Abstract
The targets of tuberculosis (TB) control programmes are to detect 70% of new sputum smear-positive cases of TB and to cure 85% of these. The Stop TB Partnership has set additional targets related to the Millennium Development Goals: to halve TB prevalence and mortality between 1990 and 2015. This paper assesses how confident we can be about the data on TB case detection, cure rates, prevalence and mortality. Countries were grouped into those with good, limited or poor information on the burden of TB (based on notification data, population surveys and vital registration systems). Of 211 countries with a total population of 6.4 billion and an estimated 8.9 million cases of TB, 27 countries with a total population of 2.2 billion and an estimated 1.8 million cases of TB had estimates based on good information (i.e. a good-quality surveillance system detecting > 70% of all cases, or a good-quality TB prevalence survey). Of the 22 countries with a high burden of TB and bearing 80% of the global burden, none had a good surveillance system in 1997. Vital registration systems were good in 81 countries with a total population of 2.7 billion. This paper suggests that globally and in the 22 countries with a high burden of TB there is considerable uncertainty about indicators to measure progress towards the Millennium Development Goals. Routine surveillance and vital registration systems need to be strengthened. We recommend that national TB prevalence surveys be performed in selected high-burden countries, in Africa in particular.
Résumé
Les programmes nationaux de lutte antituberculeuse ont pour objectifs de détecter 70% des nouveaux cas de tuberculose (TB) à frottis positif et de guérir 80% des cas détectés. Dans le cadre de la réalisation des objectifs du Millénaire pour le développement, le Partenariat Halte à la tuberculose a défini un objectif supplémentaire : diviser par deux, entre 1990 et 2015, la prévalence de la tuberculose et la mortalité due à cette maladie. Le présent article évalue le degré de fiabilité des données concernant la détection des cas de TB, les taux de guérison, la prévalence de la TB et la mortalité due à cette maladie. Les pays ont été répartis en trois catégories selon que l’on disposait de données satisfaisantes, limitées ou insatisfaisantes sur la charge de TB qu’ils supportent (d’après les données de notification, les enquêtes en population et les systèmes d’enregistrement des données d’état-civil). Parmi 211 pays représentant une population totale de 6,4 milliard d’habitants et un nombre total de cas de TB estimé à 8,9 millions, 27 (regroupant au total 2,2 milliard d’habitants et un nombre de cas de TB estimé à 1,8 million) disposaient d’estimations reposant sur des données satisfaisantes (c’est-à-dire fournies par un bon système de surveillance, détectant plus de 70% des cas, ou par une enquête sur la prévalence de la TB de bonne qualité). Parmi les 22 pays supportant une forte charge de TB et totalisant à eux seuls 80% de la charge mondiale de tuberculose, aucun ne pouvait compter en 1997 sur un bon système de surveillance. Les systèmes d’enregistrement des données d’état-civil étaient satisfaisants dans 81 pays regroupant au total 2,7 milliards d’habitants. L’article fait observer qu’à l’échelle mondiale et dans les 22 pays fortement touchés par la TB, les indicateurs servant à mesurer les progrès dans la réalisation des objectifs du Millénaire pour le développement sont entachés d’une grande incertitude. La surveillance de routine et les systèmes d’enregistrement des données d’état-civil doivent être renforcés. Nous recommandons de procéder à des enquêtes sur la prévalence nationale de la TB dans un certain nombre de pays fortement touchés par la tuberculose, notamment en Afrique.
Resumen
Las metas de los programas de control de la tuberculosis consisten en detectar un 70% de los nuevos casos bacilíferos y curar el 85% de esos casos. La Alianza Alto a la Tuberculosis ha establecido nuevas metas relacionadas con los Objetivos de Desarrollo del Milenio: reducir a la mitad la prevalencia de tuberculosis y la mortalidad por esa causa entre 1990 y 2015. En este artículo se evalúa la fiabilidad de los datos sobre la detección de casos de tuberculosis y las tasas de curación, la prevalencia y la mortalidad correspondientes. Se clasificó a los países en función de la calidad de su información -buena, escasa o mala- sobre la carga de tuberculosis (calibrada a partir de los datos de notificación, las encuestas de población y los sistemas de registro civil). De 211 países con una población total de 6400 millones de personas y una cifra estimada de 8,9 millones de casos de tuberculosis, 27 países con una población total de 2200 millones de habitantes y unos 1,8 millones de casos estimados disponían de estimaciones basadas en una buena información (es decir, un sistema de vigilancia de buena calidad que detectaba más del 70% de todos los casos, o una encuesta de buena calidad sobre la prevalencia de la tuberculosis). De los 22 países con una alta carga de tuberculosis, que suponen el 80% de la carga mundial, ninguno tenía un buen sistema de vigilancia en 1997. Los sistemas de registro civil eran satisfactorios en 81 países que totalizaban una población de 2700 millones de personas. En el presente artículo se sugiere que tanto a nivel mundial como en los 22 países que presentan una alta carga de tuberculosis existe una incertidumbre considerable sobre los indicadores empleados para medir los progresos hacia los Objetivos de Desarrollo del Milenio. Es preciso reforzar la vigilancia sistemática y los sistemas de registro civil. Recomendamos que se lleven a cabo encuestas nacionales sobre la prevalencia de la tuberculosis en algunos de los países con alta carga de la enfermedad, en África en particular.
ملخص
تـتمثـَّل أەداف برامج مكافحة السل بكشف 70 بالمئة من الحالات الإيجابية للطاخات البلغم وشفاء 85% منەا. وقد وضعت شراكة دحر السل أەدافاً أخرى متعلِّقة بالمرامي الإنمائية للألفية وەي: الوصول بمعدلات انتشار ووفيات السل إلى نصف ما كانت عليە عام 1990، وذلك بحلول عام 2015. وتقيِّم ەذە الورقة المدى الذي يمكن لنا الوثوق بە بالمعطيات الخاصة بكشف السل؛ وبمعدلات الشفاء، والانتشار والوفيات. وقد قُسِّمتْ البلدان إلى مجموعات ەي مجموعة ذات معلومة جيدة، ومجموعة ذات معلومات محدودة، ومجموعة ذات معلومات سيئة؛ وذلك وفقاً لما لديەا من معلومات حول عبء السل (ويرتكز التقسيم على الإبلاغ بالمعطيات، والمسوحات السكانية ونُظُم التسجيل للأحوال المدنية) ومن بين 211 بلداً يعيش فيەا 6.4 بليون نسمة، وفيەا 8.9 مليون حالة سل، كان ەناك تقديرات مرتكزة على معطيات جيدة في 27 بلداً يعيش فيەا 2.2 مليون نسمة وفيەا 1.8 مليون حالة سل، (وذلك يعني أن لديەا نظام تـرصُّد عالي الجودة يكشف أكثر من 70 بالمئة من مجمل الحالات، أو أن لديەا مسوحات عالية الجودة لانتشار السل). ولم يكن بين 22 بلداً من البلدان التي تـتحمَّل عبئاً مرتفعاً للسل يبلغ 80 بالمئة من العبء العالمي، لم يكن بينەا أي بلد لديە نظام تـرصُّد جيد في عام 1997. وقد كانت نُظُم تسجيل الأحوال المدنية جيدة في 81 بلداً يعيش فيەا 2.7 بليون نسمة. وتقتـرح ەذە الورقة أن ەناك ارتياباً حول المؤشِّرات التي تقيس التقدُّم الـمُحرز نحو المرامي الإنمائي للألفية في 22 بلداً من البلدان التي ترزح تحت عبء ثقيل من السل. وينبغي تقوية النُظُم الروتينية لتسجيل الأحوال المدنية وللتـرصُّد. ونوصي بإجراء مسوحات وطنية لمعدلات انتشار السل في بلدان منتقاة تنوء تحت عبء ثقيل من السل ولاسيَّما في أفريقيا.
Introduction
Tuberculosis (TB) is an important cause of illness and death globally, accounting for an estimated 8.8 million new cases and 1.6 million deaths each year.1 Mycobacterium tuberculosis, the organism that causes TB, is found almost exclusively in humans, and is spread by patients with pulmonary tuberculosis, in particular those with sputum smears in which M. tuberculosis has been identified. The most important control strategy is the early identification and treatment of infectious cases of TB. It has been suggested that if 70% of new smear-positive cases were detected and 85% of these were cured, a substantial impact on the prevalence of TB, and thus on its transmission and future incidence, would be expected.2–4
Rates of case detection and cure, as well as prevalence and mortality, have been included as indicators for measuring progress towards achieving the Millennium Development Goals.5 It was expected that the targets of the Stop TB Partnership, i.e. to halve the prevalence and mortality of TB between 1990 and 2015, would be possible if the targets for case detection and cure were reached by 2005, based on the model described by Dye et al.3 This model fitted fairly well with data from the Netherlands. However, whether important parameters such as risk of disease after infection (which may depend on factors such as the immune status of the host, nutritional status and smoking) can be extrapolated from the Netherlands to other settings is uncertain. Consequently, we do not know whether achieving the targets of case detection and cure will have the expected impact on TB incidence.
Whether or not countries are achieving the targets for case detection and cure is often uncertain. The case detection rate, which has notifications in the numerator and estimated TB incidence in the denominator, is difficult to estimate. The reported cure rate is generally based on routine surveillance data, but may require validation because the success of TB control programmes may be overestimated by their managers at national or lower levels. Estimates of TB incidence, prevalence and mortality by country are made annually by the World Health Organization, making the best use of information available.6 However, WHO acknowledges that the accuracy of these estimates is uncertain because assumptions are required to obtain the estimates from the available data and the data themselves contain uncertainty.7
The aim of this paper is to describe how confident we can be about the available data on TB case detection, cure rates, prevalence and mortality. It gives special attention to the 22 countries with a heavy burden of TB and in which approximately 80% of the global burden of TB is found.
Methods
Case detection rates
WHO’s estimates of case detection rate by country are reported annually for new smear-positive cases and for all forms of TB. Since the Stop TB strategy8 emphasizes the detection and treatment of all forms of TB, we focus on the case detection rate of all forms of TB in this analysis. To assess the quality of these estimates, we used the initial publication of the global burden of tuberculosis, which is the foundation for the current WHO estimates.6 Countries were divided into three groups according to the basis for these estimates: expert opinion, tuberculin survey(s) and TB prevalence surveys.
Since the initial publication,1 re-estimates of the prevalence and incidence of TB have been made for 39 countries. We received information about the method of re-estimation and the background documents from the WHO Stop TB Department. Information used for the re-estimation consisted of expert knowledge, recently performed tuberculin or TB prevalence surveys, or vital registration data. For these 39 countries, we used this updated information to assess data quality.
Two epidemiologists assessed the TB prevalence surveys in publications used by Dye et al.6 for estimating TB prevalence and incidence, and the surveys used for the re-estimates; the surveys were categorized as eligible or not eligible for estimation of the national prevalence of TB. A survey was considered to be eligible if: it was conducted not more than 15 years before the year of estimation; probability sampling was used, leading to a nationally representative sample; sputum examination was performed either for all cases or for those with chest X-ray abnormalities; and cases were bacteriologically confirmed. If the available information was inadequate to judge eligibility, eligibility was recorded as unknown.
Next, countries were categorized into three groups. The first group was countries for which good information on the burden of TB was available, in the form of a good national TB prevalence survey or a good surveillance system, as judged by expert opinion,6 and capturing at least 70% of cases with all forms of TB.1 The second group comprised countries for which limited information on the burden of TB was available; this included countries with a TB prevalence survey classified as not eligible, with data from a tuberculin survey or vital registration systems available or surveillance systems considered to be good, but capturing less than 70% of cases with all forms of TB. The third group included countries for which information on the burden of TB was poor, the surveillance system was not considered to be good by experts6 and no additional information from population surveys or vital registration systems was available.
Cure rates
Cure rates tend to be accepted as reported by TB control programmes. We conducted a literature search in PubMed using the search terms “tuberculosis” and “cure rate” to identify articles that assessed the quality of reported cure rates.
Rates of mortality from TB
A recent review of death registration systems worldwide9 was used to classify countries into three groups with respect to completeness of cause-of-death registration: (1) good – cause of death registration completeness > 70% and ill-defined codes < 20%; (2) limited – cause of death registration completeness 50–70%; or completeness > 70% and ⩽ 20% ill-defined codes; and (3) poor – death registration completeness < 50%, or no information submitted to WHO by December 2003.9 Completeness refers to the percentage of deaths registered; ill-defined causes of death are specified by ICD code in the original reference.9 If information about completeness was not available, estimated coverage (total number of deaths reported from the vital registration system for a country in a given year divided by the total number of deaths estimated by WHO for that year for the national population) was used.
Annual reported data on mortality statistics by cause of death (ICD-9 and ICD-10) as obtained from civil registration systems were downloaded from the WHO web site.10 Using this database, we calculated the number of deaths attributable to tuberculosis per country per year. The total number of deaths from TB in 2004 or the most recent reported year was compared with the estimated number of deaths from TB in 2004 as reported in the WHO 2006 report1 for countries with a good death registration system.
Results
Case detection rates and estimates of TB prevalence
For 13 countries, the results of a TB prevalence survey were used to estimate the incidence and prevalence of TB in 2004. For Macau, a Special Administrative Region of China, and Mongolia, the results of the TB prevalence survey in China11 were used; and for Palau the results of the survey in the Philippines were used.12 Of the ten surveys, the four performed in the WHO Western Pacific Region were assessed as eligible for estimation of the national prevalence of TB (Table 1). The survey in the WHO Eastern Mediterranean Region and the surveys in the WHO South-East Asia Region were considered to be not eligible, or information provided in the document that described the survey was inadequate to judge eligibility.
Table 1. Assessment of the quality of surveys used to estimate the prevalence and incidence of all forms of tuberculosis in the WHO 2006 report1.
| WHO region/country | Information used for quality assessment | Overall result of quality assessmenta | Reference | ||||
|---|---|---|---|---|---|---|---|
| Survey conducted ⩽ 15 years before the year of estimation (year of survey) | Probability sampling used, leading to a nationally representative sample | Sputum examination performed either in all cases, or in those with radiological abnormalities | Cases bacteriologically confirmed | ||||
| Eastern Mediterranean | |||||||
| Pakistan | Yes (1987–1988) | ? | No | Yes | Not eligible | 22 | |
| South-East Asia | |||||||
| Bangladesh | Yes (1987–1988) | Yes | No | Yes | Not eligible | 23 | |
| Indonesia | Yes (2004) | Yes | No | Yes | Not eligible | 24 | |
| Nepal | No (1978) | ? | No | Yes | Not eligible | 25 | |
| Thailand | Yes (1991) | ? | ? | ? | Unknown | 26 | |
| Western Pacific | |||||||
| Brunei Darussalamb | – | – | – | – | Unknown | ||
| Cambodia | Yes (2002) | Yes | Yes | Yes | Eligible | 17 | |
| China | Yes (2000) | Yes | Yes | Yes | Eligible | 11 | |
| Philippines | Yes (1997) | Yes | Yes | Yes | Eligible | 12 | |
| Republic of Korea | Yes (1995) | Yes | Yes | Yes | Eligible | 16 | |
?, information not provided in the background document. a The survey was considered eligible if: (a) the survey was conducted no more than 15 years before the year of estimation; (b) probability sampling was used leading to a nationally representative sample; (c) sputum examination was performed either in all cases or in those with chest X-ray abnormalities; and (c) cases were bacteriologically confirmed. If information was inadequate to judge eligibility, eligibility was recorded as “unknown”. b Background document not available.
Of 211 countries with a total population of 6.4 billion and an estimated 8.9 million cases of all forms of TB, 27 countries with a total population of 2.2 billion and an estimated 1.8 million cases of all forms of TB had TB estimates that were based on good information (Table 2). Of these 27 countries with good information, a TB prevalence survey of good quality had been conducted in 4 countries with a population of 1.5 billion and an estimated 1.7 million cases of all forms of TB. In 154 countries with a total population of 2.0 billion and an estimated 3.1 million cases of all forms of TB, TB estimates were based on poor information, i.e. a surveillance system not considered to be good and without additional information from population surveys or vital registration systems (Table 2). The proportion of the population for which information on TB was of poor quality was largest in Africa and Europe.
Table 2. Estimated incidence of all forms of tuberculosis according to quality of information, by WHO region.
| WHO region | Quality of informationa | No. of countries | Population in 2004 (millions) | Incidence |
|
|---|---|---|---|---|---|
| No. of cases (× 1000) | Rate per 100 000 population | ||||
| African | Good | 0 | 0 | 0 | 0 |
| Limited | 2 | 80 | 357 | 448 | |
| Poor | 44 | 642 | 2 216 | 345 | |
| Americas | Good | 5 | 358 | 20 | 6 |
| Limited | 4 | 165 | 97 | 59 | |
| Poor | 35 | 357 | 246 | 69 | |
| Eastern Mediterranean | Good | 1 | 31 | 34 | 110 |
| Limited | 8 | 279 | 516 | 185 | |
| Poor | 13 | 220 | 95 | 43 | |
| European | Good | 13 | 171 | 28 | 16 |
| Limited | 6 | 67 | 9 | 13 | |
| Poor | 33 | 643 | 408 | 63 | |
| South-East Asia | Good | 1 | 0.3 | 0.2 | 49 |
| Limited | 7 | 1 589 | 2 910 | 183 | |
| Poor | 3 | 44 | 57 | 130 | |
| Western Pacific | Good | 7 | 1 603 | 1 717 | 107 |
| Limited | 3 | 89 | 156 | 175 | |
| Poor | 26 | 48 | 53 | 110 | |
| Global | Good | 27 | 2 163 | 1 799 | 83 |
| Limited | 30 | 2 269 | 4 044 | 178 | |
| Poor |
154 |
1 955 |
3 075 |
157 |
|
| Total | 211 | 6 387 | 8 918 | 140 | |
a Good = good national TB prevalence of disease survey or a good surveillance system according to expert opinion6 and capturing at least 70% of all cases – as in most industrialized countries1; Limited = neither good nor poor information; Poor = surveillance system not considered good by experts6 and no additional information available from population surveys or vital registration systems.
Of the 22 countries with a high burden of TB, with a total population of 4.0 billion and an estimated 7.1 million cases of all forms of TB, none had a surveillance system considered to be good in 1997. The case detection rate for all forms of TB was estimated to be more than 70% in four countries (Brazil, Myanmar, the Russian Federation and South Africa). Overall, three countries with a high burden of TB (Cambodia, China and the Philippines), with a total population of 1.4 billion and an estimated 1.6 million cases of all forms of TB, had TB estimates based on good information (Table 3, available at: http://www.who.int/bulletin). In these three countries, this was based on a TB prevalence survey.
Table 3. Information available on tuberculosis in 22 countries with a high burden of disease.
| Country | Available information |
Overall quality of available informationf | Population in 2004 (millions) | Incidence of TB in 2004 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Quality of surveillance system in 1997a | % of all TB patients captured by surveillance systemb | Assessment of TB prevalence surveyc | Tuberculin surveyd | Quality of vital registratione | No of cases (× 1000) | Rate per 100 000 population | |||
| Afghanistan | Low | 19 | – | Yes | Poor | Limited | 29 | 95 | 333 |
| Bangladesh | Low | 31 | Not eligible | – | Poor | Limited | 139 | 319 | 229 |
| Brazil | Intermediate | 79 | – | – | Intermediate | Poor | 184 | 110 | 60 |
| Cambodia | Intermediate | 44 | Eligible | – | Poor | Good | 14 | 70 | 510 |
| China | Intermediate | 60 | Eligible | – | Intermediate | Good | 1 308 | 1 325 | 101 |
| Democratic Republic of the Congo | Low | 46 | – | – | Poor | Poor | 56 | 204 | 366 |
| Ethiopia | Low | 46 | – | – | Poor | Poor | 76 | 267 | 353 |
| India | Intermediate | 62 | – | Yes | Good | Limited | 1 087 | 1 824 | 168 |
| Indonesia | Low | 39 | Not eligible | – | Poor | Limited | 220 | 539 | 245 |
| Kenya | Low | 49 | – | – | Poor | Poor | 33 | 207 | 619 |
| Mozambique | Low | 35 | – | – | Poor | Poor | 19 | 89 | 460 |
| Myanmar | Low | 113 | – | Yes | Poor | Limited | 50 | 85 | 171 |
| Nigeria | Low | 15 | – | – | Poor | Poor | 129 | 374 | 290 |
| Pakistan | Low | 36 | Unknown | – | Poor | Limited | 155 | 281 | 181 |
| Philippines | Intermediate | 55 | Eligible | – | Good | Good | 82 | 239 | 293 |
| Russian Federation | Intermediate | 73 | – | – | Good | Poor | 144 | 166 | 115 |
| South Africa | Low | 78 | – | – | Intermediate | Limited | 47 | 339 | 718 |
| Thailand | Intermediate | 61 | Unknown | – | Intermediate | Limited | 64 | 91 | 142 |
| Uganda | Low | 39 | – | – | Poor | Poor | 28 | 112 | 402 |
| United Republic of Tanzania | Intermediate | 48 | – | – | Poor | Poor | 38 | 131 | 347 |
| Viet Nam | Intermediate | 67 | – | Yes | Poor | Limited | 83 | 147 | 176 |
| Zimbabwe | Intermediate | 64 | – | – | Poor | Poor | 13 | 87 | 674 |
| Total | 3 993 | 7 102 | 178 | ||||||
TB, tuberculosis; –, no data on prevalence of disease or infection used for estimating TB incidence and prevalence. a According to expert opinion.6 b Case detection rate of 2004 for all forms of TB.1 c A prevalence of disease survey was considered eligible if: (1) the survey was conducted no more than 15 years before the year of estimation; (2) probability sampling was used leading to a nationally representative sample; (3); sputum examination was performed either in all cases or in cases with chest X-ray abnormalities; and (4) cases were bacteriologically confirmed. If information was inadequate to judge eligibility, eligibility was recorded as “unknown”. d Information from tuberculin surveys was used by Dye et al.6 to estimate incidence and prevalence of TB. The tuberculin surveys in India were performed between 2000 and 2003. In Viet Nam, they were performed between 1988 and 1990. For Afghanistan, the reference document was not available. For Myanmar, the reference document did not state the year of the survey. e Based on a recent review of death registration systems worldwide by Mathers et al.11: Good = cause of death registration completeness > 70% and ill-defined codes < 20%; Intermediate = cause of death registration completeness 50–70%, or completeness > 70% and ⩾ 20% ill-defined codes; Poor = death registration completeness < 50% or no information submitted to WHO by December 2003. f Good = good national TB prevalence-of-disease survey or a good surveillance system according to expert opinion6 and capturing at least 70% of all TB cases (as in most industrialized countries);1 Limited = neither good nor poor information; Poor = surveillance system not considered good by experts6 and no additional information available from population surveys or vital registration systems.
Cure rates
We did not identify any formal evaluations of cure rates. Therefore, this review was unable to evaluate the quality of data on cure rates.
Estimates of mortality attributable to TB
Vital registration systems were considered to be good in 81 countries with a total population of 2.7 billion (Table 4). Most of the population of the WHO European and South-East Asia Regions and the Region of the Americas was covered by good vital registration systems. However, this proportion was low (< 20%) in the African, Eastern Mediterranean and Western Pacific Regions (Table 4). Of the 22 countries with a high burden of TB, only three (India, Philippines and the Russian Federation) with a total population of 1.3 billion were considered to have good vital registration systems.
Table 4. Quality of death registration systems covering countries and population in 2004, by WHO region.
| WHO region | Quality of death registration systema |
Total | ||
|---|---|---|---|---|
| Good | Intermediate | Poor | ||
| African | ||||
| Number of countries (%) | 2 (4) | 2 (4) | 42 (91) | 46 (100) |
| Population 2004, in millions (%) | 1 (0.2) | 80 (11) | 641 (89) | 722 (100) |
| Americas | ||||
| Number of countries | 24 (55) | 7 (16) | 13 (30) | 44 (100) |
| Population 2004, in millions (%) | 567 (64) | 275 (31) | 38 (4) | 880 (100) |
| Eastern Mediterranean | ||||
| Number of countries | 2 (9) | 7 (32) | 13 (59) | 22 (100) |
| Population 2004, in millions (%) | 5 (1) | 177 (33) | 348 (66) | 530 (100) |
| European | ||||
| Number of countries | 41 (79) | 9 (17) | 2 (4) | 52 (100) |
| Population 2004, in millions (%) | 735 (83) | 146 (17) | 0.1 (0.0) | 881 (100) |
| South-East Asia | ||||
| Number of countries | 1 (9) | 3 (27) | 7 (64) | 11 (100) |
| Population 2004, in millions (%) | 1 087 (67) | 85 (5) | 461 (28) | 1 633 (100) |
| Western Pacific | ||||
| Number of countries | 11 (31) | 8 (22) | 17 (47) | 36 (100) |
| Population 2004, in millions (%) | 313 (18) | 1 309 (75) | 117 (7) | 1 740 (100) |
| Global | ||||
| Number of countries | 81 (38) | 36 (17) | 94 (40) | 211 (100) |
| Population 2004, in millions (%) | 2 710 (42) | 2 071 (32) | 1 606 (25) | 6 387 (100) |
Source: Mathers et al.9 a Good = cause of death registration completeness > 70% and ill-defined codes < 20%; Intermediate = cause of death registration completeness 50–70%; or completeness > 70% and ⩾ 20% ill-defined codes; Poor = death registration completeness < 50% or no information submitted to WHO by December 2003.
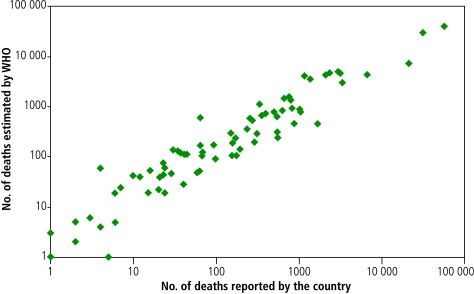
Seventy-seven of the 81 countries with a good vital registration system reported data on mortality statistics using ICD-9 or ICD-10 to WHO. Mortality statistics were not available from the Cook Islands, India, Oman and Tonga. The estimated mortality corresponds well with the reported data (R = 0.89; Fig. 1).
Fig. 1.
Comparison of number of deaths from tuberculosis estimated by WHO and number of deaths from tuberculosis reported by countries with a good death registration system Regression line is 2.5 + 7.9x, R = 0.89
Sources: WHO estimates of mortality from tuberculosis were obtained from the WHO 2006 Report.1 The numbers of deaths from tuberculosis reported by country were obtained from the WHO database.10 Analysis and interpretations were performed by the authors.
Discussion
This paper has shown that globally, and in the 22 countries with a high burden of TB, there is uncertainty about the disease’s incidence, prevalence, mortality, case detection rates and perhaps cure rates. While WHO makes the best use of the available information, more data are needed to reduce the uncertainty in these estimates. A large proportion of the global population is not covered by high-quality surveillance systems that capture more than 70% of TB cases. Independent confirmation of cure rates is not usually obtained. National TB prevalence surveys of sufficient quality have only been conducted in a few countries. And finally, vital registration systems have poor coverage in parts of the world with a high burden of TB, including the WHO African, Eastern Mediterranean and Western Pacific Regions. The problem of uncertainty is largest in Africa: Africa has the world’s highest burden of TB and experiences the most serious impact from the HIV epidemic; however, independent information on the burden of TB is limited and vital registration systems are poor. Therefore the need for TB prevalence surveys is of highest priority in Africa.
The quality of a notification system has two important aspects: consistency and completeness. Consistency can be, and is, checked fairly easily. Indicators include the proportion of years and subunits (e.g. districts or provinces) for which data are available, and year-to-year variation. However, the completeness or coverage of case detection remains uncertain in the absence of data sources independent from TB notifications. Such independent data sources may include laboratory and hospital discharge information to be used for capture–recapture analysis,13–15 or, where appropriate, population surveys.11,12,16,17 It would be particularly helpful to assess case detection from independent data sources in two instances: high-burden countries with inadequate surveillance systems, and countries that are thought to have a case detection rate of 70% or more, to certify that that target has indeed been met. In countries with inadequate surveillance systems, such assessments may need to be repeated every ten years or so, until it is shown that trends in notification rates provide reliable estimates of trends in incidence in the country concerned.
Prevalence surveys provide essential information on the burden of TB when the data provided by surveillance systems are of insufficient consistency or completeness. In this review, a large proportion of identified surveys could not be used to derive national estimates owing to limitations in sampling or data collection. This suggests the need to establish standard guidelines, and, where appropriate, to have access to technical assistance. The WHO Regional Office for the Western Pacific and partners are now preparing guidelines for the planning, conduct and analysis of TB prevalence surveys in order to increase their utility and comparability.
Repeated surveys of the prevalence of TB disease or infection can provide valuable trend estimates if rigorous methods are used that have a constant sensitivity (e.g. chest-X-ray for identification of suspects). We showed that few countries had performed at least one survey on the prevalence of disease or infection that was of sufficient quality. This suggests that surveys should be performed urgently, in particular in Africa, and be repeated by 2015 to assess progress towards achieving the Millennium Development Goals and Stop TB Partnership targets.
While well-conducted surveys of disease prevalence can be used to estimate national prevalence, estimating incidence requires additional assumptions and involves uncertainties. For this reason, it has been argued that detection rate may be a more appropriate indicator than the proportion of patients detected.18
We used data about the quality of notification systems as judged by expert opinion in 1997.6 Since notification systems have probably improved since this time, we attempted to update the quality assessment of the notification systems by defining uniform criteria that can be used to judge the quality of surveillance systems using the information available in the annual country reports. It appeared that this was not possible. Thus a new round of expert consultation (as was done in 1997)6 or collection of information that can be used to assess the quality of the notification system would be required to re-assess the quality of the surveillance system.
No formal evaluations of the cure rate were identified. Informal assessments are often part of technical assistance visits and programme reviews. We believe certification that the cure rate is 85% or more in countries claiming this success would be extremely informative, since managers of TB control programmes may be tempted to overestimate treatment success. A standard protocol would need to be developed for this certification.
Data on TB mortality would in principle be best obtained from routine vital registration systems. Unfortunately, these systems are least developed in the countries with the highest TB rates, so further development of vital registration systems is clearly the way forward. In some countries, sample vital registrations may be set up in representative areas of the country as an intermediate step. In the short term in many countries, mortality attributable to TB may need to be estimated with the use of simple models. Such models require information on the burden of TB and the rate of case detection or proportion of cases detected. As suggested above, the latter information may be obtained at least in part from prevalence surveys in high-burden countries.
The impact of HIV on TB estimates is important and has been taken into account by Corbett et al., who used parameter estimates from literature review and HIV estimates provided by the Joint United Nations Programme on HIV/AIDS (UNAIDS).19 These methods are included in the annual WHO TB burden estimates. The estimates of TB in countries with a high burden of HIV are less certain than those in other countries, since there is additional uncertainty about the HIV estimates and about the exact impact of HIV on TB incidence, mortality and case detection. HIV has had the biggest impact in Africa, reinforcing the conclusion that uncertainty about the burden of TB is greatest in Africa.
One of the parameters used to estimate the incidence of TB in the presence of HIV is the relative risk of developing TB in HIV-infected individuals who are co-infected with M. tuberculosis. In the global estimates it is assumed that HIV-infected individuals are six times more likely to develop TB than are individuals who are not infected with HIV.19 While this is a reasonable overall assumption, it is also clear that the rate ratio varies between settings and depends on the stage of the HIV epidemic, and that the impact of HIV depends on the degree of clustering of TB and HIV in specific risk groups. Further information on this is likely to become available in the near future with the uptake of diagnostic counselling and testing in Africa,20,21 and from TB prevalence surveys in which TB patients and a sample of controls are offered testing for HIV.
We conclude that routine surveillance and vital registration systems need strengthening in most countries with a high burden of TB in order to assess progress towards the Millennium Development Goals. In the short term, we recommend the wider use of national TB prevalence surveys to provide information on the burden of TB, especially in Africa. ■
Acknowledgements
The authors thank the Stop TB Department, and especially Catherine J Watt, for assisting with collection of the background documents and for providing information.
Competing interests
None declared.
References
- 1.Global tuberculosis control: surveillance, planning, financing: WHO report 2006 Geneva: WHO; 2006 (WHO/HTM/TB/2006.362).
- 2.Styblo K, Bumgarner JR. Tuberculosis can be controlled with existing technologies: evidence The Hague: Tuberculosis Surveillance Research Unit; 1991:60-72. [Google Scholar]
- 3.Dye C, Garnett GP, Sleeman K, Williams BG. Prospects for worldwide tuberculosis control under the WHO DOTS strategy. Directly observed short-course therapy. Lancet. 1998;352:1886–91. doi: 10.1016/S0140-6736(98)03199-7. [DOI] [PubMed] [Google Scholar]
- 4.Borgdorff MW, Floyd K, Broekmans JF. Interventions to reduce tuberculosis mortality and transmission in low- and middle-income countries. Bull World Health Organ. 2002;80:217–27. [PMC free article] [PubMed] [Google Scholar]
- 5.Millennium Development Goals Indicators New York: United Nations; 2006. Available at: http://unstats.un.org/unsd/mdg/default.aspx
- 6.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 7.Dye C, Watt CJ, Bleed DM, Hosseini SM, Raviglione MC. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. JAMA. 2005;293:2767–75. doi: 10.1001/jama.293.22.2767. [DOI] [PubMed] [Google Scholar]
- 8.Raviglione MC, Uplekar MW. WHO’s new Stop TB Strategy. Lancet. 2006;367:952–5. doi: 10.1016/S0140-6736(06)68392-X. [DOI] [PubMed] [Google Scholar]
- 9.Mathers CD, Fat DM, Inoue M, Rao C, Lopez AD. Counting the dead and what they died from: an assessment of the global status of cause of death data. Bull World Health Organ. 2005;83:171–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Mortality data Geneva: WHO; 2007. Available at: http://www.who.int/healthinfo/statistics/mortality/en/
- 11.China Tuberculosis Control Collaboration The effect of tuberculosis control in China. Lancet. 2004;364:417–22. doi: 10.1016/S0140-6736(04)16764-0. [DOI] [PubMed] [Google Scholar]
- 12.Tupasi TE, Radhakrishna S, Rivera AB, Pascual ML, Quelapio MI, Co VM, et al. The 1997 nationwide tuberculosis prevalence survey in the Philippines. Int J Tuberc Lung Dis. 1999;3:471–7. [PubMed] [Google Scholar]
- 13.van Hest NA, Smit F, Baars HW, De Vries G, De Haas PE, Westenend PJ, et al. Completeness of notification of tuberculosis in the Netherlands: how reliable is record-linkage and capture-recapture analysis? Epidemiol Infect. doi: 10.1017/S0950268806007540. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baussano I, Bugiani M, Gregori D, van Hest R, Borraccino A, Raso R, et al. Undetected burden of tuberculosis in a low-prevalence area. Int J Tuberc Lung Dis. 2006;10:415–21. [PubMed] [Google Scholar]
- 15.Tocque K, Bellis MA, Beeching NJ, Davies PD. Capture-recapture as a method of determining the completeness of tuberculosis notifications. Commun Dis Public Health. 2001;4:141–3. [PubMed] [Google Scholar]
- 16.Hong YP, Kim SJ, Lew WJ, Lee EK, Han YC. The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis. 1998;2:27–36. [PubMed] [Google Scholar]
- 17.National TB prevalence survey. Cambodia 2002 Phnom Penh: National Tuberculosis Control Program of Cambodia; 2005.
- 18.Borgdorff MW. New measurable indicator for tuberculosis case detection. Emerg Infect Dis. 2004;10:1523–8. doi: 10.3201/eid1009.040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–21. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 20.De Cock KM, Mbori-Ngacha D, Marum E. Shadow on the continent: public health and HIV/AIDS in Africa in the 21st century. Lancet. 2002;360:67–72. doi: 10.1016/S0140-6736(02)09337-6. [DOI] [PubMed] [Google Scholar]
- 21.Corbett EL, Marston B, Churchyard GJ, De Cock KM. Tuberculosis in sub-Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet. 2006;367:926–37. doi: 10.1016/S0140-6736(06)68383-9. [DOI] [PubMed] [Google Scholar]
- 22.Dolin P. Report of a visit to Tehran, Iran, July 6–12, 1998 Geneva: WHO; 1998. [Google Scholar]
- 23.Report on the national prevalence survey on tuberculosis in Bangladesh, 1987–1988 Dhaka: Directorate General of Health Services, Office of the Director, TB & Leprosy Control Services; 1989.
- 24.Tuberculosis prevalence survey in Indonesia, 2004 Jakarta: National Institute of Health Services and Development, Ministry of Health, and the Directorate General of Communicable Disease Control and Environmental Health, Ministry of Health, and WHO, and Project DOTS Expansion GFATM; 2005.
- 25.National tuberculosis programme review Geneva: WHO and Kingdom of Nepal; 1994.
- 26.Tuberculosis programme review Geneva: Thailand Ministry of Public Health and WHO; 1995.