Abstract
Intracellular phosphorylation of dCK on Ser-74 results in increased nucleoside kinase activity. We mimicked this phosphorylation by a Ser-74-Glu mutation in bacterially produced dCK and investigated kinetic parameters using various nucleoside substrates. The S74E mutation increases the kcat values 11-fold for dC, and 3-fold for the anti-cancer analogues dFdC and AraC. In contrast, the rate is decreased for the purine substrates. In HEK293 cells, we found that by comparing transiently transfected dCK(S74E)-GFP and wild-type dCK-GFP, mimicking the phosphorylation of Ser-74 has no effect on cellular localisation. We note that phosphorylation may represent a mechanism to enhance the catalytic activity of the relatively slow dCK enzyme.
Keywords: Deoxycytidine kinase, Nucleoside analogues, Prodrug phosphorylation, Protein phosphorylation, Subcellular localisation
1. Introduction
Mammalian cells have four nucleoside kinases, thymidine kinases (TK1 and TK2), deoxyguanosine kinase (dGK) and deoxycytidine kinase (dCK), which partake in the first phosphorylation step of the nucleotide salvage-pathway providing resting cells with deoxynucleotides for mitochondrial DNA synthesis and nuclear DNA repair [1–3]. Of these dCK has the broadest substrate specificity phosphorylating the natural deoxynucleosides, deoxyadenosine (dA), deoxycytidine (dC) and deoxyguanosine (dG) [4]. Additionally, dCK phosphorylates a number of anti-viral and anti-cancer nucleoside analogue prodrugs. Chemotherapy agents such as the cytidine analogues Gemcitabine (dFdC) and Cytarabine (Ara-C), the adenosine analogue Cladribine (CldA) [5] and anti-viral cytidine analogues such as 3TC and ddC used to treat HIV infection [5,6], are converted by dCK in the first step of intracellular activation from their pharmacologically inactive form (prodrug) to their 5′-phosphorylated species. Deficiencies in dCK expression, or loss of dCK activity, are common factors that reduce efficacy of such prodrugs. Several studies have previously shown that drug sensitivity can be restored and cellular toxicity enhanced by increasing dCK activity through gene transfer [7].
Until recently, the intracellular control mechanisms of dCK activity were largely unknown. It has now been shown that phosphorylation and dephosphorylation of dCK is important in the regulation of dCK activity [8]. A total of four potential in vivo phosphorylation sites were identified: Thr-3, Ser-11, Ser-15 and Ser-74. Following mutational analyses of these four sites, the Ser-74 residue, which lies in the surface-exposed insert region of dCK [9], was shown to be the only phosphorylation site that influences intracellular activity. The authors developed a phospho-Ser74 antibody raised to the phospho-peptide (EELTMpSQKNGG) which comprises amino acids 69–79 of dCK. Using this antibody, a direct correlation between an elevated phosphorylation state of Ser-74 and increased dCK activity was shown in both dCK overexpressed in HEK 293 cells and endogenous dCK expressed in human leukemic lymphocytes [8,10]. Although phosphorylation of dCK appears to be an important post-translation regulatory mechanism, the protein kinase which phosphorylates dCK has yet to be identified by a biochemical approach. However, a recent elegant proteomic study, which aimed to analyse proteins phosphorylated on specific consensus sequences following cellular DNA damage, may aid in identifying the kinase responsible for the phosphorylation of Ser-74 on dCK [11]. The authors selected to identify proteins phosphorylated on Ser-Gln (SQ) or Thr-Gln (TQ) motifs following the induction of cellular DNA damage by ionizing radiation. Both SQ and TQ motifs are substrates for ATM (ataxia telangiectasia mutated) and ATR (ATM and Rad3 related) kinases which are rapidly initiated when double-strand breaks in chromosomal DNA arise in a cell. This study showed that when cells are subjected to ionizing radiation human dCK is phosphorylated on Ser-74 and this phosphorylation is ATM-dependant.
As dCK activity plays a critical role in the sensitivity to nucleoside analogue prodrugs, in this study we mimicked phosphorylation of Ser-74 on dCK by serine-to-glutamic acid (S74E) or serine-to-aspartic acid (S74D) mutations and analysed the effects on enzymatic activity to both dFdC and Ara-C, and to the natural nucleoside substrates dC, dG and dA. Furthermore, to study the influence of side-chain length and charge on Ser-74, enzymatic activities of dCK with serine-to-glutamine (S74Q) and serine-to-alanine (S74A) mutations were analysed. We also investigated the potential influence of phosphorylation on cellular localisation of overexpressed dCK.
2. Materials and methods
2.1. Plasmid construction and site-directed mutagenesis
The human dCK Ser-74 mutants were constructed using the PCR-fusion method and wild-type human dCK cDNA as the template [9]. The PCR products were subcloned via the 5′-Nde I/3′-Bam HI sites into the pET14b vector (Novagen) providing a His6 tag at the N-terminus. Wild-type dCK, and mutants dCK(S74E) and dCK(S74A) were subcloned into mammalian green fluorescent protein (GFP) expression vector pEGFP-C1 (Clontech) via Nde I/Sal I sites. The N-terminal nuclear import signal of dCK WT and dCK (S74E) was abolished by a double mutation (K6N and R7G) in the bipartite nuclear import signal region (residues: 6–18) by PCR mutagenesis [12] and subcloned into the pEGFP-C1 vector. All plasmid DNA was purified using a Nucleo-Bond® kit (Macherey-Nagel) according to the manufacturer’s instructions.
2.2. Protein expression and purification
BL21 (DE3) Escherichia coli was transformed with the dCK constructs in the pET14b vector, grown in 2YT media and induced with 0.1 mM IPTG over 4 h at 37 °C [9]. Cells were harvested and the pellet lysed by sonication. Lysates were cleared by centrifugation at 10000 × g for 30 min at 4 °C and subjected to batch purification with Protino® Ni-TED (Macherey-Nagel) following the supplier’s protocol. After elution with imidazole, the protein samples were aliquoted, snap-frozen and stored at −80 °C. Samples of purified enzyme were prepared in Laemmli buffer, heated at 95 °C and analysed on a 10% SDS–PAGE gel.
2.3. Kinetic assay
The activities of the dCK WT and dCK Ser-74 mutants were determined using an NADH-dependent enzyme-coupled assay [13] and a Uvikon 943 spectrophotometer. All measurements were done at 25 °C in a buffer containing 100 mM Tris, pH 7.5, 100 mM KCl, 10 mM MgCl2 and 2 mM ATP. For kcat measurements, the nucleosides dC, dA, dG and nucleoside analogues Gemcitabine (dFdC) or AraC were used at saturating concentrations. For Km measurements, varying ranges of nucleoside concentrations were used with, all other assaying conditions kept constant: dC (0.01–1 mM), dA (0.05–4 mM), dG (0.05–1 mM), dFdC (0.005–1 mM) or AraC (0.05–1 mM). Enzyme concentrations varied between 0.3 and 1.6 μM depending on the nucleoside analog to be measured. Kinetic experiments carried out at 37 °C yielded kcat ratios for the S74E mutant and the wild-type enzyme that were very similar to those obtained at 25 °C (data not shown).
2.4. Cell culture, transient transfection and confocal laser-scanning microscopy
Human embryonic kidney (HEK293) cells were maintained at 37 °C in an atmosphere of 5% CO2/95% humidity in complete Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 0.1% penicillin/streptomycin (10000 U/ml), L-glutamine (2 mM) and 10% fetal calf serum. Transfection of HEK293 cells was done using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Transfected cells were seeded onto 12 mm glass cover-slips 1 day prior to fixation. The cover-slips were washed with ice-cold HEPES-buffered saline (HBS) and cells fixed in 3.5% paraformaldehyde (PFA) for 5 min at 4 °C and for 10 min at room temperature. The cover-slips were washed three times with HBS and subjected further to fixation and permeabilisation steps with methanol for 6 min at −20 °C, washed once with HBS and mounted in Mowiol [14]. Images of transfected cells were taken using a Leica TCS SP5 confocal microscope.
3. Results and discussion
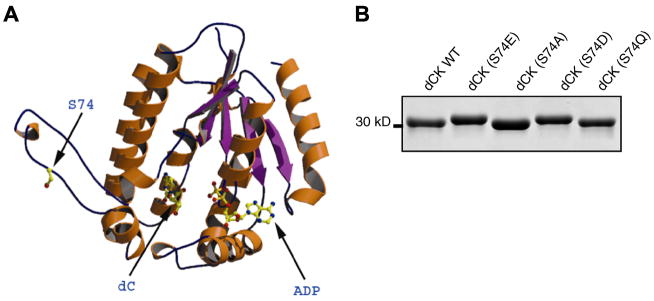
Human dCK is a homodimeric protein of a calculated sub-unit mass of 30.5 kDa (260 amino acids) [15]. The 3D structure of human dCK (at 1.6Å resolution) bound to ADP and to its natural nucleoside substrate dC, or to the nucleoside analogues Ara-C and dFdC, has been solved by Sabini et al. [9]. This structure reveals that the putative Ser-74 phosphorylation site of dCK lies in a surface-exposed insert region which would be easily accessible to cellular protein kinases (Fig. 1A). In fact, in several other crystal structures of dCK the entire insert region could not be modeled, indicating the high flexibility of this region. Furthermore, it has been postulated that phosphorylation of dCK and the resultant increase in activity is due to a conformational change of the enzyme [16,17]. The exact nature of this conformational change is unknown. We did not study the role of three N-terminally located, minor phosphorylation sites (Thr-3, Ser-11, Ser-15) that were identified previously and were shown to have no effect on dCK activity [8]. Since the N-terminal region containing these residues is not conserved among different species [8] and could not be seen in our crystal structure [9] we omit it from Fig. 1A.
Fig. 1.
(A) Ribbon diagram of a dCK monomer highlighting the Ser-74 phosphorylation site in the insert region accessible to cellular protein kinases. (B) Coomassie blue-stained SDS–PAGE gel showing the reduced mobility of the dCK(S74E) and dCK(S74D) mutant proteins mimicking S74 phosphorylation.
Upon SDS–PAGE analysis, decreased electrophoretic mobilities (‘band-shifts’) have been reported for many proteins and kinases upon phosphorylation [18,19]. In these cases, decreases in electrophoretic mobility were correlated to an elevation of the phosphorylation status of the protein and to a concomitant increase in its catalytic activity. Such ‘band-shifts’ have also been previously shown for phosphorylated and activated dCK [8]. We observed, following the separation by SDS–PAGE of the various N-terminally His-tagged dCK mutants Ser-74-Glu (S74E), Ser-74-Asp (S74D), Ser-74-Ala (S74A) and Ser-74-Gln (S74Q), that the phospho-mimicking S74E and S74D mutant enzymes have a slightly decreased electrophoretic mobility (Fig. 1B). These observed properties indicated that both our E. coli produced S74E and S74D mutants of dCK are akin to the Ser-74 phosphorylation of dCK in intact cells.
Next, we set out to investigate the effect of mimicking the phosphorylation of Ser-74 on the enzyme kinetics of recombinant dCK using different natural deoxynucleoside substrates and nucleoside analogues. Here, we found that the most marked changes in kcat occurred with the S74E variant. Compared to WT, the S74E mutation resulted in an 11-fold increase in kcat for dC, and ~3-fold increase for the cytidine analogues dFdC and Ara-C (Table 1a). No significant change was observed for the natural substrate dA, although the kcat for the purine substrate dG decreased 2-fold. As with S74E, the S74D mutation can potentially mimic the charged phosphoserine. However, the increase in rate was not as dramatic with the S74D mutation for dC (4-fold), dFdC (2-fold) and Ara-C (2-fold) (Table 1a). The S74Q mutation, generated in order to observe the sole effect of side-chain length, did not result in a significant change in kcat for any of the substrates. The S74A mutation also revealed no significant changes in kcat for any of the substrates (Table 1a). Finally, as the S74E mutation resulted in an increased rate for dC and the dC analogues dFdC and AraC, we determined the Km values and calculated the kcat/KM ratios for the three physiological substrates and for the two nucleoside analogues (Table 1b). The KM for dC and Ara-C increased ~4-fold for dCK-S74E variant compared to dCK-WT. Thus, the specificity constant, as given by the kcat/KM ratio, remains nearly unchanged in the case of AraC and dFdC and increases about 2-fold for deoxycytidine. In contrast, it drops about 5-fold in the case of dA and dG, leading to a considerably lower activity of the S74E variant on purine substrates. It is important to realize that the improved kcat is the overriding parameter that determines catalytic effectiveness at intracellular substrate concentrations around or above the mutant’s KM values.
Table 1.
| Nucleoside |
kcat (s−1) values |
|||||
|---|---|---|---|---|---|---|
| WT | S74E | Ratio S74E/WT | S74D | S74Q | S74A | |
| (a) Catalytic activities of wild-type dCK and dCK phosphorylation site Ser-74 dCK mutants | ||||||
| dC | 0.04 ± 0.005 | 0.45 ± 0.03 | 11.25 | 0.16 ± 0.003 | 0.04 ± 0.03 | 0.04 ± 0.003 |
| dA | 0.77 ± 0.07 | 0.69 ± 0.04 | 0.90 | 1.00 ± 0.07 | 0.90 ± 0.08 | 1.12 ± 0.10 |
| dG | 0.73 ± 0.05 | 0.36 ± 0.01 | 0.49 | 0.57 ± 0.05 | 0.65 ± 0.03 | 0.68 ± 0.04 |
| dFdC | 0.44 ± 0.03 | 1.21 ± 0.05 | 2.75 | 0.90 ± 0.02 | 0.45 ± 0.06 | 0.50 ± 0.03 |
| AraC | 0.32 ± 0.02 | 1.06 ± 0.04 | 3.31 | 0.57 ± 0.02 | 0.30 ± 0.08 | 0.33 ± 0.05 |
|
KM (μM) |
kcat/KM (M−1 s−1) |
|||
|---|---|---|---|---|
| WT | S74E | WT | S74E | |
| (b) Steady-state kinetic parameters KM and kcat/KM of dCK WT and dCK(S74E) | ||||
| dC | 1.7 ± 0.2 | 8.9 ± 0.3 | 23.1 × 103 | 50.6 × 103 |
| dA | 116.9 ± 11.5 | 517.9 ± 92.7 | 6.6 × 103 | 1.3 × 103 |
| dG | 245.8 ± 18.8 | 601.8 ± 80.2 | 2.9 × 103 | 0.6 × 103 |
| dFdC | 6.2 ± 1.3 | 15.0 ± 3.1 | 71.0 × 103 | 80.7 × 103 |
| AraC | 5.9 ± 1.5 | 24.1 ± 12.1 | 54.2 × 103 | 44.0 × 103 |
All kinetic measurements were performed at 25 °C with 2 mM ATP as the phosphoryl donor and, as indicated, with different nucleoside substrates.
Calculated kcat values include standard error (n = 4).
KM values were determined with constant 2 mM ATP and varying concentrations of substrates. All measurements were performed at 25 °C. The KM values are an average of at least three experiments and were calculated using Origin (Hyperbolic function). The kcat values from Table 1a were used to calculate the kcat/KM values presented here.
How does our data on the recombinantly produced enzymes compare to activity measurements done in mammalian cells? Smal et al. [8] expressed dCK in HEK 293T cells and observed that the dCK activity in cell lysates (measured with ATP and dC as substrates) decreases by approximately a factor of 6 after phosphatase treatment. In contrast, specific enzymatic activity as determined in lysates of cells expressing wild-type dCK or dCK-S74E increased 1.5–2-fold in the mutant enzyme, whereas the S74A mutant showed a 6–8-fold lower activity than wild-type [8]. The interpretation here is that in these experiments wild-type dCK represents a mixture of phosphorylated and unphosphorylated enzyme, S74E the fully phosphorylated form, and the S74A version completely unphosphorylated dCK. Taken together, the difference in activity of the totally unphosphorylated enzyme and the phosphorylation mimic amounts to a factor of 10–12 in the HEK 293T cellular system. This compares remarkably well to our data obtained on bacterially produced wild-type and dCK-S74E showing a ratio of 11 for the ATP/dC substrate pair.
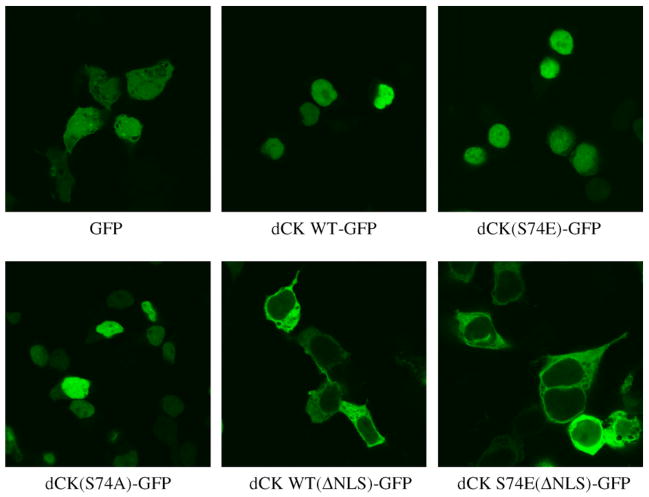
As the intracellular phosphorylation of some proteins can result in their subcellular re-localisation, we analysed this issue by transiently transfecting both wild-type dCK and the dCK(S74E) phosphorylation mutant fused to GFP [designated dCK WT-GFP and dCK(S74E)-GFP, respectively] into HEK293 cells (Fig. 2). Specific activity of the bacterially over-produced and purified dCK-GFP proteins was about half of that of the unfused species (unpublished data). Both dCK WT-GFP and dCK(S74E)-GFP were found mainly in the nucleus in contrast to the GFP only control, which was observed throughout the cell. In order to rule out that the phosphorylation of Ser-74 influences localisation, we overexpressed dCK(S74A)-GFP in HEK293 cells. The transiently transfected dCK(S74A)-GFP was also observed as being mainly nuclear (Fig. 2). Previously, it had been reported that endogenous dCK is in fact cytosolic [20] and is only nuclear following overexpression, and this was independent of the absence [20] or presence [21] of C-terminally fused GFP. This nuclear redistribution of overexpressed dCK was attributed to a nuclear localisation signal (NLS) found in the extreme N-terminal region of dCK [20]. Furthermore, it was postulated that a binding partner exists which keeps endogenous dCK in the cytosol whereas overexpressed dCK is observed also in the nucleus due to an imbalance of the binding partner/dCK ratio. Therefore, we created further mutants dCK WT(ΔNLS)-GFP and dCK S74E(ΔNLS)-GFP to abolish the nuclear localisation due to the NLS. Our rationale was to observe whether mimicking Ser-74 phosphorylation would have any effect on cytosolic overexpressed dCK-GFP as opposed to the normally nuclear overexpressed dCK (Fig. 2). We found that both dCK WT(ΔNLS)-GFP and dCK S74E(ΔNLS)-GFP were distributed uniformly in the cytosol. Thus, we conclude that there is no effect on the localization of dCK upon phosphorylation of S74.
Fig. 2.
Comparison of the intracellular localisation of transiently expressed dCK WT, dCK(S74E), dCK(S74A), and nuclear import signal mutants of dCK WT and dCK(S74E). HEK293 cells were transfected with the indicated N-terminal green fluorescent protein (GFP)-fused dCK constructs; dCK WT-GFP, dCK(S74E)-GFP, dCK(S74A)-GFP and dCK WT(ΔNLS)-GFP or dCKS74E(ΔNLS)-GFP where the nuclear import signal (NLS) has been abolished. Empty pEGFP-C1 vector was used as the GFP control.
In summary, the data from our biochemical analysis of the phosphorylation mimic dCK(S74E) suggest that the enzyme’s catalytic activity can be upregulated about 10-fold, and rendered more selective for dC substrates through phosphorylation of a single site. Since expression of the mammalian dCK gene, in contrast to TK1 which shows highest level during the S-phase, does not fluctuate during the cell cycle, this post-translational modification may be considered as a mechanism for modulating its intracellular activity. Moreover, enhancement of the first, and often critical, phosphorylation step in the activation pathway of deoxycytidine analog prodrugs protects them from rapid deamination, and, thus, deactivation We are presently studying the structural consequences of phosphorylation and its impact on protein stability and interaction with potential binding partners, as well as cellular effects resulting from overexpression of dCK variants in human cells and treating them with nucleoside analogues.
Acknowledgments
This work was supported by an NIH Grant (AI046943) to A.L. and M.K, and by the Max-Planck-Society. We thank Ursula Welscher-Altschäffel for technical assistance.
References
- 1.Arner ES, Flygar M, Bohman C, Wallstrom B, Eriksson S. Deoxycytidine kinase is constitutively expressed in human lymphocytes: consequences for compartmentation effects, unscheduled DNA synthesis, and viral replication in resting cells. Exp Cell Res. 1988;178:335–342. doi: 10.1016/0014-4827(88)90403-x. [DOI] [PubMed] [Google Scholar]
- 2.Gower WR, Jr, Carr MC, Ives DH. Deoxyguanosine kinase. Distinct molecular forms in mitochondria and cytosol. J Biol Chem. 1979;254:2180–2183. [PubMed] [Google Scholar]
- 3.Eriksson S, Munch-Petersen B, Kierdaszuk B, Arner E. Expression and substrate specificities of human thymidine kinase 1, thymidine kinase 2 and deoxycytidine kinase. Adv Exp Med Biol. 1991;309B:239–243. doi: 10.1007/978-1-4615-7703-4_53. [DOI] [PubMed] [Google Scholar]
- 4.Sarup JC, Fridland A. Identification of purine deoxyribonucleoside kinases from human leukemia cells: substrate activation by purine and pyrimidine deoxyribonucleosides. Biochemistry. 1987;26:590–597. doi: 10.1021/bi00376a034. [DOI] [PubMed] [Google Scholar]
- 5.Van Rompay AR, Johansson M, Karlsson A. Substrate specificity and phosphorylation of antiviral and anticancer nucleoside analogues by human deoxyribonucleoside kinases and ribonucleoside kinases. Pharmacol Ther. 2003;100:119–139. doi: 10.1016/j.pharmthera.2003.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee H, Hanes J, Johnson KA. Toxicity of nucleoside analogues used to treat AIDS and the selectivity of the mitochondrial DNA polymerase. Biochemistry. 2003;42:14711–14719. doi: 10.1021/bi035596s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hapke DM, Stegmann AP, Mitchell BS. Retroviral transfer of deoxycytidine kinase into tumor cell lines enhances nucleoside toxicity. Cancer Res. 1996;56:2343–2347. [PubMed] [Google Scholar]
- 8.Smal C, Vertommen D, Bertrand L, Ntamashimikiro S, Rider MH, Van Den Neste E, Bontemps F. Identification of in vivo phosphorylation sites on human deoxycytidine kinase. Role of Ser-74 in the control of enzyme activity. J Biol Chem. 2006;281:4887–4893. doi: 10.1074/jbc.M512129200. [DOI] [PubMed] [Google Scholar]
- 9.Sabini E, Ort S, Monnerjahn C, Konrad M, Lavie A. Structure of human dCK suggests strategies to improve anticancer and antiviral therapy. Nat Struct Biol. 2003;10:513–519. doi: 10.1038/nsb942. [DOI] [PubMed] [Google Scholar]
- 10.Smal C, Van Den Neste E, Maerevoet M, Poire X, Theate I, Bontemps F. Positive regulation of deoxycytidine kinase activity by phosphorylation of Ser-74 in B-cell chronic lymphocytic leukaemia lymphocytes. Cancer Lett. 2007;253:68–73. doi: 10.1016/j.canlet.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Matsuoka S, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 12.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal KC, Miech RP, Parks RE., Jr Guanylate kinases from human erythrocytes, hog brain, and rat liver. Meth Enzymol. 1978;51:483–490. doi: 10.1016/s0076-6879(78)51066-5. [DOI] [PubMed] [Google Scholar]
- 14.Brock R, Hamelers IH, Jovin TM. Comparison of fixation protocols for adherent cultured cells applied to a GFP fusion protein of the epidermal growth factor receptor. Cytometry. 1999;35:353–362. doi: 10.1002/(sici)1097-0320(19990401)35:4<353::aid-cyto8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson S, Cederlund E, Bergman T, Jornvall H, Bohman C. Characterization of human deoxycytidine kinase. Correlation with cDNA sequences. FEBS Lett. 1991;280:363–366. doi: 10.1016/0014-5793(91)80332-w. [DOI] [PubMed] [Google Scholar]
- 16.Keszler G, Spasokoukotskaja T, Csapo Z, Talianidis I, Eriksson S, Staub M, Sasvari-Szekely M. Activation of deoxycytidine kinase in lymphocytes is calcium dependent and involves a conformational change detectable by native immunostaining. Biochem Pharmacol. 2004;67:947–955. doi: 10.1016/j.bcp.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Keszler G, Virga S, Spasokoukotskaja T, Bauer PI, Sasvari-Szekely M, Staub M. Activation of deoxycytidine kinase by deoxyadenosine: implications in deoxyadenosine-mediated cytotoxicity. Arch Biochem Biophys. 2005;436:69–77. doi: 10.1016/j.abb.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Kuroda F, Moss J, Vaughan M. Regulation of brefeldin A-inhibited guanine nucleotide-exchange protein 1 (BIG1) and BIG2 activity via PKA and protein phosphatase 1gamma. Proc Natl Acad Sci USA. 2007;104:3201–3206. doi: 10.1073/pnas.0611696104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacKenzie SJ, et al. Long PDE4 cAMP specific phosphodiesterases are activated by protein kinase A-mediated phosphorylation of a single serine residue in Upstream Conserved Region 1 (UCR1) Br J Pharmacol. 2002;136:421–433. doi: 10.1038/sj.bjp.0704743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatzis P, Al-Madhoon AS, Jullig M, Petrakis TG, Eriksson S, Talianidis I. The intracellular localization of deoxycytidine kinase. J Biol Chem. 1998;273:30239–30243. doi: 10.1074/jbc.273.46.30239. [DOI] [PubMed] [Google Scholar]
- 21.Johansson M, Brismar S, Karlsson A. Human deoxycytidine kinase is located in the cell nucleus. Proc Natl Acad Sci USA. 1997;94:11941–11945. doi: 10.1073/pnas.94.22.11941. [DOI] [PMC free article] [PubMed] [Google Scholar]