF, Das C, Eaton S, Calame K, Tucker PW. Novel protein-DNA interactions associated with increased immunoglobulin transcription in response to antigen plus interleukin-5. Mol Cell Biol. 1991;11:5197–5205. doi: 10.1128/mcb.11.10.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajaiya J, Hatfield M, Nixon JC, Rawlings DJ, Webb CF. Bruton’s tyrosine kinase regulates immunoglobulin promoter activation in association with the transcription factor Bright. Mol Cell Biol. 2005;25:2073–2084. doi: 10.1128/MCB.25.6.2073-2084.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang WY, Desiderio S. BAP-135, a target for Bruton’s tyrosine kinase in response to B cell receptor engagement. Proc Natl Acad Sci USA. 1997;94:604–609. doi: 10.1073/pnas.94.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novina CD, Kumar S, Bajpai U, Cheriyath V, Zhang KM, Pillai S, Wortis HH, Roy AL. Regulation of nuclear localization and transcriptional activity of TFII-I by Bruton’s tyrosine kinase. Mol Cell Biol. 1999;19:5014–5024. doi: 10.1128/mcb.19.7.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajaiya J, Nixon JC, Ayers N, Desgranges ZP, Roy AL, Webb CF. Induction of immunoglobulin heavy chain transcription through the transcription factor Bright requires TFII-I. Mol Cell Biol. 2006;26:4758–4768. doi: 10.1128/MCB.02009-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rawlings DJ, Saffran DC, Tsukada S, Largaespada DA, Grimaldi JC, Cohen L, Mohr RN, Bazan JF, Howard M, Copeland NG, Jenkins NA, Witte ON. Mutation of the unique region of Bruton’s tyrosine kinase in immunodeficient XID mice. Science. 1993;261:358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 11.Thomas JD, Sideras P, Smith CIE, Vorechovsky I, Chapman V, Paul WE. Colocalization of X-linked agammaglobulinemia and X-linked immunodeficiency genes. Science. 1993;261:355–358. doi: 10.1126/science.8332900. [DOI] [PubMed] [Google Scholar]
- 12.Khan WN, Alt FW, Gerstein RM, Malynn BA, Larsson I, Rathbun G, Davidson L, Muller S, Kantor AB, Herzenberg LA, Rosen FS, Sideras P. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283–299. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 13.Klaus GGB, Holman M, Johnson-Léger C, Elgueta-Karstegl C, Atkins C. A re-evaluation of the effects of X-linked immunodeficiency (xid) mutation on B cell differentiation and function in the mouse. Eur J Immunol. 1997;27:2749–2756. doi: 10.1002/eji.1830271102. [DOI] [PubMed] [Google Scholar]
- 14.Fluckiger AC, Li ZM, Kato RM, Wahl MI, Ochs HD, Longnecker R, Kinet JP, Witte ON, Scharenberg AM, Rawlings DJ. Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J. 1998;17:1973–1985. doi: 10.1093/emboj/17.7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forssell J, Nilsson A, Sideras P. Bruton’s tyrosine-kinase-deficient murine B lymphocytes fail to enter S phase when stimulated with anti-immunoglobulin plus interleukin-4. Scand J Immunol. 1999;49:155–161. doi: 10.1046/j.1365-3083.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- 16.Brown M, Stenzel-poore M, Rittenberg M. Immunologic memory to phosphocholine VII. Lack of T15 V1 gene utilization in Xid anti-PC hybridomas. J Immunol. 1985;135:3558–3563. [PubMed] [Google Scholar]
- 17.Fruman DA, Satterthwaite AB, Witte ON. Xid-like phenotypes: a B cell signalosome takes shape. Immunity. 2000;13:1–3. doi: 10.1016/s1074-7613(00)00002-9. [DOI] [PubMed] [Google Scholar]
- 18.Jefferies CA, O’Neill LA. Bruton’s tyrosine kinase (Btk)-the critical tyrosine kinase in LPS signalling? Immunol Lett. 2004;92:15–22. doi: 10.1016/j.imlet.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Conley ME, Broides A, Hernandez-Trujillo V, Howard V, Kanegane H, Miyawaki T, Shurtleff SA. Genetic analysis of patients with defects in early B-cell development. Immunol Rev. 2005;203:216–234. doi: 10.1111/j.0105-2896.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 20.Hinman RM, Bushanam JN, Nichols WA, Satterthwaite AB. B cell receptor signaling down-regulates forkhead box transcription factor class O 1 mRNA expression via phosphatidylinositol 3-kinase and Bruton’s tyrosine kinase. J Immunol. 2007;178:740–747. doi: 10.4049/jimmunol.178.2.740. [DOI] [PubMed] [Google Scholar]
- 21.Hirano M, Kikuchi Y, Nisitani S, Yamaguchi A, Satoh A, Ito T, Iba H, Takatsu K. Bruton’s tyrosine kinase (Btk) enhances transcriptional co-activation activity of BAM11, a Btk-associated molecule of a subunit of SWI/SNF complexes. Int Immunol. 2004;16:747–757. doi: 10.1093/intimm/dxh076. [DOI] [PubMed] [Google Scholar]
- 22.Webb CF, Yamashita Y, Ayers N, Evetts S, Paulin Y, Conley ME, Smith EA. The transcription factor Bright associates with Bruton’s tyrosine kinase, the defective protein in immunodeficiency disease. J Immunol. 2000;165:6956–6965. doi: 10.4049/jimmunol.165.12.6956. [DOI] [PubMed] [Google Scholar]
- 23.Webb CF, Smith EA, Medina KL, Buchanan KL, Smithson G, Dou S. Expression of Bright at two distinct stages of B lymphocyte development. J Immunol. 1998;160:4747–4754. [PubMed] [Google Scholar]
- 24.Gregory SL, Kortschak RD, Kalionis B, Saint R. Characterization of the dead ringer gene identifies a novel, highly conserved family of sequence-specific DNA-binding proteins. Mol Cell Biol. 1996;16:792–799. doi: 10.1128/mcb.16.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shandala T, Kortschak RD, Gregory S, Saint R. The Drosophila dead ringer gene is required for early embryonic patterning through regulation of argos and buttonhead expression. Development. 1999;126:4341–4349. doi: 10.1242/dev.126.19.4341. [DOI] [PubMed] [Google Scholar]
- 26.Callery EM, Smith JC, Thomsen GH. The ARID domain protein dril1 is necessary for TGFbeta signaling in Xenopus embryos. Dev Biol. 2005;278:542–559. doi: 10.1016/j.ydbio.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Nixon JC, Rajaiya J, Webb CF. Mutations in the DNA-binding domain of the transcription factor Bright act as dominant negative proteins and interfere with immunoglobulin transactivation. J Biol Chem. 2004;279:52465–52472. doi: 10.1074/jbc.M403028200. [DOI] [PubMed] [Google Scholar]
- 28.Sauer B. Inducible gene targeting in mice using the Cre/lox system. Methods. 1998;14:381–392. doi: 10.1006/meth.1998.0593. [DOI] [PubMed] [Google Scholar]
- 29.Maas A, Dingjan GM, Grosveld F, Hendriks RW. Early arrest in B cell development in transgenic mice that express the E41K Bruton’s tyrosine kinase mutant under the control of the CD19 promoter region. J Immunol. 1999;162:6526–6533. [PubMed] [Google Scholar]
- 30.Shankar M, Nixon JC, Maier S, Workman J, Farris AD, Webb CF. Anti-nuclear antibody production and autoimmunity in transgenic mice that over-express the transcription factor Bright. J Immunol. 2007;178:2996–3006. doi: 10.4049/jimmunol.178.5.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Briles DE, Nahm M, Schroer K, Davie J, Baker P, Kearney J, Barletta R. Antiphosphocholine antibodies found in normal mouse serum are protectie against intravenous infection with type 3 Steptococcus pneunmoiae. J Exp Med. 1981;153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–620. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 33.Gunn KE, Brewer JW. Evidence that marginal zone B cells possess an enhanced secretory apparatus and exhibit superior secretory activity. J Immunol. 2006;177:3791–3798. doi: 10.4049/jimmunol.177.6.3791. [DOI] [PubMed] [Google Scholar]
- 34.Sato S, Steeber DA, Jansen PJ, Tedder TF. CD19 expression levels regulate B lymphocyte development: human CD19 restores normal function in mice lacking endogenous CD19. J Immunol. 1997;158:4662–4669. [PubMed] [Google Scholar]
- 35.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 36.McDonnell TJ, Deane N, Platt FM, Nunez G, Jaeger V, McKearn JP, Korsmeyer SJ. Bcl-2 transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1998;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 37.Hardy RR. B-1 B cells: development, selection, natural autoantibody and leukemia. Curr Opin Immunol. 2006;18:1–9. doi: 10.1016/j.coi.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Riggs J, Howell K, Matechin B, Matlack R, Pennello A, Chiasson R. X-chromosome-linked immune-deficient mice have B-1b cells. Immunology. 2003;108:440–451. doi: 10.1046/j.1365-2567.2003.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knoops L, Louahed J, Renauld JC. IL-9-induced expansion of B-1b cells restores numbers but not function of B-1 lymphocytes in xid mice. J Immunol. 2004;172:6101–6106. doi: 10.4049/jimmunol.172.10.6101. [DOI] [PubMed] [Google Scholar]
- 40.Martin F, Kearney JF. B1 cells: similarities and differences with other B cell subsets. Curr Opin Immunol. 2001;13:195–201. doi: 10.1016/s0952-7915(00)00204-1. [DOI] [PubMed] [Google Scholar]
- 41.Contreras CM, Halcomb KE, Randle L, Hinman RM, Gutierrez T, Clarke SH, Satterthwaite AB. Btk regulates multiple stages in the development and survival of B-1 cells. Mol Immunol. 2007;44:2719–2728. doi: 10.1016/j.molimm.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engel P, Zhou LJ, Ord DC, Sato S, Koller B, Tedder TF. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 1995;3:39–50. doi: 10.1016/1074-7613(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 43.Petro JB, Gerstein RM, Lowe J, Carter RS, Shinners N, Khan WN. Transitional type 1 and 2 B lymphocyte subsets are differentially responsive to antigen receptor signaling. J Biol Chem. 2002;277:48009–48019. doi: 10.1074/jbc.M200305200. [DOI] [PubMed] [Google Scholar]
- 44.Su TT, Rawlings DJ. Transitional B lymphocyte subsets operate as distinct checkpoints in murine splenic B cell development. J Immunol. 2002;168:2101–2110. doi: 10.4049/jimmunol.168.5.2101. [DOI] [PubMed] [Google Scholar]
- 45.Brorson K, Brunswick M, Ezhevsky S, Wei DG, Berg R, Scott D, Stein KE. XID affects events leading to B cell cycle entry. J Immunol. 1997;159:135–143. [PubMed] [Google Scholar]
- 46.Fukuyo Y, Mogi K, Tsunematsu Y, Nakajima T. E2FBP1/hDril1 modulates cell growth through downregulation of promyelocytic leukemia bodies. Cell Death Differ. 2004;11:747–759. doi: 10.1038/sj.cdd.4401412. [DOI] [PubMed] [Google Scholar]
- 47.Ma KW, Araki K, Ichwan SJA, Suganuma T, Tamamori-Adachi M, Ikeda MA. E2FBP1/DRIL1, an AT-rich interaction domain-family transcription factor, is regulated by p53. Cell Growth Differ. 2003;1:438–444. [PubMed] [Google Scholar]
- 48.Perlmutter RM, Crews ST, Douglas R, Sorensen G, Johnson N, Nivera N, Gearhart PJ, Hood L. The generation of diversity in phosphorylcholine-binding antibodies. Adv Immunol. 1984;35:1–37. doi: 10.1016/s0065-2776(08)60572-6. [DOI] [PubMed] [Google Scholar]
- 49.Kenny JJ, Stall AM, Sieckmann DG, Lamers MC, Finkelman FD, Finch L, Longo DL. Receptor-mediated elimination of phosphocholine-specific B cells in X-linked immune-deficient mice. J Immunol. 1991;146:2568–2577. [PubMed] [Google Scholar]
- 50.Buchanan KL, Smith EA, Dou S, Corcoran LM, Webb CF. Family-specific differences in transcription efficiency of Ig heavy-chain promoters. J Immunol. 1997;159:1247–1254. [PubMed] [Google Scholar]
- 51.Johnston CM, Wood AL, Bolland DJ, Corcoran AE. Complete sequence assembly and characterization of the C57BL/6 mouse Ig heavy chain V region. The Journal of Immunology. 2006;176:4221–4234. doi: 10.4049/jimmunol.176.7.4221. [DOI] [PubMed] [Google Scholar]
- 52.Casellas R, Jankovic M, Meyer G, Gazumyan A, Luo Y, Roeder RG, Nussenzweig MC. OcaB is required for normal transcription and V(D)J recombination of a subset of immunoglobulin kappa genes. Cell. 2002;110:575–585. doi: 10.1016/s0092-8674(02)00911-x. [DOI] [PubMed] [Google Scholar]
- 53.Martin F, Chen XJ, Kearney JF. Development of VH81X transgene-bearing B cells in fetus and adult: Sites for expansion and deletion in conventional and CD5/B1 cells. International Immunology. 1997;9:493–505. doi: 10.1093/intimm/9.4.493. [DOI] [PubMed] [Google Scholar]
- 54.Wilson EL, Sherwood EM, King AM, Riley RL. A phenotypically distinct subset of immature B cells exhibits partial activation, increased survival, and preferential expression of VhS107. Eur J Immunol. 2003;33:3398–3408. doi: 10.1002/eji.200324324. [DOI] [PubMed] [Google Scholar]