Abstract
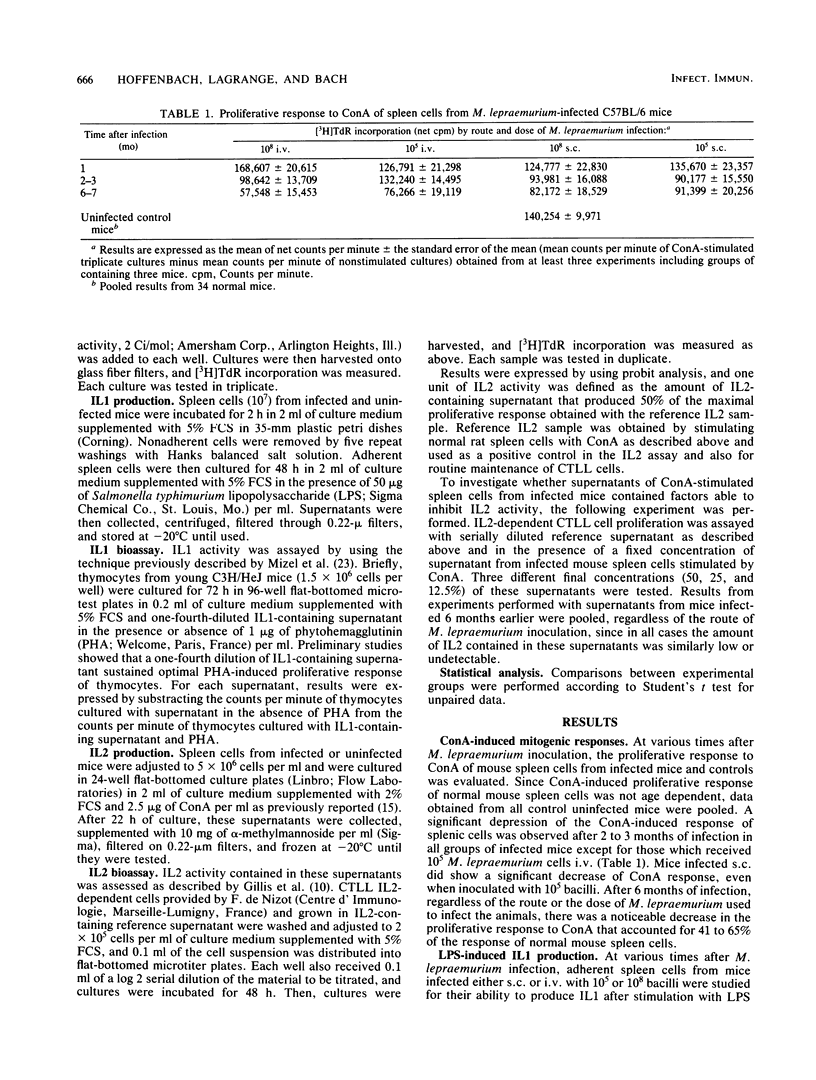
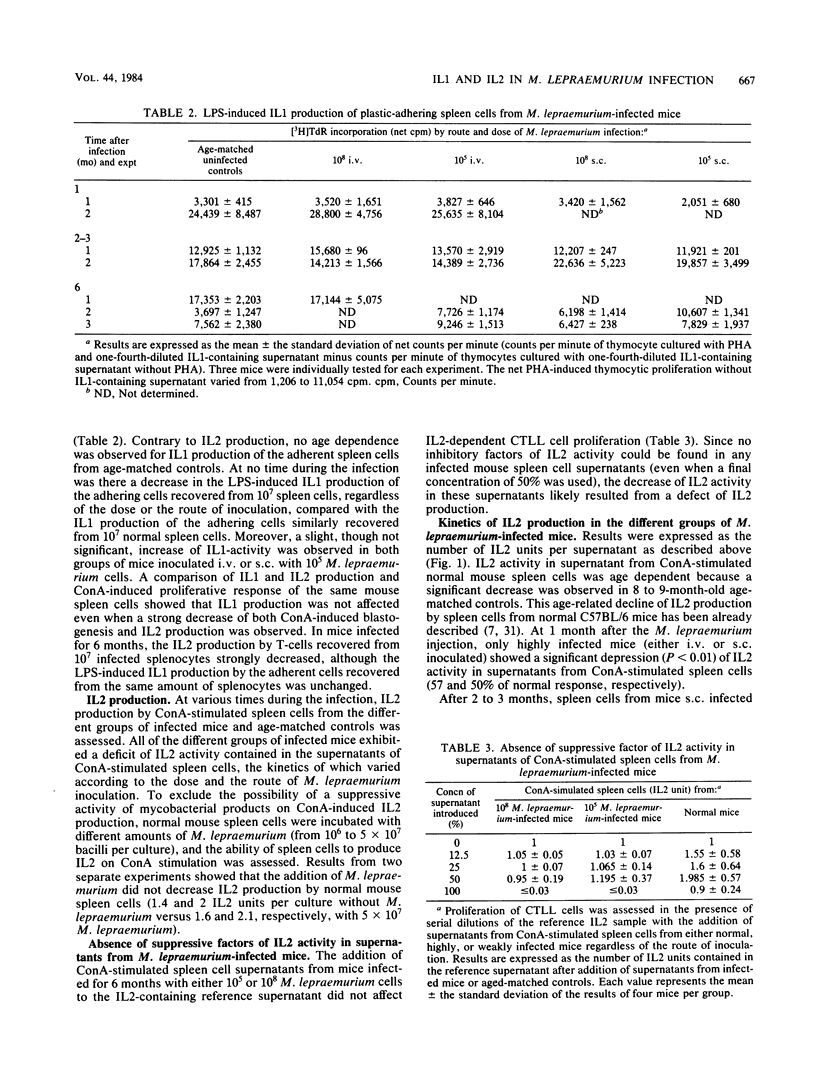
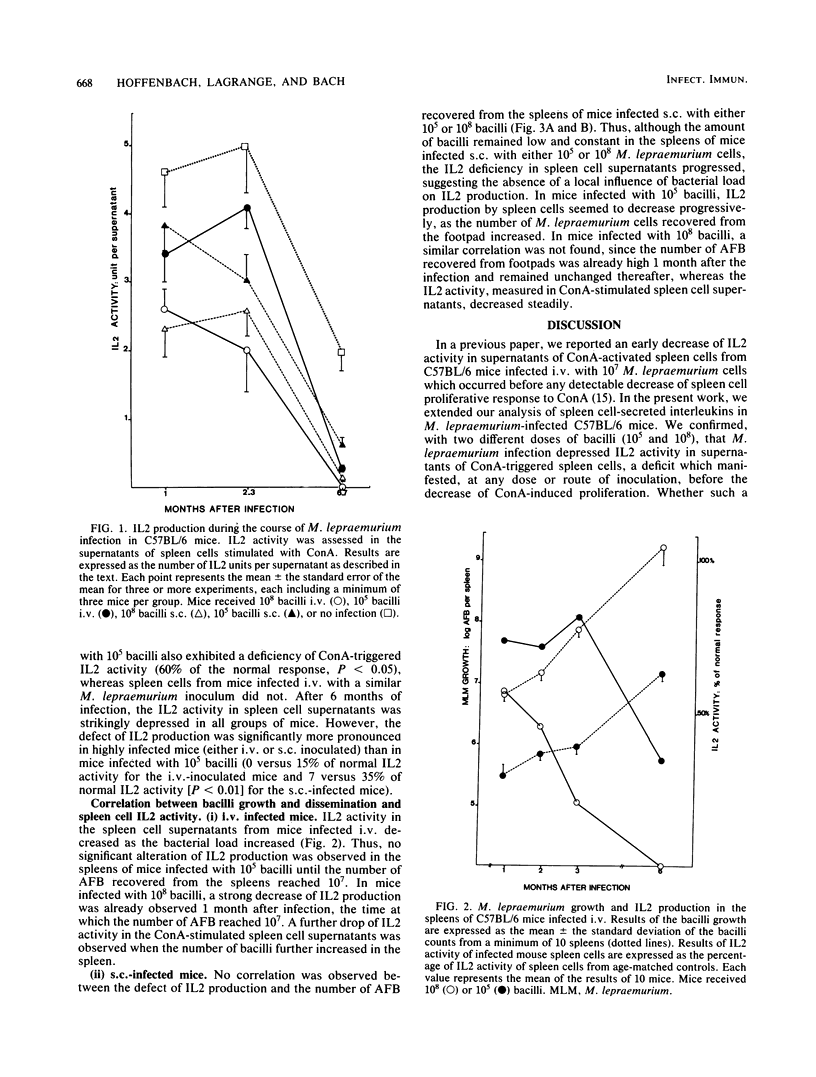
Groups of C57BL/6 mice were infected either intravenously or subcutaneously with 10(5) or 10(8) Mycobacterium lepraemurium cells, and the ability of their splenic macrophages and T-cells to produce, respectively, interleukin 1 on lipopolysaccharide stimulation and interleukin 2 on concanavalin A stimulation was assessed during the course of infection. In all groups of infected mice, interleukin 1 production remained unaffected during the entire observation period, whereas interleukin 2 activity decreased as the infection progressed. Heavily infected mice (10(8) M. lepraemurium cells) showed an earlier and stronger deficiency interleukin 2 production by concanavalin A-stimulated spleen cells than did mice infected with a lower dose (10(5) bacilli), without detectable influence by the route of inoculation. In mice receiving 10(5) bacilli, minor differences were seen according to the route of infection, with a slight delay in interleukin 2 decrease in mice injected intravenously. In subcutaneously inoculated mice, the failure of spleen cells to produce interleukin 2 after concanavalin A stimulation did not correlate with the number of bacilli developing in the spleen, suggesting the existence of suppressor mechanisms acting at a distance from the site of inoculation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adu H. O., Curtis J., Turk J. L. The resistance of C57BL/6 mice to subcutaneous infection with Mycobacterium lepraemurium is dependent on both T cells and other cells of bone marrow origin. Cell Immunol. 1983 Jun;78(2):249–256. doi: 10.1016/0008-8749(83)90279-4. [DOI] [PubMed] [Google Scholar]
- Alexander J. Adoptive transfer of immunity and suppression by cells and serum in early Mycobacterium lepraemurium infections of mice. Parasite Immunol. 1979 Summer;1(2):159–166. doi: 10.1111/j.1365-3024.1979.tb00703.x. [DOI] [PubMed] [Google Scholar]
- Bullock W. E., Carlson E. M., Gershon R. K. The evolution of immunosuppressive cell populations in experimental mycobacterial infection. J Immunol. 1978 May;120(5):1709–1716. [PubMed] [Google Scholar]
- Bullock W. E., Jr Perturbation of lymphocyte circulation in experimental murine leprosy. I. Description of the defect. J Immunol. 1976 Oct;117(4):1164–1170. [PubMed] [Google Scholar]
- Closs O. Experimental murine leprosy: growth of Mycobacterium lepraemurium in C3H and C57/BL mice after footpad inoculation. Infect Immun. 1975 Sep;12(3):480–489. doi: 10.1128/iai.12.3.480-489.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs O. Experimental murine leprosy: induction of immunity and immune paralysis to Mycobacterium lepraemurium in C57BL mice. Infect Immun. 1975 Oct;12(4):706–713. doi: 10.1128/iai.12.4.706-713.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauphinée M. J., Kipper S. B., Wofsy D., Talal N. Interleukin 2 deficiency is a common feature of autoimmune mice. J Immunol. 1981 Dec;127(6):2483–2487. [PubMed] [Google Scholar]
- Farrar J. J., Benjamin W. R., Hilfiker M. L., Howard M., Farrar W. L., Fuller-Farrar J. The biochemistry, biology, and role of interleukin 2 in the induction of cytotoxic T cell and antibody-forming B cell responses. Immunol Rev. 1982;63:129–166. doi: 10.1111/j.1600-065x.1982.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Gery I., Gershon R. K., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972 Jul 1;136(1):128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Godal T. Immunological aspects of leprosy--present status. Prog Allergy. 1978;25:211–242. [PubMed] [Google Scholar]
- Gullberg M., Larsson E. L. Studies on induction and effector functions of concanavalin A-induced suppressor cells that limit TCGF production. J Immunol. 1982 Feb;128(2):746–750. [PubMed] [Google Scholar]
- Haregewoin A., Godal T., Mustafa A. S., Belehu A., Yemaneberhan T. T-cell conditioned media reverse T-cell unresponsiveness in lepromatous leprosy. Nature. 1983 May 26;303(5915):342–344. doi: 10.1038/303342a0. [DOI] [PubMed] [Google Scholar]
- Harel-Bellan A., Joskowicz M., Fradelizi D., Eisen H. Modification of T-cell proliferation and interleukin 2 production in mice infected with Trypanosoma cruzi. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3466–3469. doi: 10.1073/pnas.80.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffenbach A., Lagrange P. H., Bach M. A. Deficit of interleukin 2 production associated with impaired T-cell proliferative responses in Mycobacterium lepraemurium infection. Infect Immun. 1983 Jan;39(1):109–116. doi: 10.1128/iai.39.1.109-116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffenbach A., Lagrange P. H., Bach M. A. Surface Lyt phenotype of suppressor cells in C57BL/6 mice infected with Mycobacterium lepraemurium. Clin Exp Immunol. 1983 Oct;54(1):151–157. [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y., Matsuoka M., Kawatsu K., Homma J. Y., Abe C. Susceptibility to murine leprosy bacilli of nude mice. Jpn J Exp Med. 1976 Jun;46(3):167–180. [PubMed] [Google Scholar]
- Lagrange P. H., Hurtrel B. Local immune response to Mycobacterium lepraemurium in C3H and C57Bl/6 mice. Clin Exp Immunol. 1979 Dec;38(3):461–474. [PMC free article] [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Influence of dose and route of antigen injection on the immunological induction of T cells. J Exp Med. 1974 Mar 1;139(3):528–542. doi: 10.1084/jem.139.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J., Mackaness G. B. Suppression of immunity to Mycobacterium lepraemurium infection. Infect Immun. 1977 Nov;18(2):363–369. doi: 10.1128/iai.18.2.363-369.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J., Patel P. J., Poulter L. W., Mackaness G. B. Induction of cell-mediated immunity to Mycobacterium lepraemurium in susceptible mice. Infect Immun. 1977 Dec;18(3):654–659. doi: 10.1128/iai.18.3.654-659.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkovsky M., Asherson G. L., Stockinger B., Watkins M. C. Nonspecific inhibitor released by T acceptor cells reduces the production of interleukin-2. Nature. 1982 Dec 16;300(5893):652–655. doi: 10.1038/300652a0. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosenstreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by the macrophage cell line, P388D1. I. Enhancement of LAF production by activated T lymphocytes. J Immunol. 1978 May;120(5):1497–1503. [PubMed] [Google Scholar]
- Patel P. J. Antibacterial resistance in mice infected with Mycobacterium lepraemurium. Clin Exp Immunol. 1981 Sep;45(3):654–661. [PMC free article] [PubMed] [Google Scholar]
- Preston P. M. Macrophages and protective immunity in Mycobacterium lepraemurium infections in a 'resistant' (C57Bl) and a 'susceptible' (BALB/c) mouse strain. Clin Exp Immunol. 1982 Feb;47(2):243–252. [PMC free article] [PubMed] [Google Scholar]
- Ptak W., Gaugas J. M., Rees R. J., Allison A. C. Immune responses in mice with murine leprosy. Clin Exp Immunol. 1970 Jan;6(1):117–124. [PMC free article] [PubMed] [Google Scholar]
- Reiner N. E., Finke J. H. Interleukin 2 deficiency in murine Leishmaniasis donovani and its relationship to depressed spleen cell responses to phytohemagglutinin. J Immunol. 1983 Sep;131(3):1487–1491. [PubMed] [Google Scholar]
- Shepard C. C., McRae D. H. A method for counting acid-fast bacteria. Int J Lepr Other Mycobact Dis. 1968 Jan-Mar;36(1):78–82. [PubMed] [Google Scholar]
- Shepard C. C., Walker L. L., Van Landingham R. M., Ye S. Z. Sensitization or tolerance to Mycobacterium leprae antigen by route of injection. Infect Immun. 1982 Nov;38(2):673–680. doi: 10.1128/iai.38.2.673-680.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoman M. L., Weigle W. O. Lymphokines and aging: interleukin-2 production and activity in aged animals. J Immunol. 1981 Nov;127(5):2102–2106. [PubMed] [Google Scholar]
- Turcotte R. Influence of route of Mycobacterium lepraemurium injection on susceptibility to mouse leprosy and on lymphoblastic transformation. Infect Immun. 1980 Jun;28(3):660–668. doi: 10.1128/iai.28.3.660-668.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk J. L., Bryceson A. D. Immunological phenomena in leprosy and related diseases. Adv Immunol. 1971;13:209–266. doi: 10.1016/s0065-2776(08)60185-6. [DOI] [PubMed] [Google Scholar]
- Wadee A. A., Rabson A. R. Binding of phosphatidylethanolamine and phosphatidylinositol to OKT8+ lymphocytes activates suppressor cell activity. J Immunol. 1983 May;130(5):2271–2276. [PubMed] [Google Scholar]
- Watson J., Mochizuki D. Interleukin 2: a class of T cell growth factors. Immunol Rev. 1980;51:257–278. doi: 10.1111/j.1600-065x.1980.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Watson S. R., Collins F. M. Development of suppressor T cells in mice heavily infected with mycobacteria. Immunology. 1980 Mar;39(3):367–373. [PMC free article] [PubMed] [Google Scholar]


