Abstract
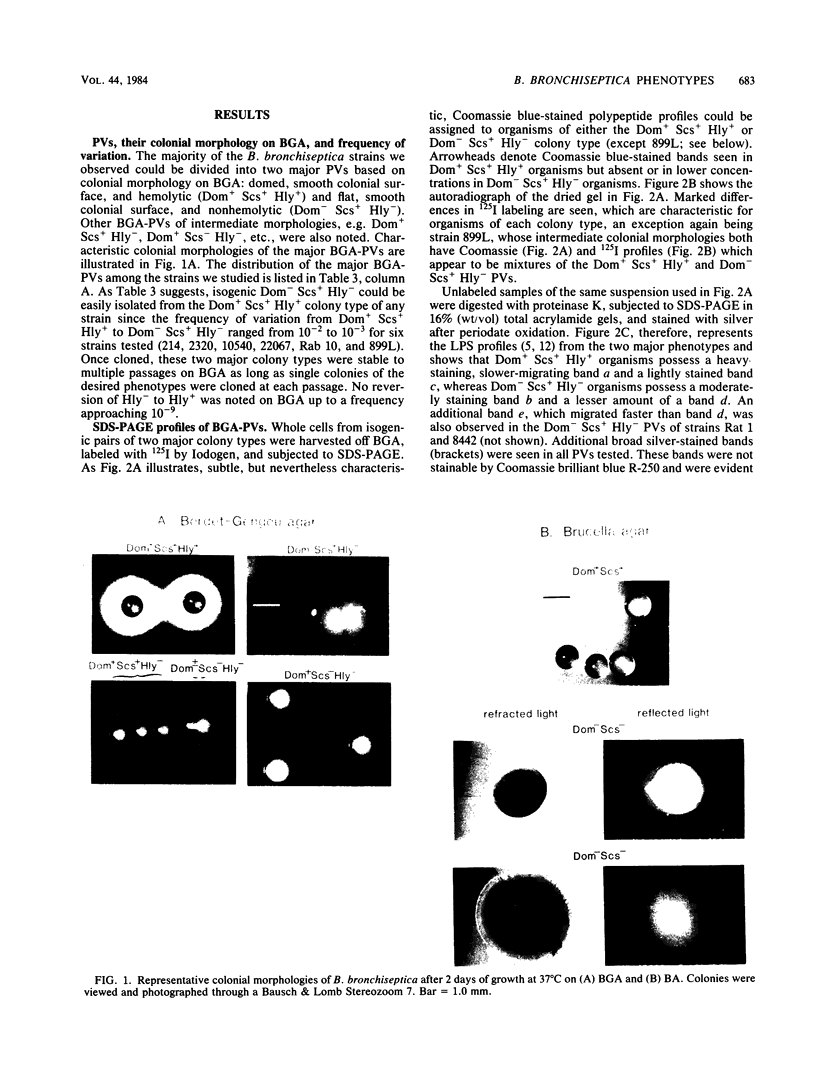
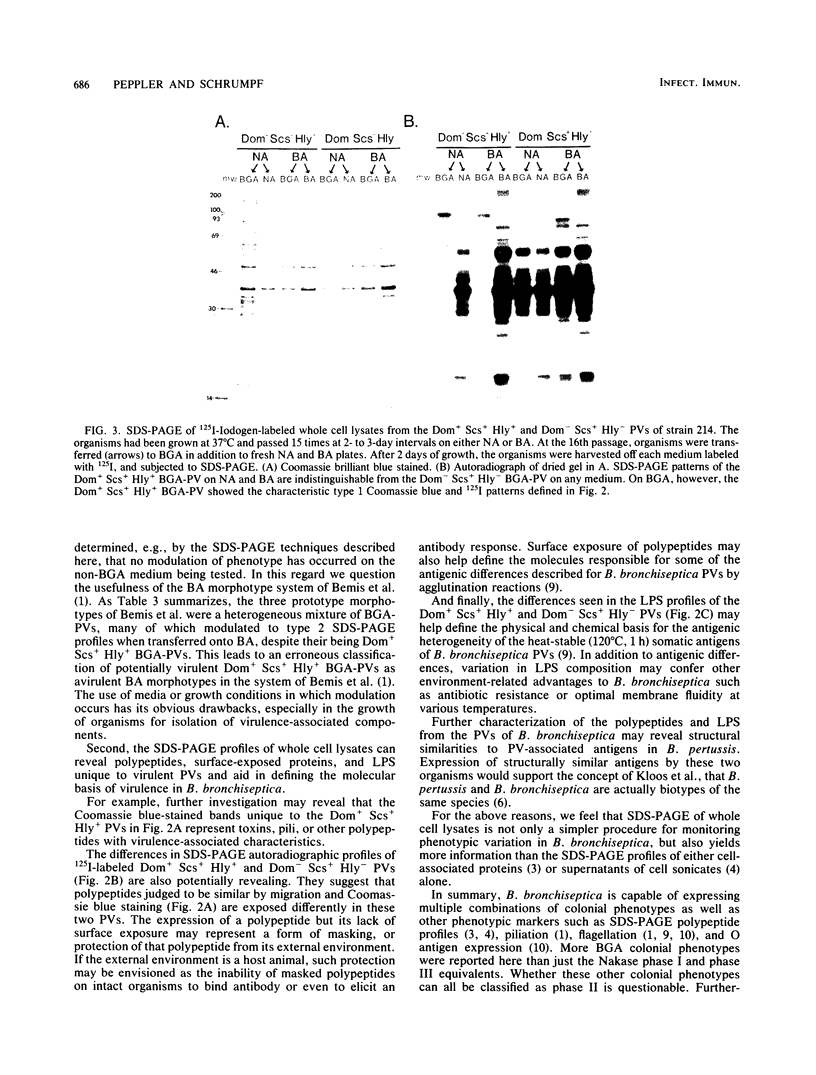
Most of the isolates of Bordetella bronchiseptica obtained by this laboratory possessed a characteristic colonial morphology when grown on Bordet- Gengou agar (BGA) at 37 degrees C. The colonies appeared domed (Dom+) with a smooth colonial surface (Scs+) and a clear zone of hemolysis ( Hly +). From these Dom+ Scs+ Hly + BGA colony types arose flat (Dom-), smooth colonial surface (Scs+) and nonhemolytic ( Hly -) variants at frequencies of 10(-2) to 10(-3). Isogenic pairs of Dom+ Scs+ Hly + and Dom- Scs+ Hly - BGA phenotype variants (BGA-PVs) were picked from 11 strains of B. bronchiseptica, and their whole cell lysates were compared with each other by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Characteristic SDS-PAGE profiles were observed for each of the Dom+ Scs+ Hly + and Dom- Scs+ Hly - BGA-PVs with regard to (i) surface-exposed proteins, based on autoradiographs of 125I- Iodogen -labeled organisms, (ii) polypeptide differences, based on gels stained with Coomassie brilliant blue R-250, and (iii) lipopolysaccharide differences based on gels stained with silver after oxidation with periodic acid. SDS-PAGE profiles were then used to monitor the phenotypes expressed by Dom+ Scs+ Hly + and Dom- Scs+ Hly - BGA-PVs transferred and grown on brucella agar, Trypticase soy agar, and nutrient agar. When grown on non-BGA media, the Dom+ Scs+ Hly + BGA-PVs from six of eight strains showed SDS-PAGE profiles identical to those of Dom- Scs+ Hly - BGA-PVs. This phenotypic change was reversible even after 15 subcultures on the non-BGA media, since Dom+ Scs+ Hly + organisms passed back onto BGA expressed both Dom+ Scs+ Hly + colonial morphology and Dom+ Scs+ Hly + SDS-PAGE profiles. The influence of cultural conditions on maintenance of virulence is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bemis D. A., Greisen H. A., Appel M. J. Bacteriological variation among Bordetella bronchiseptica isolates from dogs and other species. J Clin Microbiol. 1977 Apr;5(4):471–480. doi: 10.1128/jcm.5.4.471-480.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endoh M., Takezawa T., Nakase Y. Adenylate cyclase activity of Bordetella organisms. I. Its production in liquid medium. Microbiol Immunol. 1980;24(2):95–104. doi: 10.1111/j.1348-0421.1980.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Ezzell J. W., Dobrogosz W. J., Kloos W. E., Manclark C. R. Phase-shift markers in Bordetella: alterations in envelope proteins. J Infect Dis. 1981 Apr;143(4):562–569. doi: 10.1093/infdis/143.4.562. [DOI] [PubMed] [Google Scholar]
- Goodnow R. A., Causey S. C., Geary S. J., Wren W. S. Comparison of an infective avirulent and canine virulent Bordetella bronchiseptica. Am J Vet Res. 1983 Feb;44(2):207–211. [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Peppler M. S. Isolation and characterization of isogenic pairs of domed hemolytic and flat nonhemolytic colony types of Bordetella pertussis. Infect Immun. 1982 Mar;35(3):840–851. doi: 10.1128/iai.35.3.840-851.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppler M. S. Two physically and serologically distinct lipopolysaccharide profiles in strains of Bordetella pertussis and their phenotype variants. Infect Immun. 1984 Jan;43(1):224–232. doi: 10.1128/iai.43.1.224-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Yokomizo Y., Shimizu T. Adherence of Bordetella bronchiseptica to swine nasal epithelial cells and its possible role in virulence. Res Vet Sci. 1979 Jul;27(1):15–21. [PubMed] [Google Scholar]






