Abstract
The Global Plan to Stop TB 2006–2015 is a road map for policy-makers and managers of national programmes. It sets out the key actions needed to achieve the targets of the Millennium Development Goals relating to tuberculosis (TB): to halve the prevalence and deaths by 2015 relative to 1990 levels and to save 14 million lives. Developed by a broad coalition of partners, the plan presents a model approach combining interventions that can feasibly be supplied on the ground. The main areas of activity set out in the plan are: scaling up interventions to control tuberculosis; promoting the research and development of improved diagnostics, drugs and vaccines; and engaging in related activities for advocacy, communications and social mobilization.
Scenarios for the planning process were developed; these looked at issues both globally and in seven epidemiological regions. The scenarios made ambitious but realistic assumptions about the pace of scale-up and implementation coverage of the activities. A mathematical model was used to estimate the impact of scaling up current interventions based on data from studies of tuberculosis biology and from experience with tuberculosis control in diverse settings.
The estimated costs of the activities set out in the Global Plan were based on implementing interventions and researching and developing drugs, diagnostics and vaccines; these costs were US$ 56 billion over 10 years. When translated into cost per disability adjusted life year averted, these costs compare favourably with those of other public health interventions. This approach to planning for global tuberculosis control is a valuable example of developing plans to improve global health that has relevance for other health issues.
Résumé
Le plan mondial Halte à la tuberculose 2006-2015 fournit des lignes directrices aux décideurs politiques et aux directeurs de programmes nationaux. Il présente les interventions clés nécessaires à la réalisation des objectifs du Millénaire pour le développement concernant la tuberculose (TB), à savoir faire baisser de moitié, entre 1990 et 2015, la prévalence de cette maladie et la mortalité lui étant imputable et épargner 14 millions de vies. Mis au point par un vaste groupement de partenaires, le plan propose un modèle de stratégie associant des interventions faciles à mettre en œuvre sur le terrain. Les principaux domaines d’activité prévus sont : le passage à l’échelle supérieure des interventions de lutte antituberculeuse, la promotion des travaux de recherche et développement concernant des outils diagnostiques, des médicaments et des vaccins plus performants et l’engagement dans des activités connexes de plaidoyer, de communication et de mobilisation sociale.
Des scénarios ont été développés pour aider au processus de planification : ils considèrent les problèmes à l’échelle mondiale et dans sept régions épidémiologiques. Ces scénarios reposent sur des hypothèses ambitieuses, mais réalistes, quant au rythme de passage à l’échelle supérieure et de développement de la couverture des activités. Un modèle mathématique a été utilisé pour évaluer l’impact du passage à l’échelle supérieure des interventions en cours à partir de données d’études biologiques sur la tuberculose et de l’expérience acquise dans divers contextes en matière de lutte antituberculeuse.
Les estimations de coûts pour les activités prévues par le Plan mondial correspondent à la mise en œuvre des interventions et aux travaux de recherche et développement de médicaments, d’outils diagnostiques et de vaccins améliorés ; elles se montent à US$ 56 milliard sur 10 ans. Une fois convertis en coûts par année de vie corrigée de l’incapacité (DALY), ces chiffres supportent favorablement la comparaison avec ceux d’autres interventions de santé publique. Cette stratégie de planification de la lutte contre la tuberculose au niveau mondial est un exemple utile de programme de développement pour l’amélioration de la santé dans le monde, qui intéresse d’autres problèmes sanitaires.
Resumen
El Plan Mundial para Detener la Tuberculosis 2006-2015 es una hoja de ruta para instancias normativas y gestores de programas nacionales. En él se establecen las principales intervenciones necesarias para alcanzar las metas de los Objetivos de Desarrollo del Milenio relacionadas con la tuberculosis: reducir a la mitad la prevalencia de esta enfermedad y la mortalidad por esa causa para 2015 en comparación con los niveles de 1990 y salvar así 14 millones de vidas. Elaborado por una amplia coalición de asociados, el plan presenta un modelo que combina diversas intervenciones que pueden aplicarse de forma viable sobre el terreno. Las áreas principales de actividad contempladas en el plan son las siguientes: expansión de las intervenciones de lucha antituberculosa; promoción de la investigación y el desarrollo de mejores medios diagnósticos, medicamentos y vacunas; y participación en las actividades relacionadas con la promoción, las comunicaciones y la movilización social.
Se desarrollaron distintos escenarios para el proceso de planificación, considerando los problemas a nivel mundial y en siete regiones epidemiológicas. Los escenarios partían de hipótesis ambiciosas pero realistas sobre el ritmo de expansión y la cobertura de aplicación de las actividades. Se utilizó un modelo matemático para estimar la repercusión de la expansión de las intervenciones actuales sobre la base de datos procedentes de estudios de la biología de la tuberculosis y de la experiencia de la lucha antituberculosa en diversos entornos.
Los costos estimados de las actividades establecidas en el Plan Mundial -correspondientes a la ejecución de las intervenciones y la investigación y el desarrollo de medicamentos, medios de diagnóstico y vacunas- ascendían a US$ 56 000 millones a lo largo de 10 años. Traducidos al costo por AVAD (años de vida ajustados en función de la discapacidad) evitado, la cifra es más baja que la de otras intervenciones de salud pública. Este método de planificación del control mundial de la tuberculosis brinda un valioso ejemplo para elaborar planes de mejora de la salud mundial que revistan interés para otros problemas sanitarios.
ملخص
تُعد الخطة العالمية لدحر السل 2006 – 2015 بمثابة خارطة طريق لأصحاب القرار السياسي ومديري البرامج الوطنية؛ فەي توطِّد الأنشطة الرئيسية اللازمة لتحقيق المرامي الإنمائية للألفية وأەدافەا المتعلِّقة بالسل؛ وەي الوصول بمعدلات الانتشار والوفيات عام 2015 إلى نصف ما كانت عليە عام 1990، وإنقاذ حياة 14 مليون نسمة. وتمثـِّل الخطة العالمية التي شارك في إعدادەا تحالف واسع النطاق من الشركاء أسلوباً نموذجياً يضم التدخلات التي يمكن تنفيذەا بسەولة في الواقع. أما مجالات العمل الرئيسية التي وطَّدتەا الخطة العالمية لدحر السل 2006 – 2015 فەي: النەوض بالتدخلات لمكافحة السل، وتعزيز البحوث وابتكار مواد تشخيصية وأدوات ولقاحات مُحَسَّنة، والمساەمة في الأنشطة المتعلِّقة بالحملات الإعلامية وبأنشطة التواصل، واستنەاض المجتمع لمكافحة السل.
وقد أُعِدَّت السيناريوەات لعملية التخطيط، وتـتناول ەذە السيناريوەات القضايا على الصعيد العالمي وعلى صعيد الأقاليم الإبيديميولوجية السبعة. وقد وَضَعَتْ السيناريوەات افتـراضاتٍ طموحةً ولكنەا واقعية حول سرعة النەوض بالأنشطة وحول التغطية بتنفيذەا، كما استُخْدِم نموذج حسابي لتقدير أثر النەوض بالتدخلات الحالية اعتماداً على المعطيات التي جُمِعَتْ من دراسات حول بيولوجيا السل ومن الخبرات حول مكافحة السل في مواقع واسعة التباين.
وقد ارتكزت التكاليف المقدَّرة للأنشطة التي وضعتەا الخطة العالمية لدحر السل 2006 – 2015، على تنفيذ التدخلات وعلى البحوث وعلى ابتكار الأدوية والمواد التشخيصية واللقاحات، وقُدِّرَتْ ەذە التكاليف بـ 56 مليون دولار على مدى عشر سنوات، أما عند تـرجمتەا إلى التكاليف وفق سنوات العمر المصحِّحة باحتساب مُدَد العجز التي يمكن تفاديەا، فإن ەذە التكاليف تفوق التدخلات الأخرى للصحة العمومية عند مقارنتەا بەا. إن ەذا الأسلوب من التخطيط لمكافحة السل على الصعيد العالمي يُعد مثالاً ەاماً لإعداد الخطط لتحسين الصحة في العالم والتي قد يكون لەا علاقة مع القضايا الصحية الأخرى.
Introduction
“When the elephants fight, the grass gets trampled”– this aphorism exemplifies the vigorous debate over the best approach to planning for development. The debate positions Jeffrey Sachs,1 a proponent of a comprehensive supply-side blueprint, against William Easterly,2 a proponent of more specific solutions that respond to local demands. In the trampled grass lies an approach to planning that combines the benefits – and minimizes the drawbacks – of both these approaches. Amartya Sen recommends using an optimal planning process whereby interventions that can potentially be supplied are matched against ground-level explorations of what is feasible.3 This is exemplified by the global planning for tuberculosis (TB) control over the next decade that has been undertaken by the diverse coalition of stakeholders in the Stop TB Partnership.
The Stop TB Partnership is a global movement to accelerate social and political action against tuberculosis. The network of partners includes international organizations, countries, donors (from both the public and private sectors), individuals and governmental and nongovernmental organizations. The partnership was established in 2000 to realize the goal of eliminating tuberculosis as a public health problem by 2050. The partnership’s secretariat is housed within WHO. The partnership has seven working groups, each providing a focus for coordinated action in a particular area of activity (Box 1).
Box 1. The Stop TB Partnership’s working groups.
| Working group | Purpose |
|---|---|
| DOTS expansion | To expand the coverage of DOTS, WHO’s recommended strategy for tuberculosis control |
| Multidrug-resistant TB | To integrate surveillance for drug resistance and the management of multidrug-resistant TB into routine components of TB control by providing access to diagnosis and treatment for all patients and engaging all health-care providers |
| TB/HIV | To reduce the global burden of HIV-related TB by implementing effective collaboration between TB and HIV programmes and communities, and by promoting evidence-based collaborative activities to control TB and HIV |
| Advocacy, communication and social mobilization | To achieve TB-free communities by using global and country-level advocacy, as well as communication and social mobilization techniques |
| New TB diagnostics | To implement research, advocacy and/or operational activities to develop tools to diagnose TB and to meet the aims of the partnership |
| New TB vaccines | To bring together the wide range of international groups with an interest in developing a TB vaccine; to accelerate the identification and introduction of the most effective vaccination strategy |
| New TB drugs | To ensure that scientists, academics, pharmaceutical companies, donors, multilateral agencies and patients are working together to speed the development of new drugs for TB |
As a road map both for policy-makers and managers of national programmes, the Global Plan to Stop TB 2006–20154 sets out the key actions needed to implement interventions to control tuberculosis, such as case-finding, treatment and preventive treatment for high-risk groups,5 as well as actions needed for research and development of improved tools such as diagnostics, drugs and vaccines. The plan is oriented towards achieving the international targets linked to the Millennium Development Goals: to halve prevalence and deaths by 2015 relative to 1990 levels.6 These targets represent a step towards the Stop TB Partnership’s vision of a tuberculosis-free world by 2050.7
In this paper we outline the general rationale for planning for control of communicable diseases, describe briefly the process and outcomes of global planning for tuberculosis control, and then review the policy implications of long-term planning for tuberculosis control to illustrate the value of the planning process.
Background
Communicable diseases constitute a large proportion of the global burden of morbidity and mortality, accounting in 2002 for 24% of the global burden of disability-adjusted life years (DALYs) and 19% of global deaths.8 Globalization not only poses threats regarding communicable disease control; it also provides opportunities.9 Awareness of the global burden and threat of communicable diseases as well as opportunities for control have led to the development of many international partnerships and alliances to coordinate efforts to control such diseases. Good examples include the global campaign in the 1960s and 1970s that eradicated smallpox,10 the programme since 1974 to control onchocerciasis in west Africa11 and, more recently, the development of the GAVI Alliance (formerly the Global Alliance for Vaccines and Immunization)12 and the Stop TB Partnership.13
The effectiveness and efficiency of international collaborations depends on sound planning. Examples of plans for global control of an acute communicable disease include those related to poliomyelitis;14 an example related to a chronic communicable disease includes those plans related to leprosy.15,16 Long-term planning is necessary to control epidemics of chronic communicable diseases, such as leprosy and tuberculosis, that change relatively slowly owing to the specific dynamics of disease transmission. Tuberculosis exacts an annual global toll of 8.8 million new cases and 1.6 million deaths.17 The main areas of activity set out in the plan are: scaling up the use of interventions for tuberculosis control; promoting research and development of improved diagnostics, drugs and vaccines; and promoting related activities for advocacy, communications and social mobilization, including the crucial parts played by patients and communities in implementing the plan. These activities dovetail with WHO’s new Stop TB Strategy18 and are oriented towards achieving the international targets for 2015. The Stop TB Strategy builds on the DOTS strategy (the policy package comprising political commitment, bacteriological case detection, standardized supervised treatment, effective drug supply and an evaluation system).19 As recognized in the Stop TB Strategy, scaling up implementation of interventions for tuberculosis control involves both collaboration across the health sector and the need to focus on special populations.
Methods
Each of the partnership’s seven working groups developed a strategic plan of activities to contribute to the overall plan. Three of the working groups are concerned with implementing interventions: the DOTS expansion group, the multidrug-resistant tuberculosis (MDR-TB) group and the tuberculosis/HIV group (Box 1). They developed regional and global scenarios to assess the impact and costs of the planned scale-up of activities from 2006 to 2015. Scenarios were developed with the input of a coalition of partners to assess the global impact and costs as well as the impact in seven epidemiological regions (excluding the established market economies and central Europe, which account for only 1.7% of the global total of TB cases.). The scenarios involved ambitious but realistic assumptions about the pace of scale-up and the implementation coverage of activities. In the breadth of the coalition’s representation (which included groups of activists and representatives of civil society groups, governments, academics, United Nations organizations, nongovernmental organizations and technical experts), this coalition made a unique contribution to Sen’s favoured approach to global planning. The groups concerned with research and development (diagnostics, drugs and vaccines) and the advocacy, communications and social mobilization group developed strategic plans based on each group’s consensus view, but they did not estimate these activities’ expected epidemiological impacts.
A mathematical model was used – based on previous modelling20,21 – to estimate the potential impact of scaling up interventions; levels of tuberculosis case detection and treatment outcomes were estimated for the next 10 years. Data from studies of tuberculosis biology and from tuberculosis control experience in diverse settings were brought together to model the impact on tuberculosis prevalence, incidence and death rates in relation to the 2015 targets. The two main components in estimating costs – funding and funding gaps – were analysed in relation to the implementation of interventions and the research and development of new diagnostics, drugs and vaccines.22
Findings
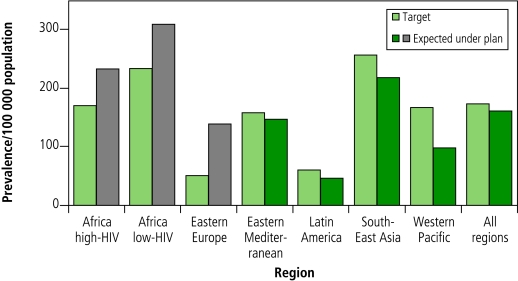
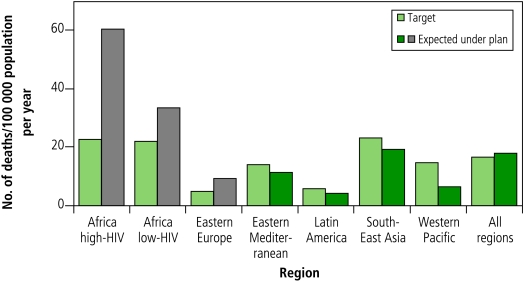
The modelling outcomes were represented in terms of the number of people benefiting from interventions and the progress towards targets (i.e. the impact on disease burden). From 2006 to 2015, a total of about 50 million people will be treated for tuberculosis under the Stop TB Strategy. This total includes 800 000 patients with MDR-TB and 3 million patients infected with both tuberculosis and HIV who will be enrolled in antiretroviral therapy. Implementing the plan would result in 14 million lives saved compared with a situation in which the DOTS strategy is not implemented. In terms of the impact of the planned scale-up, estimates indicate that the strategy would be expected to achieve the global target for 2015 for prevalence and deaths (measured against the 1990 baseline) with considerable progress made in all regions (measured over the planning period 2006–2015). (Fig.1 and Fig. 2.)
Fig. 1.
Expected prevalence of all forms of tuberculosis in 2015 under implementation of the Global Plan to Stop TB 2006–2015 in comparison with targetsa (countries included in each region can be found in the text of the plan)
a The target is light green. The number expected under plan is dark green when the target is likely to be met, and grey when it is not likely to be met.
Fig. 2.
Expected number of deaths from all forms of tuberculosis in 2015 under implementation of the Global Plan to Stop TB 2006–2015 in comparison with targetsa (countries included in each region can be found in the text of the plan)
a The target is light green. The number expected under plan is dark green when the target is likely to be met, and grey when it is not likely to be met.
Because of the surge in tuberculosis in Africa since 1990 (as a consequence of the HIV epidemic) and in eastern Europe (as a consequence of socioeconomic turmoil in addition to the emergence of MDR-TB), these regions are likely to achieve these targets after 2015. Additional scenarios identified the measures needed to achieve the targets in these two regions. There would need to be massive improvements in the general health systems, a 50% reduction in HIV incidence and rapid availability of new tools to increase diagnostic capacity, substantially shorten treatment duration, and effectively prevent tuberculosis. Even substantial amounts of additional funding and greater effort are unlikely to succeed in overcoming these constraints by 2015.
The Global Plan to Stop TB 2006–2015 builds on the track record of the first Global Plan (2001–2005), in which US$ 5 billion was budgeted, raised and spent, with a doubling of patients treated under the DOTS strategy from 2 million in 2000 to more than 4 million in 2004. The total US$ 56 billion cost of the current plan represents a threefold increase in annual investment in tuberculosis control compared with the first Global Plan. As shown in Table 1, this total includes US$ 47 billion for scaling up interventions and US$ 9 billion for research and development. The total estimated funding gap is US$ 31 billion, since an estimated US$ 25 billion is likely to be available based on projections of trends in funding (both domestic and external). These projections include the contributions made by various funding sources to the budgets of national tuberculosis control programmes, including the World Bank, bilateral donors and the Global Fund to Fight AIDS, Tuberculosis and Malaria.
Table 1. Total costs, available funding and funding gaps in the Global Plan to Stop TB 2006–2015, as compared with the first Global Plan, 2001–2005.
| Global Plan to Stop TB
(10-year plan)a |
First Global Plan
(5-year plan) |
||||
|---|---|---|---|---|---|
| Costs | Available funding | Funding gap | Costs | ||
| Total implementation | 47.2 | 22.5 | 24.7 | 8.0 | |
| Country costs | 44.3 | 21.8b | 22.5 | – | |
| DOTS expansion | 28.9 | – | – | 6.0 | |
| Strategy for multidrug-resistant tuberculosis | 5.8 | – | – | 1.1 | |
| TB/HIV collaborative activities | 6.7 | – | – | 0.6 | |
| Advocacy, communication and social mobilization programmes | 2.9 | – | – | 0.0 | |
| International agencies (technical cooperation)c | 2.9 | 0.7 | 2.2 | 0.3 | |
| Total new tools | 9.0 | 2.8 | 6.1 | 0.9 | |
| Vaccinesd | 3.6 | 2.1 | 1.5 | 0.4 | |
| Drugs | 4.8 | 0.6 | 4.2 | 0.3 | |
| Diagnostics |
0.5 |
0.1 |
0.4 |
0.2 |
|
| Total costs | 56.2 | 25.3 | 30.8 | 9.1 | |
a All amounts shown in billions of US$. Column totals may not add up exactly due to rounding. b The figures for domestic funding assume that governments’ commitments in 2005 are sustained and increase in line with inflation; commitments from the Global Fund to Fight AIDS, Tuberculosis and Malaria are based on results. c Technical cooperation includes strategic and technical support, capacity building, monitoring and evaluation, operational research, policy development and working groups. d This category includes costs for maintenance of the current BCG (bacille Calmette–Guérin) vaccination programme.
Out of a total need of US$ 47 billion to scale up interventions (Table 1), the funding likely to be available is estimated at US$ 22.5 billion, leaving a US$ 25 billion funding gap, which requires increased funding commitments from nations and donors. The shortfall in funding available for scaling up increases from US$ 1.4 billion in 2006 to US$ 3.1 billion in 2015. At a total scale-up cost of US$ 47 billion, the global cost-effectiveness is US$ 157 per DALY averted. (Details of this analysis are available from the corresponding author upon request.) The average global cost per patient treated under the plan is US$ 448, ranging from US$ 151 in WHO’s South-East Asia Region to US$ 1732 in eastern Europe. Calculating the cost of scale-up on a per-capita population basis, the cost per person per year is lowest in Latin America, South-East Asia, the Western Pacific and the Eastern Mediterranean; these are areas where it ranges from US$ 0.10 to US$ 0.30. In African countries with a low prevalence of HIV and in eastern Europe, costs range from US$ 0.90 to US$ 1.10. In countries in Africa with a high prevalence of HIV, costs range from US$ 1.30 to US$ 1.50.
Discussion
From 2006 to 2015, a total of 50 million people will be treated for tuberculosis under the Stop TB Strategy. Treating an average of 5 million people per year over the next 10 years is feasible and realistic: 26 million people with tuberculosis (both new cases and relapsed cases) were treated under the DOTS strategy from 1995 to 2005, of whom 4.9 million were treated in 2005.
The key financial outcomes of the process of developing the plan included estimating the global cost of activities (US$ 56 billion) that will be oriented towards achieving the partnership’s targets for 2015 and estimating the likely funding gap (US$ 31 billion). There are two major reasons why the funding gap increases from US$ 1.4 billion in 2006 to US$ 3.1 billion in 2015. The first is that the estimates of funding are based on the assumptions that domestic funding will be sustained at 2005 levels and that donor funding (excluding funding from the Global Fund to Fight AIDS, Tuberculosis and Malaria) will be sustained at 2004 levels. The second is that the plan includes large investments in new approaches, which is in keeping with the Stop TB Strategy. These investments will include new approaches to implementing DOTS as well as more widespread implementation of the management of MDR-TB, collaborative activities to control tuberculosis and HIV, and advocacy, communication and social mobilization activities. The plan also includes much more investment in technical cooperation (at around an average of US$ 290 million per year), which is needed to support this substantial increase in both the number and scale of interventions.
These additional large investments will require increased funding commitments both from governments of countries with a high burden of tuberculosis and from foreign donors. Given the existing distribution of funding for tuberculosis control and the size of the funding gap, it is likely that governments in countries where there is a high burden will need to finance a large proportion of this gap themselves. Filling the funding gap would require donor funding to increase about eight times, whereas domestic funding would need only to double.
The global cost-effectiveness of US$ 157 per DALY averted compares favourably with that of other health interventions, as described in the Disease Control Priorities Project.23 For nearly all regions, the cost per DALY averted is less than US$ 1 dollar per day. The exception is in eastern Europe, where the cost per DALY averted is close to US$ 2100 owing to extensive reliance on relatively expensive hospitalization of patients during treatment for drug-susceptible tuberculosis and because of the much higher costs of managing MDR-TB. Tuberculosis control in South-East Asia and the Western Pacific is particularly cost-effective at about US$ 60–70 per DALY averted. The DOTS strategy is among the most cost-effective of all health interventions. The tuberculosis control interventions in the plan represent an enhanced DOTS strategy that is being used as part of the new Stop TB Strategy; despite being more comprehensive, this strategy is still cost-effective.
In order to realize the plan’s expected gains, global strategic directions will need to be translated into national-level action and funding through the use of more detailed planning. This planning must be tailored to regions’ and countries’ specific needs. Long-term regional and national plans, based on estimates of impact and costs of proposed actions, will provide powerful tools for policy-makers and programmes. The lack of long-term national planning was identified as a barrier to progress in tuberculosis control, spurring the adoption of the 2005 World Health Assembly resolution on sustainable financing for tuberculosis prevention and control.24
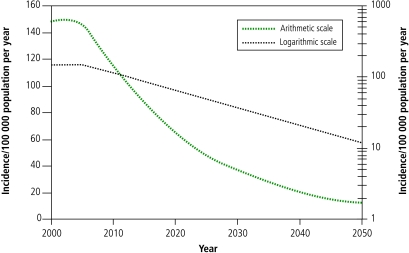
The planning outcomes have implications for the future prospects of long-term tuberculosis control. Projections show that continued implementation of the planned interventions at the level of scale-up reached in 2015 will not result in the partnership’s vision of eliminating tuberculosis by 2050 (Fig. 3). The average rate of decline in tuberculosis incidence that is expected to be reached globally between 2010 and 2015 is 5–6% per year. Under this plan, the incidence in 2050 will still be about 100 times greater than the elimination target of 1 case per million population. In addition to scaling up interventions, new tools will be needed to meet this long-term goal by more effectively reducing transmission and thereby preventing infection. They are also needed to prevent tuberculosis among people already infected, by decreasing reactivation of latent infection. This may be achieved through mass treatment of latent infection (ideally by specifically targeting those infected people most likely to develop tuberculosis) or mass vaccination.25 The development pathway for each new tool has a high attrition rate. The chances of a successful product emerging from the end of the development pipeline depend in part on the number of potential products entering the pipeline.26 To keep the pipeline stocked, increased investment is necessary not only in product research and development, but also in basic research on pathogenesis and immunology.
Fig. 3.
Projected tuberculosis incidence 2000–2050 plotted on a linear scale and a logarithmic scale
Although the planning process was successful, “the proof of the pudding is in the eating” and the plan’s success depends on how it is implemented, specifically on the extent to which the plan helps mobilize the needed funds for planned activities and how successful countries are in implementing activities. Critical evaluation of progress in implementing the plan will reveal the degree of success or failure encountered in translating into action the political commitment mobilized in developing and launching the plan. While the assumptions that underpinned the plan’s development were based on the best understanding of the planning and epidemiological parameters at the time, measurements of progress in implementing activities and their impact will enable testing and refining of these assumptions. The estimates of tuberculosis prevalence (Fig. 1 and Fig. 2) in the two epidemiological regions of sub-Saharan Africa (i.e. those countries with high HIV prevalence and those with low HIV prevalence) provide an example of this. The estimated tuberculosis prevalences reflect estimates of the risk of death among tuberculosis patients: the high risk of death among HIV-positive patients with tuberculosis results in a lower tuberculosis prevalence in the region of countries with high HIV prevalence, compared with the region of countries where there is low HIV prevalence. As part of measuring the plan’s impact, improved assessments of life expectancy among HIV-positive and HIV-negative patients with tuberculosis will enable testing and refining of the assumptions underpinning prevalence estimates. The need for flexibility in adopting and adapting the plan is highlighted by the problem of extensively drug-resistant tuberculosis, which has emerged as a key issue since the plan’s publication.27
Conclusion
This approach to planning global tuberculosis-control activities is valuable as an example of using planning to improve global health. Based on a wide consensus, interventions that can potentially be supplied were matched against ground-level explorations of what is feasible; this was done in keeping with the approach advocated by Sen. A consensus plan based on sound epidemiological analysis with robust budget justifications presents a convincing argument for mobilizing the resources needed for action. It also provides a means of ensuring accountability, since progress is measured against actual milestones. ■
Acknowledgements
The authors gratefully acknowledge the contributions of the Global Plan Study Team and of all contributors listed in the plan (see http://www.stoptb.org/globalplan).
Funding
The Global Plan to Stop TB’s development was funded by the Stop TB Partnership through contributions from the Netherlands Ministry of Foreign Affairs, the Open Society Institute, the United Kingdom Department for International Development, the United States Agency for International Development and the World Bank.
Competing interests
None declared.
References
- 1.Sachs J. The end of poverty: economic possibilities for our time. New York: Penguin; 2004. [DOI] [PubMed] [Google Scholar]
- 2.Easterly W. The white man’s burden: why the West’s efforts to aid the rest have done so much ill and so little good. New York: Penguin; 2006. [Google Scholar]
- 3.Sen A. The man without a plan. Foreign Affairs March/April 2006.
- 4.Stop TB. Partnership. The global plan to stop TB 2006–2015 Geneva: WHO; 2006. Available at: http://www.stoptb.org/globalplan
- 5.Treatment of tuberculosis: guidelines for national programmes 3rd ed. Geneva: WHO; 2003. [Google Scholar]
- 6.Dye C, Maher D, Weil D, Espinal M, Raviglione M. Targets for global tuberculosis control. Int J Tuberc Lung Dis. 2006;10:460–2. [PubMed] [Google Scholar]
- 7.Stop TB. Partnership. Basic framework for the Global Partnership to Stop TB Available at: http://www.stoptb.org/stop_tb_initiative/
- 8.The world health report 2004: changing history Geneva: WHO; 2004.
- 9.Yach D, Bettcher D. The globalization of public health. I: threats and opportunities. Am J Public Health. 1998;88:735–8. doi: 10.2105/ajph.88.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. The intensified smallpox eradication programme, 1967-1980. In: Smallpox and its eradication Geneva: WHO; 1988:421-538. [Google Scholar]
- 11.Hopkins DR, Richards FO, Katabarwa M. Whither onchocerciasis control in Africa? Am J Trop Med Hyg. 2005;72:1–2. [PubMed] [Google Scholar]
- 12.Jacobs L, Martin J-F, Godal T. A paradigm for international cooperation: the Global Alliance for Vaccines and Immunization (GAVI) and the Vaccine Fund. In: Levine MM, Kaper JB, Rappuoli R, Liu MA, Good MF, eds. New generation vaccines, 3rd ed. New York: Marcel Dekker; 2004. [Google Scholar]
- 13.Kumaresan J, Heitkamp P, Smith I, Billo N. Global Partnership to Stop TB: a model of an effective public health partnership. Int J Tuberc Lung Dis. 2004;8:120–9. [PubMed] [Google Scholar]
- 14.Global Polio Eradication Initiative. strategic plan 2004–2008 Geneva: WHO; 2003. Available at: http://www.polioeradication.org/content/publications/2004stratplan.pdf [PubMed]
- 15.The final push towards elimination of leprosy: strategic plan 2000–2005 Geneva: WHO; 2003 (WHO/CDS/CPE/CEE/2000.1).
- 16.Global strategy for further reducing the leprosy burden and sustaining leprosy control activities (plan period: 2006-2010) Geneva: WHO; 2005 (WHO/CDS/CPE/CEE/2005.53). Available at: http://www.who.int/lep/resources/GlobalStrategy.pdf [PubMed]
- 17.Global tuberculosis control: surveillance, planning, financing WHO report 2007. Geneva: WHO; 2007 (WHO/HTM/TB/2007.376).
- 18.Raviglione MC, Uplekar M. WHO’s new Stop TB Strategy. Lancet. 2006;367:952–5. doi: 10.1016/S0140-6736(06)68392-X. [DOI] [PubMed] [Google Scholar]
- 19.What is DOTS? Geneva: WHO; 2005.
- 20.Williams BG, Granich R, Chauhan LS, Dharmshaktu NS, Dye C. The impact of HIV/AIDS on the control of tuberculosis in India. Proc Natl Acad Sci USA. 2005;102:9619–24. doi: 10.1073/pnas.0501615102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Currie CSM, Williams BG, Cheng RCH, Dye C. Tuberculosis epidemics driven by HIV: is prevention better than cure? AIDS. 2003;17:2501–8. doi: 10.1097/00002030-200311210-00013. [DOI] [PubMed] [Google Scholar]
- 22.Floyd K, Pantoja A. The Global Plan to Stop TB, 2006-2015: methods used to estimate costs, funding and funding gaps Geneva: WHO; 2006. [Google Scholar]
- 23.Laxminarayan R, Mills AJ, Breman JG, Measham AR, Alleyne G, Claeson M, et al. Advancement of global health: key messages from the Disease Control Priorities Project. Lancet. 2006;367:1193–208. doi: 10.1016/S0140-6736(06)68440-7. [DOI] [PubMed] [Google Scholar]
- 24.Fifty-eighth World Health Assembly. resolutions and decisions Geneva: WHO; 2005 (WHA58/2005/REC/1). [Google Scholar]
- 25.Young D, Dye C. The development and impact of tuberculosis vaccines. Cell. 2006;124:683–7. doi: 10.1016/j.cell.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Glickman SW, Rasiel EB, Hamilton CD, Kubataev A, Schulman KA. A portfolio model of drug development for tuberculosis. Science. 2006;311:1246–7. doi: 10.1126/science.1119299. [DOI] [PubMed] [Google Scholar]
- 27.Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, et al. Extensively drug-resistant tuberculosis as a cause of death in patients coinfected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–80. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]