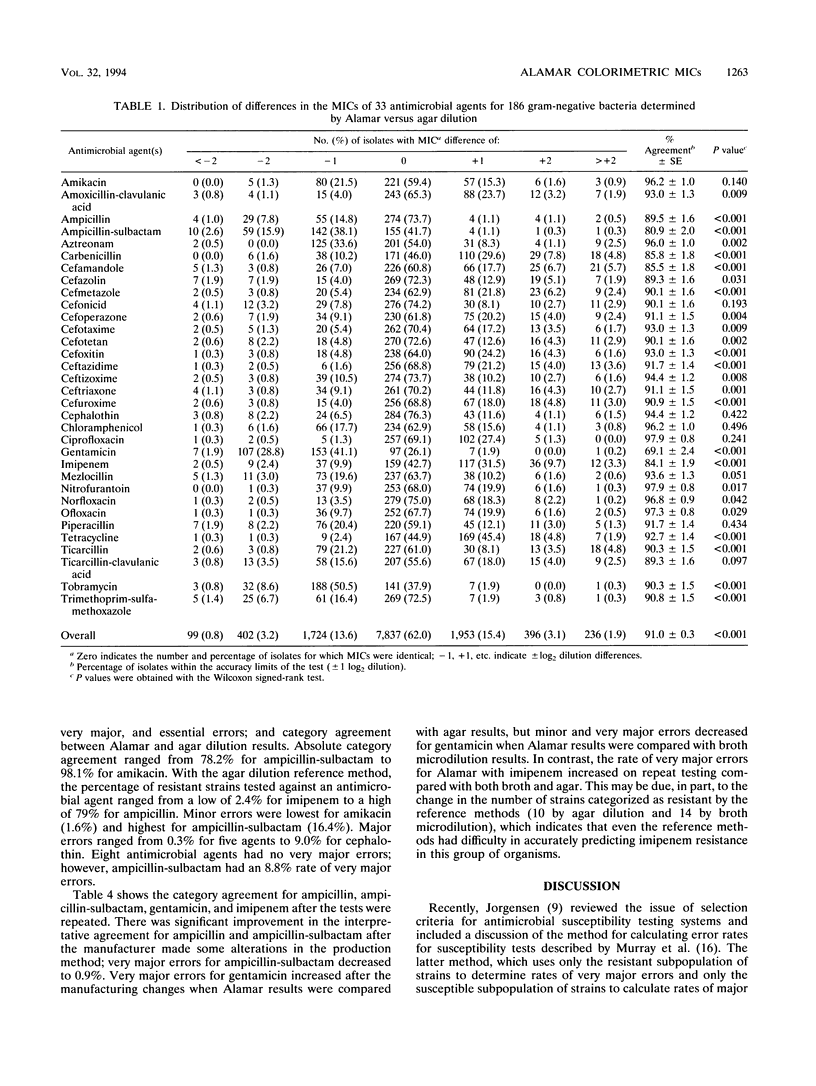
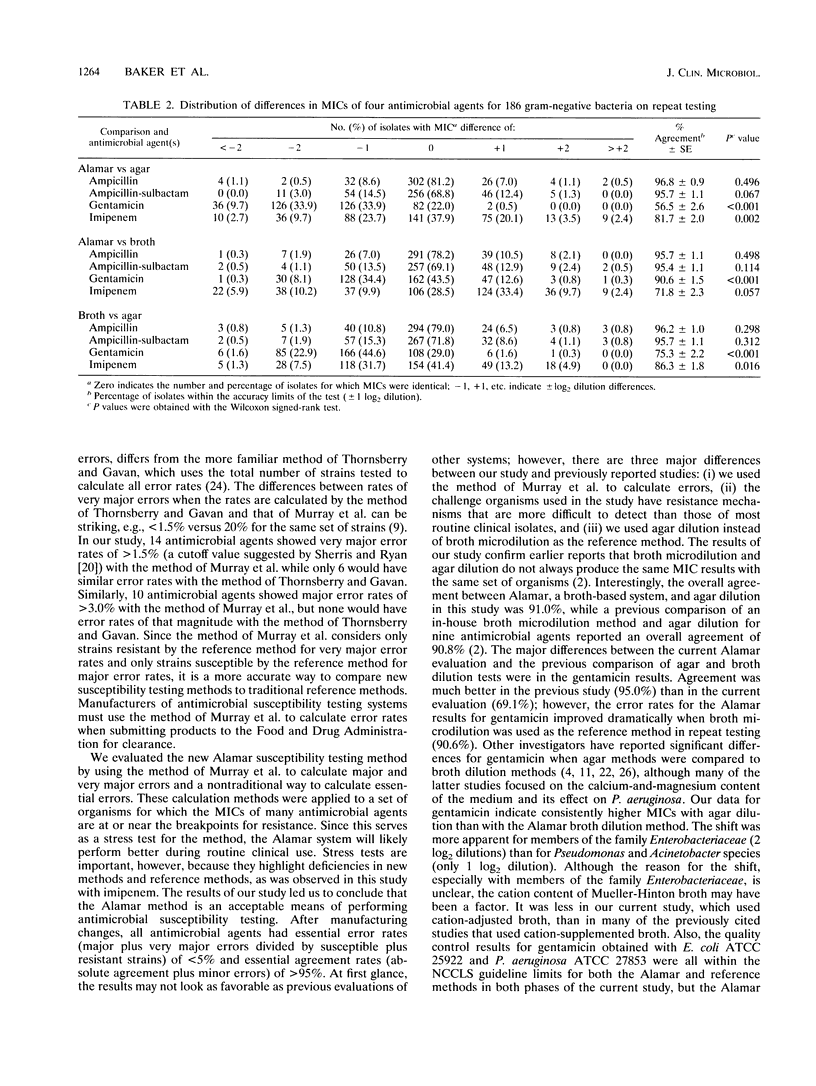
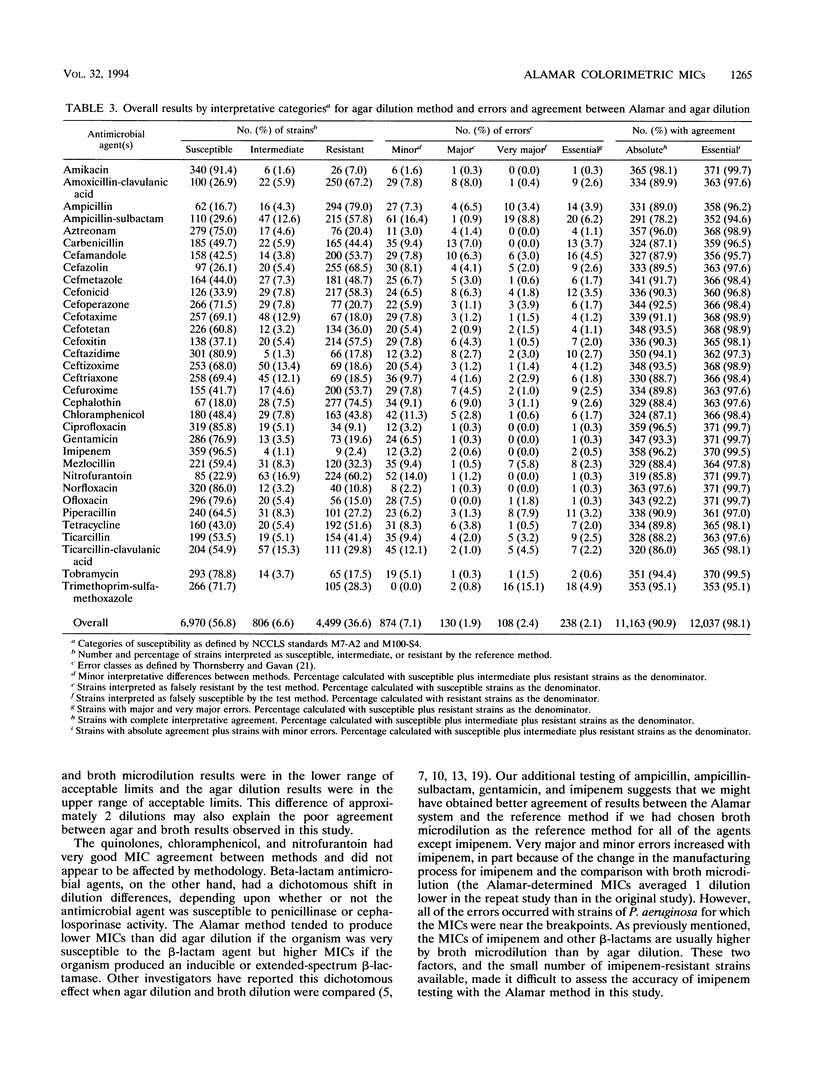
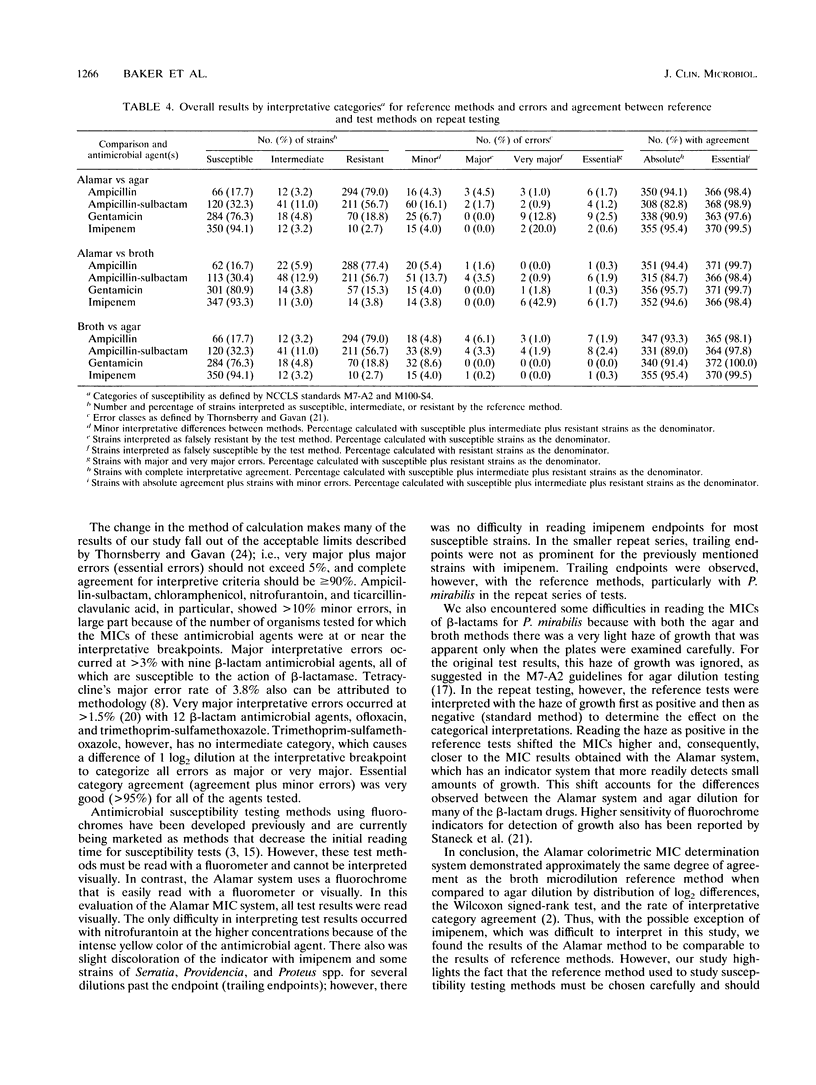
Abstract
The Alamar (Alamar Biosciences, Inc., Sacramento, Calif.) colorimetric antimicrobial susceptibility testing method is a new approach to the determination of broth microdilution MICs. The method uses a color indicator to detect growth of microorganisms within the wells of a microdilution tray. The color changes can be read visually or with a fluorometer. The system contains growth and sterility control wells and 20 antimicrobial agents per MIC tray with eight twofold dilutions for each antimicrobial agent. We tested 186 multiresistant, gram-negative bacterial isolates against 33 antimicrobial agents and compared the results to those obtained by agar dilution. Categorical agreement for all agents was 90.9% and ranged from 78.2% for ampicillin-sulbactam to 98.1% for amikacin. Percent agreement for MIC results (within +/- 1 log2 dilution) was 91.0% for all agents and ranged from 69.1% for gentamicin to 97.9% for ciprofloxacin. Most of the disagreements were with the penicillins and cephalosporins for beta-lactamase-producing strains. The Alamar MIC system is very easy to read visually and appears to be a satisfactory addition to currently used MIC determination methods.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. N., Stocker S. A., Culver D. H., Thornsberry C. Comparison of the E Test to agar dilution, broth microdilution, and agar diffusion susceptibility testing techniques by using a special challenge set of bacteria. J Clin Microbiol. 1991 Mar;29(3):533–538. doi: 10.1128/jcm.29.3.533-538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascomb S., Godsey J. H., Kangas M., Nea L., Tomfohrde K. M. Rapid antimicrobial susceptibility testing of gram-positive cocci using Baxter MicroScan rapid fluorogenic panels and autoSCAN-W/A. Pathol Biol (Paris) 1991 May;39(5):466–470. [PubMed] [Google Scholar]
- D'amato R. F., Thornsberry C., Baker C. N., Kirven L. A. Effect of calcium and magnesium ions on the susceptibility of Pseudomonas species to tetracycline, gentamicin polymyxin B, and carbenicillin. Antimicrob Agents Chemother. 1975 May;7(5):596–600. doi: 10.1128/aac.7.5.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E. J., Cherubin C. E., Shulman M. Comparison of microtiter broth dilution and agar dilution methods for susceptibility testing of Eikenella corrodens. Antimicrob Agents Chemother. 1983 Jan;23(1):42–45. doi: 10.1128/aac.23.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain Z., Lannigan R., Schieven B. C., Stoakes L., Groves D. Comparison of susceptibility results of anaerobic organisms determined by agar dilution method and Sceptor Anaerobe MIC/ID Micro Broth Dilution Panels. Diagn Microbiol Infect Dis. 1987 Oct;8(2):95–100. doi: 10.1016/0732-8893(87)90155-6. [DOI] [PubMed] [Google Scholar]
- Jorgensen J. H. Selection criteria for an antimicrobial susceptibility testing system. J Clin Microbiol. 1993 Nov;31(11):2841–2844. doi: 10.1128/jcm.31.11.2841-2844.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasemann C., Werner H., Hildenbrand G., Benning M., Brandt U., Ungerechts J. Methodological implications of testing anaerobe susceptibility to cephalosporins (cefazolin, cefamandole, cefoxitin). Infection. 1980;8 (Suppl 2):S182–S186. doi: 10.1007/BF01639888. [DOI] [PubMed] [Google Scholar]
- Lairscey R. G., Kelly M. T. Evaluation of the Precept microdilution MIC system for single-drug testing in individual trays. J Clin Microbiol. 1984 Mar;19(3):318–320. doi: 10.1128/jcm.19.3.318-320.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebers D. M., Baltch A. L., Smith R. P., Hammer M. C., Conroy J. V. Susceptibility of Legionella pneumophila to eight antimicrobial agents including four macrolides under different assay conditions. J Antimicrob Chemother. 1989 Jan;23(1):37–41. doi: 10.1093/jac/23.1.37. [DOI] [PubMed] [Google Scholar]
- Manafi M., Kneifel W., Bascomb S. Fluorogenic and chromogenic substrates used in bacterial diagnostics. Microbiol Rev. 1991 Sep;55(3):335–348. doi: 10.1128/mr.55.3.335-348.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. R., Niles A. C., Heeren R. L. Comparison of a highly automated 5-h susceptibility testing system, the Cobas-Bact, with two reference methods: Kirby-Bauer disk diffusion and broth microdilution. J Clin Microbiol. 1987 Dec;25(12):2372–2377. doi: 10.1128/jcm.25.12.2372-2377.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. A., Heifetz C. L., Sesnie J. C. Comparison of microdilution and agar dilution procedures for testing antibiotic susceptibility of Neisseria gonorrhoeae. J Clin Microbiol. 1984 Oct;20(4):828–830. doi: 10.1128/jcm.20.4.828-830.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staneck J. L., Allen S. D., Harris E. E., Tilton R. C. Automated reading of MIC microdilution trays containing fluorogenic enzyme substrates with the Sensititre Autoreader. J Clin Microbiol. 1985 Aug;22(2):187–191. doi: 10.1128/jcm.22.2.187-191.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staneck J. L., Glenn S., DiPersio J. R., Leist P. A. Wide variability in Pseudomonas aeruginosa aminoglycoside results among seven susceptibility testing procedures. J Clin Microbiol. 1989 Oct;27(10):2277–2285. doi: 10.1128/jcm.27.10.2277-2285.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Gavan T. L., Sherris J. C., Balows A., Matsen J. M., Sabath L. D., Schoenknecht F., Thrupp L. D., Washington J. A., 2nd Laboratory evaluation of a rapid, automatic susceptibility testing system: report of a collaborative study. Antimicrob Agents Chemother. 1975 Apr;7(4):466–480. doi: 10.1128/aac.7.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfrey B. F., Fox J. M., Quall C. O. Comparison of minimum inhibitory concentration values determined by three antimicrobic dilution methods for Pseudomonas aeruginosa. Am J Clin Pathol. 1981 Jan;75(1):39–44. doi: 10.1093/ajcp/75.1.39. [DOI] [PubMed] [Google Scholar]


