Abstract
Oversaturated deoxy-α2β2T4V aggregated instantly without a delay time, which is in contrast to the delay time before the generation of fibers of deoxy-HbS and deoxy-α2β2E6V, D73H. Solubility of deoxy-α2β2T4V was ~10-fold lower than that of deoxy-HbS and was similar to oxy- and deoxy-α2β2E6V,T4V. These results indicate that β4Val in HbA in the oxy and deoxy forms with or without β6Val facilitates hydrophobic interaction of the A-helix with the EF helix of adjacent molecules without forming a β4/β73 hydrogen bond. Deoxy-HbA generated crystals following aggregation as does HbC-Harlem(α2β2E6V,D73N), while α2β2T4V and α2β2D73H as well as HbS, α2β2E6V,D73H and α2β2E6V,T4V in the oxy and deoxy forms did not form crystals, indicating in addition to the strength of β6 amino acid hydrophobicity that the synergism between the β4Thr hydrogen bond and β6 hydrophobic interaction free energies on the A-helix play a critical role in formation of fibers versus crystalline nuclei during phase transition.
Keywords: Hemoglobin, Polymerization, Fiber formation, Crystallization, Hydrogen bond, Hydrophobic interaction, Sickle hemoglobin, Phase transition
The single amino acid replacement of β6Glu with a hydrophobic β6Val in the β chain of hemoglobin (HbS) leads to decreased solubility and formation of gels including long, multi-stranded fibers when HbS is deoxygenated [1, 2]. HbS fibers appear stable but over longer periods in vitro form crystals with a lower solubility [3, 4]. In addition, the single amino acid replacement of β6Glu to β6Lys (HbC) leads to crystallization in the oxy-form in vitro and in vivo [5]. Intracellular polymers or fibers which go on to form gels and crystals from soluble hemoglobin cause a significant reduction in RBC deformability and leads to membrane damage, which can obstruct flow in the microcirculation in patients with a sickle cell disorder [1, 2, 6]. Furthermore, oxy- and deoxy-HbA formed amorphous aggregates when oversaturated in vitro even though HbA has a high solubility. In addition, oversaturated deoxy-HbA generated crystals after a long incubation time in high phosphate buffers [7, 8]. In fact, despite the polymeric nature of deoxy-HbS, its assembly behaves thermodynamically like crystallization, with a well-defined solubility as measured by classic sedimentation experiments [9]. Moreover, the kinetics of deoxy-HbS polymerization is related to solubility in which the rate is proportional to the super-saturation point raised to a high power [2]. Previously we found that the solubility of hemoglobin is not unique and depends on experimental conditions and type of polymers generated [8, 10].
Growth of domains, development of deoxy-HbS polymers and their structure as well as oxy-HbC crystallization were studied by differential interference contrast (DIC) microscopic methods [11–13]. Deoxy-HbS polymerization kinetics observed by DIC microscopy indicated that domain structure depended on the kinetics of nuclei formation [12]. More recently, DIC microscopic examination of deoxy-HbS fibers after laser photolysis and temperature-jump conditions showed that domains containing fibers produced time-dependent, multi-sperulitic domains made from homogeneous nuclei by formation of dense liquid clusters during a phase transition [14–16]. These studies suggested that homogeneous nucleation prior to HbS polymer formation occurs in a single liquid cluster, which represents a precursor phase during a liquid-to-solid-phase transition. In addition, Galkin et al. showed that formation of deoxy-HbS fibers is a first-order phase transition, similar to gas-solid phase transition, and this permits the application of thermodynamic and kinetic rules of phase transition to the analysis of HbS polymerization [15]. Galkin et al. also reported that nucleation of deoxy-HbS polymers proceeds via a precursor involving a meta-stable mesoscopic cluster of a dense liquid phase in crowded HbS solution [15]. They suggested that homogeneous nucleation of HbS polymers occurs within a cluster as a precursor phase [15, 17]. Nucleation prior to fiber formation of deoxy-HbS also was suggested to proceed by a two-step process like formation of crystalline nuclei: a droplet of dense liquid forms within which a crystalline nucleus appears due to the ordering of a certain number of molecules [18, 19].
Intracellular polymers or fibers of Hb S cause a significant reduction in red blood cell deformability (sickling), leading to obstruction of flow in the microcirculation. Each polymer of Hb S assembles into 14-stranded fibers, which then form a viscous gel [1, 2]. Further analysis of the fibers demonstrated that each is composed of seven half-staggered double strands [2], suggesting that the double-stranded structure of deoxy Hb S crystals may be relevant components of fibers within cells. An important feature of the double strand is that the contact between two molecules in adjacent strands (i.e., the so-called “lateral” contact) involves β6Val and β4Thr in hydrophobic and hydrogen bond interactions, respectively. Specifically, β85Phe and β88Leu are involved with β6Val and β73Asp is involved with β4Thr in a hydrogen bond in the E and F helices of the β chain [20, 21]) as schematically shown in Fig. 1.
Figure 1. Schematic diagram showing some of the lateral Hb tetramer hydrophobic and hydrogen bond interactions during deoxy-Hb S polymer formation.
Hydrophobic interaction of β6Val (red circle) with β88Leu/β85Phe (green circle) as well as hydrogen bond formation between β4Thr (violet circle) and β73Asp (blue circle) of Hb S tetramers are shown during deoxy-HbS polymerization.
Morphological, kinetic and thermodynamic studies of fiber/domain formation using engineered HbS and HbA β4 and β73 variants suggest the importance of the β4 hydrogen bond interaction with β73Asp in deoxy-HbS fiber/domain formation [16, 22]. However, the relationship between the hydrophobic and hydrogen bond interactions on the surface of the A-helix of the β chain of hemoglobin leading to formation of amorphous aggregates, fibers and crystals of oversaturated HbA and HbS during a liquid- to solid-phase transition is not completely understood. In this report, we focus on evaluation of polymerization properties of oversaturated HbAβ4Val (α2β2T4V) and HbAβ73His (α2β2D73H) compared to those of HbA, HbS, HbSβ4Val (α2β2E6V, T4V) and HbSβ73His (α2β2E6V, D73H) in order to obtain a better understanding of the relationship between β4/β73 hydrogen bond and β6 amino acid hydrophobicity in the A-helix of hemoglobin in promoting amorphous aggregate versus fiber/domain formation following crystallization in oversaturated hemoglobin solutions. These amino acid variants were selected since β4Val maps close to β6Val in HbS and should create a region of hydrophobicity similar to the normally mutated β6Glu to Val site in HbS. Furthermore, substitution of β4Thr to Val also should disrupt the normal β4Thr-β73Asp hydrogen bond and perturb polymer formation of HbS. In addition, β73His should constrain/inhibit formation of the normal β73Asp-β4Thr hydrogen bond. Results from these variants therefore are important in helping to define determinants critical for aggregate versus crystal or fiber formation, and may help in design of targeted inhibitors of HbS polymer formation in vivo.
Materials and Methods
Purification of hemoglobins from human blood
HbS and HbA were purified from AS heterozygotes as described previously by standard chromatography on a Source 15S FPLC column (Pharmacia Biotech, Inc., Piscataway, NJ) equilibrated with 40 mM Bis-Tris buffer, pH 5.8, and eluted using a linear gradient from 0–0.2 M NaCl in the same buffer [23]. In addition, HbC-Harlem was purified from HbAC-Harlem heterozygotes as described previously [23].
Mutagenesis, expression, purification and characterization of hemoglobin variants
Recombinant HbSβ4Val (α2β2E6V, T4V), HbAβ4Val (α2β2T4V), HbSβ73His (α2β2E6V, D73H) and HbAβ73His (α2β2D73H) were expressed in bacteria after subcloning the corresponding cDNAs into pHE2, which co-expresses α- and β-globin chains as well as methionine aminopeptidase under transcriptional control of a ptac promoter [16, 24]. β-globin chain variants were constructed by site-specific mutagenesis of the normal β chain using recombination/PCR, as described [22, 25], and were co-expressed with α chains to form tetrameric HbS and HbA variants [22].
Purification of expressed hemoglobins was as described previously [22]. Sample purity was assessed by cellulose acetate electrophoresis on Titan III membranes at pH 8.6 with Super-Heme buffer and by HPLC and SDS-PAGE. A single major band was observed after HPLC purification of each variant hemoglobin. Analysis of Hb tetramers showed only two distinct bands for α-globin and the variant β-globin chains after SDS PAGE and reverse-phase HPLC. In addition, electrospray ionization mass spectrometry was performed on the purified recombinant HbS and HbA variants using a VG BioQ triple quadrapole mass spectrometer (Micromass, Altrincham, Cheshire, UK) to confirm expected molecular weights. Data analysis employed the MassLynx® software package (Micromass, Altrincham) [22] which showed expected values for the various β-chain variants and α-globin chain.
Hemoglobin concentrations were determined spectrophotometically using millimolar extinction coefficients of mE 555 = 50 for deoxyhemoglobin and mE 579= 53.6 for carbonmonoxyhemoglobin (on a tetramer basis). Purified hemoglobins were stored in the CO-liganded form at − 80° C until used. Hemoglobin solutions were concentrated using a centricon centrifugal concentrator with a membrane cutoff of 30,000 Da (Centriprep, Milipore Co., Bedford, MA). Oxy-Hb was prepared by first blowing oxygen across the surface of a CO-Hb solution in a rotary evaporator under a 150 W flood-light bulb in an ice bath for about 1 h.
Formation of aggregates, fibers and/or crystals assessed by differential contrast (DIC) microscopy and turbidity
Aggregate, fiber and/or crystal formation for deoxy-Hb and oxy-Hb in 1.0 M phosphate buffer, pH 7.4, were evaluated by DIC microscopy and by turbidity using a temperature-jump method as described [16, 22]. Deoxy-Hb was generated by the addition of sodium dithionite in 3M phosphate buffer, pH 7.4, at 4° C to oxy-HbS solutions so that the final concentration of sodium dithionite was 100 mM in 1.0 M phosphate buffer, pH 7.4 [22]. One or 2 μl of solution was inserted into a glass capillary tube (30 μm path length × 0.3 mm width with 50 mm length) (Dynamics, Inc., Rockaway, NJ) by capillary action [16]. Both ends of the tube were sealed by Mount-Quick solution (Daido Sangyo Co., LTD., Tokyo, Japan) [22], and the tube was mounted on the glass slide after a 1-h incubation on ice. Initiation of polymerization was performed using temperature jump from 0° C to room temperature (22° C). Total mass or length of polymer was analyzed by DIC microscopy by measuring pixel area of a single domain using an Olympus microscope equipped with DIC optics and a 40× (1.00 NA) immersion lens. The microscopic images obtained were transferred to a PC via an image grabber board (Universal Image Corp., Downingtown, PA) and a CCD (charge-coupled device) camera (Cohu Camera, Cohu, Inc., San Diego, CA) [26]. In order to measure the area and length of polymer fibers, images were taken of a hemocytometer with 50 μm divisions (American Optical Corp., Buffalo, NY) at 400× magnification. Using the line measurement tool in the Molecular Device Image Analysis System (Universal Image Corp.), we determined the length of the fibers or crystals by counting pixel number. Kinetics of polymerization and solubility of HbA and HbS as well as their variants were evaluated in 1.0 M phosphate buffers, pH 7.4, at 22° C [22], with solubility determined by centrifugation after completion of polymerization.
Results
Aggregates and solubility of HbAβ4Val (α2β2T4V) and HbAβ73His (α2β2D73H) in the oxy and deoxy forms
Polymerization properties of HbAβ4Val (α2β2T4V) and HbAβ73His (α2β2D73H) in the oxy and deoxy forms were evaluated by a temperature jump in 1.0 M phosphate buffer using DIC microscopic methods. Samples were pre-incubated for 1 h on ice using a sealed glass capillary tube (30 μm path length, 0.3 mm width and 50 mm length) mounted on a glass slide prior to the temperature jump from 0° to 22° C. Both oversaturated deoxy- and oxy-HbAβ4Val (α2β2T4V) generated many globular shaped aggregates instantly after a temperature jump using approximately the same critical concentration required to generate insoluble aggregates of deoxy- and oxy-HbSβ4Val (α2β2E6V, T4V) (~ 0.3 g/dl) in 1.0 M phosphate buffer. In addition, both oversaturated HbAβ73His (α2β2D73H) in the oxy and deoxy forms aggregated instantly after a temperature jump without a delay time or domain formation. The critical concentration of HbAβ4Val (α2β2T4V) and HbSβ4Val (α2β2E6V, T4V) in the oxy and deoxy forms required for over-saturation was ~0.04-fold that of HbA in the oxy and deoxy forms as well as oxy-HbS but was similar to that of deoxy-HbSβ73His (α2β2E6V, D73H). These oversaturated hemoglobin variants formed aggregates as does oversaturated HbA in the oxy and deoxy forms as well as oxy-HbS. Aggregates from deoxy- and oxy-HbAβ4Val (α2β2T4V) showed similar DIC images (Fig. 2, bottom frames of HbA). DIC images of insoluble aggregates or fibers from oxy and deoxy forms of the β73His and β4Val variants of HbA and HbS (oxy and deoxy forms of α2β2D73H, α2β2E6V,D73H, α2β2T4V and α2β2E6V, T4V) in 1.0 M phosphate buffer after a 10-min incubation at 22° C are shown in Fig. 2 compared to those of HbA and HbS. DIC images of HbSβ73His (α2β2E6V, D73H) and HbSβ4Val (α2β2E6V, T4V) in the deoxy form were reported previously [16]. Aggregate number depended on concentration; the higher the concentration, the higher the number. Only deoxy-HbS and deoxy-HbSβ73His (α2β2E6V, D73H) generated fibers with ovoid-shaped, multi-spherulitic domains after a delay time prior to fiber formation, as previously reported [16]. All of these aggregates or fibers could be solubilized after reducing the phosphate concentration, indicating they are reversible and not caused by denaturation.
Figure 2. DIC images of fibers and aggregates for HbA, HbS β73 and β4 variants as well as HbA and HbS in the deoxy and oxy form.
Top, middle and bottom rows represent DIC images of HbA and HbS in the oxy and deoxy form and their β73His and β4Val variants after a 10-min incubation after temperature jump from 0° C to 22° C. Results are shown for deoxy- and oxy-HbA (10.5 g/dl), deoxy-Hb S (3.5 g/dl) and oxy-HbS (3.5 g/dl), deoxy-and oxy- HbAβ73 His (α2β273DH) (4.5 g/dl), deoxy-HbSβ73His (α2β2E6V, D73H) (0.5 g/dl), deoxy- and oxy-HbAβ4Val (α2β2T4V) (0.3 g/dl), deoxy- and oxy-HbSβ4Val (α2β2E6V, T4V) (0.3 g/dl) in 1.0 M phosphate buffer at pH 7.4 after temperature jump to 22° C. Images of each frame represent an area of 64 × 64 μm.
Solubilities of oxy- and deoxy-HbAβ4Val (α2β2T4V) measured at the plateau of polymerization following centrifugation were 0.238 ± 0.006 g/dl and 0.208 ± 0.004 g/dl, respectively, in 1.0 M phosphate buffer, pH 7.4 at 22° C, which are similar to that of HbSβ4Val (α2β2E6V, T4V). Solubilities for deoxy-HbAβ4Val (α2β2T4V) and deoxy-HbSβ4Val (α2β2E6V, T4V) were similar and ~10-fold less than that of deoxy-HbS. Solubility of oxy-HbAβ4Valα2β2T4V) was slightly higher than that of deoxy-HbAβ4Val α2β2T4V) but much less than that of oxy-HbS like HbSβ4Val (α2β2E6V, T4V) in the deoxy and oxy forms. Solubilities after aggregate formation of oxy- and deoxy-HbAβ4Val α2β2T4V) were similar to those of HbSβ4Val α2β2E6V, T4V), which were similar to that following fiber formation of deoxy-HbSβ73His (α2β2E6V, D73H). Solubilities of oxy- and deoxy-HbAβ73His (α2β2D73H) were 3.76 ± 0.091 g/dl and 3.39 ± 0.25 g/dl, respectively. In addition, even though the solubility of deoxy-HbAβ73His (α2β2D73H) was slightly lower than that of oxy-HbAβ73His (α2β2D73H), these results were similar to oxy-HbSβ73His (α2β2E6V, D73H) (3.51± 0.025 g/dl). Solubility of oxy- and deoxy-HbAβ73His (α2β2D73H) was ~2.2 fold lower than oxy- and deoxy-HbA, respectively, but was ~11-fold higher than that of deoxy-HbSβ73His (α2β2E6V, D73H), which is similar to solubilities of HbAβ4Val (α2β2T4V) and HbSβ4Val (α2β2E6V, T4V) in the oxy and deoxy forms. Solubility of the oxy and deoxy forms of β73His and β4Val variants of HbA and HbS (α2β2D73H, α2β2E6V, D73H, α2β2T4V and α2β2E6V, T4V in the oxy and deoxy forms) compared to those of HbA and HbS under the same conditions are summarized in Fig. 3. It should be noted that solubility of all of these hemoglobins also depends on temperature; the lower the temperature the higher the solubility, even though temperature effects on solubility of hemoglobin variants also depend on amino acids at the β4, β6 and β73 positions. These results suggest that a change from β4Thr to β4Val in HbA with or without β6Val facilitates hydrophobic interaction with the EF helix of adjacent molecules, which may be similar to β6Val interactions (e.g., Ala, Phe and Leu in the β-chain F helix) in deoxy-HbS fibers. This appears to occur in the absence of hydrogen bond formation between the β4 and β73 amino acids in both deoxy-HbAβ4Val α2β2T4V) and deoxy-HbSβ4Val (α2β2E6V, T4V).
Figure 3. Solubility after polymerization of HbS β4 and β73 variants compared to those of HbA and HbS in the oxy and deoxy forms in 1.0 M phosphate buffer.
Solubility of HbA, HbS and their variants was determined by centrifugation after completion of polymerization prior to crystallization measured by turbidity after temperature jump from 0° C to 22° C.
Crystallization of HbA and HbC-Harlem (α2β2E6V, D73N)
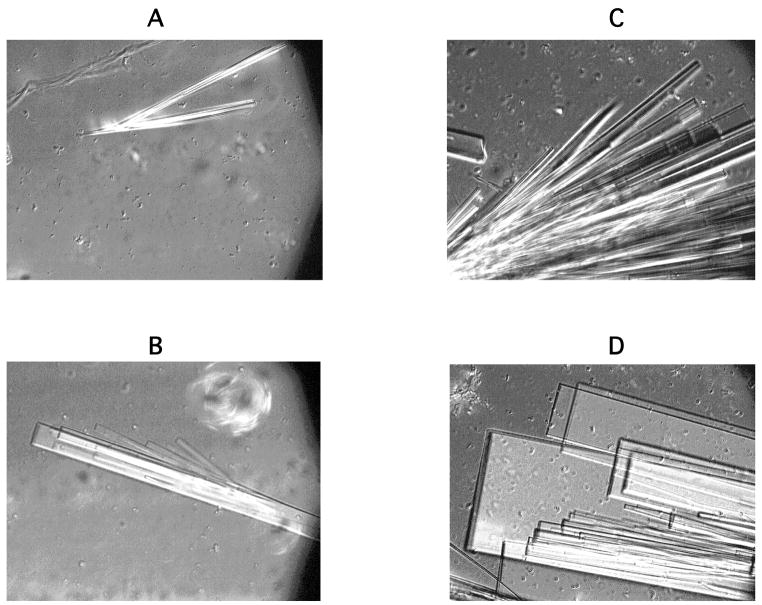
Our previous study showed that HbA and HbC-Harlem (α2β2E6V, D73N) as well as HbSβ73Leu (α2β2E6V, D73L) and HbSβ4Tyr (α2β2E6V, T4Y) in the deoxy form generated crystals after aggregate or fiber formation in high phosphate buffers as well as in 1.0 M phosphate buffer upon further incubation [7, 23]. When deoxy-HbA was over saturated (e.g., > ~7 g/dl in 1.0 M phosphate buffer at 22° C) at 10.5 g/dl in 1.0 M phosphate buffer, insoluble small aggregates were generated instantly, and no crystals were detected after a 10-h incubation. However, after 21-h some crystals are generated from the edge of existing aggregates. A DIC image of growing crystals of deoxy-HbA as a function of time is shown in Fig. 4, and growth (L) as a function of time also is shown in the Fig. 4, column A. These crystals grow to a three-dimensional shape at a rate of 2.2 × 10−3μm/s, which is ~50 times slower than growth of deoxy-HbS fibers under the same conditions [16]. After a 48-h incubation we observed larger crystals in many areas of the sealed glass capillary tube (Fig. 5). Size of deoxy-HbA crystals in the capillary tube was not consistent and depended on location in the tube. In contrast, oversaturated oxy-HbS and oxy-HbA generated similar aggregates and no crystals were observed after a 3-day incubation. In addition, we observed large, clear, needle- or board-like crystals from some of the HbC-Harlem fibers in domains after overnight incubation at room temperature (Fig. 6, frames A and B), as previously addressed [16, 22, 23]. These crystals appear to be generated on existing fibers and appear to be growing from crystalline nuclei resembling heterogeneous nuclei on fibers or aggregates [27]. Furthermore, we could not detect any liquid droplets prior to crystal formation of HbA and HbC-Harlem, in contrast to the report on formation of dense phase droplets prior to generation of HbC crystals [13]. It should be noted that crystal growth was slow like deoxy-HbA, and images of crystals were similar to those of HbA after a 48-h incubation (Fig. 6, frames C and D). However, HbAβ4Val (α2β2T4V) and HbAβ73His (α2β2D73H) as well as HbSβ4Val (α2β2E6V, T4V) and HbSβ73His (α2β2E6V, D73H) in the oxy and deoxy forms like HbA and HbS in the oxy form did not generate crystals.
Figure 4. Time course of growth of deoxy-HbA crystals in 1.0 M phosphate buffer using DIC microscopy.
DIC images show growth of deoxy-HbA (10.5 g/dl) crystals as a function of time in 1.0 M phosphate buffer. Frames A, B, C and D were taken after a 21-, 22-, 23- and 24-h incubation at 22° C, respectively. Images of each frame represent an area of 64 × 64 μm. Growth rate of length (L) of deoxy-HbA crystal was evaluated by counting the pixel number (fiber length) as a function of time.
Figure 5. DIC images of crystals for deoxy-HbA in the deoxy form after a 48-h incubation.
DIC images are shown of deoxy-HbA (10.5 g/dl) after a 48-h incubation after temperature jump to 22° C. Frames A, B, C and D represent DIC images in different areas of a glass capillary tube (30 μm path length × 0.3 mm width with 50 mm length). Images of each frame represent an area of 220 × 165 μm.
Figure 6. DIC images of crystals for deoxy-HbC-Harlem after a 24- and 48-h incubation.
DIC images are shown of deoxy-HbC-Harlem (5.5 g/dl) after a 24- (Frames A and B) and 48-h (Frames C and D) incubation after temperature jump to 22° C in a glass capillary tube (30 μm path length × 0.3 mm width with 50 mm length). Images of each frame represent an area of 220 × 165 μm.
Discussion
Our previous DIC analysis of HbS polymerization showed that not only β6Val hydrophobic interactions with β85Phe and β88Leu but also the β4Thr-β73Asp hydrogen bond in deoxy-HbS fibers was involved in the initiation of deoxy-HbS nucleation within domains and in polymerization [16, 22]. Kinetics of polymerization of deoxy-HbS were influenced by changes at the β4 and β73 amino acids. In addition, progress curves for deoxy-HbSβ73His (α2β2E6V, D73H) polymerization showed increased nucleation rates and a decreased critical concentration for fiber formation, while those for deoxy-HbC-Harlem (α2β2E6V, D73N) and deoxy-HbSβ73Leu (α2β2E6V, D73L) showed decreased nucleation following polymerization [22, 26]. Rates of nucleation prior to polymerization of the deoxy-HbSβ73 variants were affected by the β73 amino acid [22]. In addition, the inhibitory effect on polymer formation by a change from β4Thr to β4Tyr in HbS was greater than that of β4Ser but less than that of β73Asn or β73Leu [16]. Furthermore, deoxy-HbSβ4Val (α2β2E6V, T4V) promoted formation of aggregates without a delay time or domain formation, which is in contrast to the formation after a delay time of fibers with domains for HbSβ4Ser (α2β2E6V, T4S), HbSβ4Tyr (α2β2E6V, T4Y), HbSβ73His (α2β2E6V, D73H) and HbSβ73Leu (α2β2E6V, D73L) in the deoxy form [16]. We also proposed a model in which multi-nucleation rather than a single-nucleation event for general protein crystal formation occurs in a single cluster to generate numerous fibers growing from a single domain [16]. We suggested that many ordered deoxy-HbS fibers were formed with uniform growth rates in a single domain, which is in contrast to a single nucleus in a single cluster for crystal formation of protein at slow rates [18].
In our current study, formation of insoluble globular-shaped aggregates without domain and nuclei formation is documented for deoxy-HbSβ4SVal (α2β2E6V, T4V), deoxy-HbAβ4Val (α2β2T4V) and deoxy-HbAβ73His (α2β2D73H) as well as oxy-HbS and oxy-HbAβ73His (α2β2D73H) during liquid-solid phase transition. Furthermore, even though solubility of deoxy-HbAβ73His (α2β2D73H) was slightly lower than that of oxy-HbAβ73His (α2β2D73H), solubility of oxy-HbAβ73His (α2β2D73H) was ~2.2 fold lower than that of oxy- and deoxy-HbA. These values, however, were ~11-fold higher than that of deoxy-HbSβ73His (α2β2E6V, D73H). These results suggest that the hydrogen bond between β4Thr and β73Asp plays a key role in not only fiber formation of deoxy-Hb S but also during liquid-solid phase transition of Hb by aggregate formation of oxy-Hb. These results also suggest, in addition to the strength of β6 amino acid hydrophobicity, that the synergism between the β4Thr/β73Asp hydrogen bond and β6Val/β85Phe and β88Leu hydrophobic interaction free energies leading to intermolecular interactions plays a critical role in formation of fiber versus crystalline nuclei during phase transition of oxy and deoxy hemoglobin. Structural analyses of these Hb variants during and prior to fiber/aggregate formation are needed to establish further quantitative relationships between hydrophobic and hydrogen bond interactions and Hb fiber versus aggregate formation.
When the balance of hemoglobin contacts with water molecules becomes more hydrophobic by the change from β4Thr to β4Val in either HbS or HbA, the critical concentration for oversaturation is reduced nearly 10 fold compared to deoxy-HbS. This may result from enhanced hydrophobicity and absence of a hydrogen bond at the A-helix of β chains. Oversaturated hemoglobin molecules then facilitate initiation of random self aggregation without nuclei formation, resulting in numerous insoluble, globular-shaped aggregates. This result contrasts with the higher solubility of deoxy-HbAβ7Val (α2β2E7V) than deoxy-HbS but lower solubility than that of deoxy-HbA. Aggregate formation by deoxy-HbAβ7Val (α2β2E7V) is therefore promoted compared to deoxy-HbA [28]. Furthermore, oversaturated HbAβ4Val (α2β2T4V) and HbSβ4Val (α2β2E6V, T4V) in the deoxy and oxy forms as well as oxy-HbAβ73His (α2β2D73H), like oxy-HbA and oxy-HbS, did not generate crystals following additional incubation (e.g., > 3 days in 1.0 M phosphate buffer). Solubility and critical concentration required for formation of aggregates for both deoxy- and oxy-HbSβ4Val (α2β2E6V, T4V) were similar to those of deoxy-HbSβ73His (α2β2E6V, D73H) but ~10-fold lower than those of deoxy-HbS. These results are consistent with the presence of crystalline nuclei leading to additional crystal formation after a longer incubation time, as previously proposed to explain crystallization of HbA, HbS and HbC in high phosphate buffers (> 1.8M) [8]. These findings suggest that rates of crystalline nuclei formation of oversaturated hemoglobin also depend on β4 and β73 amino acids as well as the β6 amino acid to facilitate intermolecular hydrophobic and/or hydrogen bond interactions. These crystalline nuclei then may form at low rates from soluble oligomers after initiation of a liquid-solid phase change and serve as heterogeneous nuclei for generating additional crystals from existing aggregates or fibers [27]. In addition, the growth rate of deoxy-HbA crystals after nucleation is ~50-times slower than that of deoxy-HbS or deoxy-C-Harlem fibers. Thus, the formation of many ordered Hb fibers with uniform growth rates in a single domain, which is controlled by nuclei for fibers like deoxy-HbS and deoxy-HbC-Harlem, is very different from the formation of single nuclei for crystals. Furthermore, soluble oligomers in oversaturated solution can generate nuclei for fibers or crystals characterized by multi-nuclei for fibers and a single nucleus for crystals in a cluster [17, 18]. These results indicate that nuclei for crystals are different from those for fibers in oversaturated hemoglobin solution, and that nuclei formation for crystals depends on the stability of initial aggregates or fibers during liquid-solid phase transition.
In conclusion, oversaturated hemoglobin generates either insoluble aggregates or soluble oligomers during a liquid-solid phase transition, as shown in Figure 7. Nuclei for fibers can form soluble multi-nuclei in a cluster to generate multi-fibers like deoxyHbS and deoxy-HbC-Harlem following domain formation instead of quick insoluble aggregate formation. In contrast, oversaturated oxy- and deoxy-HbA, in addition to some HbA and Hb β4 and β73 variants in the oxy and deoxy forms as well as oxy-HbAβ73His (α2β2D73H)] and oxy-HbS, can generate insoluble aggregates without nuclei formation. After generation of aggregates and fibers for deoxy-HbA and deoxy-HbC-Harlem, respectively, they can generate crystals after a longer incubation time, since the β6 hydrophobic and β4 hydrogen bonds are relatively unstable compared to deoxy-HbS. However, it is noteworthy that deoxy-HbS can crystallize upon stirring [3, 29]. Such crystallization may be promoted through different reactions than fiber formation by generation of soluble oligomers for single crystalline nuclei [18]. Stability of a crystalline nucleus of hemoglobin in a single cluster also would be facilitated by interaction with existing aggregates or fibers functioning as seeds for crystal formation.
Figure 7. Schematic representation of reactions during a liquid-solid phase transition of oversaturated oxy- and deoxy-hemoglobin.
When the free energy for monomer-water equilibration required to maintain hemoglobin as soluble is exceeded, super-saturated hemoglobin generates an equilibrium between monomers and insoluble aggregates through reaction A [e.g., HbA, HbAβ4Val (α2β2T4V) and HbSβ4Val (α2β2E6V, T4V) in the oxy and deoxy forms as well as oxy-HbS and oxy-HbAβ73His (α2β2D73H)] or between self-associated soluble oligomers through reaction B. This may facilitate formation of pre-nuclei for fibers or crystals through reaction C or E, respectively [e.g., fiber nuclei for HbS, HbSβ73His (α2β2E6V, D73H) and HbC-Harlem in the deoxy form or crystalline nuclei for crystals (e.g., deoxy-HbA, deoxy-HbC-Harlem and oxy-HbC)]. If the free energy of hydrophobic and hydrogen bonds at the A-helix of the β chains is relatively high and these interactions are unstable, the result is formation of unstable aggregates or fibers of oversaturated hemoglobin and generation of soluble oligomers at slow rates through reactions B, E and F. This will lead to crystal formation after a longer incubation time, even though soluble oligomers can generate aggregates or fibers through reaction A. In contrast, increases in hydrophobicity and/or the absence of hydrogen-bond interactions at the A-helix of β chain of super-saturated hemoglobin in the oxy or deoxy forms facilitates generation of small, insoluble aggregates and inhibits nuclei formation for fiber or crystal growth. Deoxy-HbS can crystallize at low temperature with stirring through reactions B, E and F instead of forming fibers through reactions B, C and D.
Even though normal HbA in the oxy and deoxy forms is soluble and does not form insoluble aggregates in vivo, understanding polymerization and self-assembly properties of oversaturated HbA compared to HbS during liquid- and solid-phase transition as well as the high solubility of HbA is important. Such knowledge will lead to a better understanding of deoxy-HbS fiber formation with domains and oxy-HbC crystal formation in vivo, which may aid in the design of structure-based anti-HbS polymerization molecules, including hemoglobin variants and peptides based on protein-protein interactions of HbS polymers [30, 31].
Supplementary Material
Acknowledgments
We acknowledge J. Fithian for editorial assistance on the manuscript.
This research was supported in part by grants from the National Institutes of Health (HL70596, HL 69256 and DK61692) and the Cardeza Foundation for Hematologic Research.
Abbreviations
- Hb
hemoglobin
- HbS
Sickle hemoglobin (α2β2E6V)
- HbC
Hemoglobin C(α2β2E6K)
- HbC-Harlem
Hemoglobin C-Harlemα2β2E6V, D73N)
- SCD
sickle cell disease
- RBC
Red Blood Cell
- DIC
differential interference contrast
- CCD
charge-coupled device
- PCR
polymerase chain reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bunn HF, Forget BG. Hemoglobin: Molecular and Clinical Aspects. Sanders; Philadelphia: 1996. [Google Scholar]
- 2.Eaton WA, Hofrichter J. Adv Protein Chem. 1990;40:63–279. doi: 10.1016/s0065-3233(08)60287-9. [DOI] [PubMed] [Google Scholar]
- 3.Pumphrey JG, Steinhardt J. J Mol Biol. 1977;112:359–75. doi: 10.1016/s0022-2836(77)80187-3. [DOI] [PubMed] [Google Scholar]
- 4.Jones MM, Steinhardt J. J Mol Biol. 1979;129:83–91. doi: 10.1016/0022-2836(79)90061-5. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch RE, Raventos-Suarez C, Olson JA, Nagel RL. Blood. 1985;66:775–7. [PubMed] [Google Scholar]
- 6.Charache S, Conley CL, Waugh DF, Ugoretz RJ, Spurrell JR. J Clin Invest. 1967;46:1795–811. doi: 10.1172/JCI105670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adachi K, Asakura T. J Biol Chem. 1979;254:12273–6. [PubMed] [Google Scholar]
- 8.Adachi K, Asakura T. J Biol Chem. 1981;256:1824–30. [PubMed] [Google Scholar]
- 9.Ross PD, Hofrichter J, Eaton WA. J Mol Biol. 1977;115:111–34. doi: 10.1016/0022-2836(77)90093-6. [DOI] [PubMed] [Google Scholar]
- 10.Adachi K, Kim J, Kinney TR, Asakura T. J Biol Chem. 1987;262:10470–4. [PubMed] [Google Scholar]
- 11.Samuel RE, Salmon ED, Briehl RW. Nature. 1990;345:833–5. doi: 10.1038/345833a0. [DOI] [PubMed] [Google Scholar]
- 12.Briehl RW. J Mol Biol. 1995;245:710–23. doi: 10.1006/jmbi.1994.0057. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q, Vekilov PG, Nagel RL, Hirsch RE. Biophys J. 2004;86:1702–12. doi: 10.1016/S0006-3495(04)74239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galkin O, Vekilov PG. J Mol Biol. 2004;336:43–59. doi: 10.1016/j.jmb.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Galkin O, Nagel RL, Vekilov PG. J Mol Biol. 2007;365:425–39. doi: 10.1016/j.jmb.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Adachi K, Ding M, Surrey S. Biochemistry. 2008;47:5441–9. doi: 10.1021/bi800149u. [DOI] [PubMed] [Google Scholar]
- 17.Pan W, Galkin O, Filobelo L, Nagel RL, Vekilov PG. Biophys J. 2007;92:267–77. doi: 10.1529/biophysj.106.094854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kashchiev D, Vekilov PG, Kolomeisky AB. J Chem Phys. 2005;122:244706. doi: 10.1063/1.1943389. [DOI] [PubMed] [Google Scholar]
- 19.Vekilov PG. Br J Haematol. 2007;139:173–84. doi: 10.1111/j.1365-2141.2007.06794.x. [DOI] [PubMed] [Google Scholar]
- 20.Harrington DJ, Adachi K, Royer WE., Jr J Mol Biol. 1997;272:398–407. doi: 10.1006/jmbi.1997.1253. [DOI] [PubMed] [Google Scholar]
- 21.Harrington DJ, Adachi K, Royer WE., Jr J Biol Chem. 1998;273:32690–6. doi: 10.1074/jbc.273.49.32690. [DOI] [PubMed] [Google Scholar]
- 22.Adachi K, Ding M, Wehrli S, Reddy KS, Surrey S, Horiuchi K. Biochemistry. 2003;42:4476–4484. doi: 10.1021/bi026740x. [DOI] [PubMed] [Google Scholar]
- 23.Adachi K, Asakura T. J Mol Biol. 1980;144:467–80. doi: 10.1016/0022-2836(80)90332-0. [DOI] [PubMed] [Google Scholar]
- 24.Shen TJ, Ho NT, Simplaceanu V, Zou M, Green BN, Tam MF, Ho C. Proc Natl Acad Sci U S A. 1993;90:8108–12. doi: 10.1073/pnas.90.17.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adachi K, Konitzer P, Kim J, Welch N, Surrey S. J Biol Chem. 1993;268:21650–6. [PubMed] [Google Scholar]
- 26.Ivanova M, Jasuja R, Krasnosselskaia L, Josephs R, Wang Z, Ding M, Horiuchi K, Adachi K, Ferrone FA. J Mol Biol. 2001;314:851–61. doi: 10.1006/jmbi.2001.5163. [DOI] [PubMed] [Google Scholar]
- 27.Ferrone FA, Hofrichter J, Eaton WA. J Mol Biol. 1985;183:611–31. doi: 10.1016/0022-2836(85)90175-5. [DOI] [PubMed] [Google Scholar]
- 28.Yamashiro DJ, Adachi M, Konitzer P, Surrey S, Adachi K. J Biol Chem. 1994;269:23996–9. [PubMed] [Google Scholar]
- 29.Pumphrey JG, Steinhardt J. Biochem Biophys Res Commun. 1976;69:99–105. doi: 10.1016/s0006-291x(76)80278-1. [DOI] [PubMed] [Google Scholar]
- 30.Adachi K, Ding M, Surrey S, Rotter M, Aprelev A, Zakharov M, Weng W, Ferrone FA. J Mol Biol. 2006;362:528–38. doi: 10.1016/j.jmb.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 31.Akbar MG, Tamura Y, Ding M, Ding H, Rosenblatt MM, Reddy KS, Surrey S, Adachi K. Biochemistry. 2006;45:8358–67. doi: 10.1021/bi0604734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.