Abstract
Long-term success in lung transplantation is limited by obliterative bronchiolits (OB), while the mechanism for this disease is not well-understood. Chemokine SDF-1 and its receptor CXCR4 have been reported to be involved in several fibrogenic processes by recruiting inflammatory and fibroblast progenitor cells into injured tissues. We hypothesized that SDF-1/CXCR4 axis also plays a role in the pathogenesis of OB. Using the mouse heterotopic allogeneic airway transplant model, we transplanted mouse tracheas from Balb/C donors into C57BL/6 recipients. At day 10 after transplant, we found high expression of SDF-1 in cells in sub-epithelial layers of the allograft. Approximately 26% of cells infiltrating the allograft were CD45+CXCR4+ as was determined by flow cytometry analysis. Treatment of the recipients with a CXCR4 antagonist, TN 14003, decreased cell infiltration into the grafts at day 10 post implantation. At day 42 a significant reduction of the luminal occlusion was found in the TN14003 treated animals compared to controls (57.40% versus 98.21%, p<0.01). To demonstrate the relevance of SDF-1/CXCR4 axis in OB, sections of lung tissue obtained from lung transplanted patients with OB, were examined for SDF-1 and CXCR4 expression. We found higher number of CXCR4 and SDF-1 positive cells in samples from patients with OB compared with normal lungs. These findings provide new insights into the mechanisms of lung chronic rejection and may lead to new intervention tools for the treatment of OB.
Introduction
Obliterative bronchiolitis (OB) is the major long-term complication affecting lung transplant recipients. OB is characterized by a decrease in the mid-portion of the expiratory flow curve, progressive dyspnea accompanied by a non-productive cough, and a clear chest radiograph. Pathologically, there is chronic inflammation involving the bronchial epithelium, leading to gradual obliteration of small airways by inflammatory infiltrates, proliferating fibroblasts, collagen and matrix deposition(1). Commonly, OB is steadily progressive and fatal. Although treatment usually consists of intensifying immunosuppressive therapy, there is no therapy for OB (2).
The chemokine, stromal cell-derived factor-1 (SDF-1, also called CXCL-12) is a chemoattractant for a broad range of cell types (3–5) and is involved in the mobilization of cells from BM to peripheral blood and thence to injured tissues. Studies from others and our lab have shown the role of SDF-1/CXCR4 axis in lung injury and fibrosis induced by bleomycin (6, 7).
SDF-1/CXCR4 axis might participate in the development of inflammation. AMD3100, a potent CXCR4 antagonist, attenuates allergic lung inflammation and airway hyperreactivity as well as autoimmune joint inflammation in IFN-γ receptor-deficient mice(8, 9). Furthermore, several reports support a role for this pathway in the tissue migration of leukocytes, including neutrophils, in animal models of acute lung injury induced by LPS (10).
Based upon the importance of SDF-1/CXCR4 axis in many cellular injury processes, we hypothesize that SDF-1/CXCR4 axis takes part in OB in recruiting inflammatory cells and that CXCR4 inhibition would ameliorate OB in mouse trachea allografts.
Materials and Methods
Trachea grafting
Animals were transplanted as previously described (11, 12) Briefly, Balb/c donor mice (female) were euthanized and the trachea was resected and immediately placed in ice-cold PBS with penicillin G sodium (100 U/ml), streptomycin sulfate (100 μg/ml) (Life Technologies). Balb/c and C57BL/6 recipient mice were anesthetized with ketamine/xylazine (100 and 2 mg/kg i.p.; Phoenix Pharmaceuticals, St. Joseph, MO), and a 0.5-cm horizontal incision was made, and subcutaneous pockets were formed by blunt dissection. Two trachea grafts were placed heterotopically into the pockets, and the wound was closed with suture. No immunosuppressive agents were given to any graft recipient.
Experimental groups
Mice were divided into three groups: isograft, allograft, and allograft plus CXCR4 antagonist TN14003. There were 18 recipient mice and 36 transplanted tracheas in each group. Fourteen recipient mice were sacrificed on day 10 and the rest on day 42. At least 4 trachea samples were examined in each study setting. TN14003 was administered to mice daily intraperitoneally at a dose of 160ng/g initiated one day before the transplantation procedure.
Flow cytometry
Two or three tracheal grafts were pooled for each flow cytometry determination. The tracheal transplants were diced with a clean blade and then incubated in collagenase A (Roche) and DNase I (Sigma-Aldrich) at 37°C for 60 minutes to create a single cell suspension. The suspension was filtered through a 70 μM cell strainer (BD Bioscience, Mountain View, CA). Trachea cells were harvested by centrifugation for 5 minutes at 500g. The following conjugated antibodies were used for the staining: PE anti-mouse CD45, streptavidin-PerCP-Cy5.5 plus biotinylated anti-mouse CXCR4 (BD-Pharmingen, Mountain View, CA). A mouse IgG was used as isotype control. At least 50,000 events were collected using the FACSCalibur flow cytometer (BD Biosciences, Mountain View, CA) and analyzed using FlowJo (Tree Star, San Carlos, CA) software.
Western blot
Transplanted tracheas were homogenized in protein extract solution containing 0.1% Triton X-100, 100 mM NaCl, 10 mM Hepes, pH 7.9, 1 mM EDTA, and 0.5 mM PMSF (Sigma-Aldrich) on ice, and centrifuged at 13,000 g for 10 min at 4°C. The protein concentrations in the lysates were determined using the Bradford method (Bio-Rad Laboratories). Samples (20 μg protein per lane) were run on 4–20% SDS-PAGE gels (Invitrogen, Carlsbad, CA) and then transferred to nitrocellulose membranes. Blots were then incubated with a goat anti-mouse CXCR4 antibody (Abcam, Cambridge, MA) or a mouse anti-β-actin antibody (Sigma, St. Louis, MO). And recognized by horseradish peroxidase-conjugated anti-goat antibody (Amersham Biosciences, Piscataway, NJ). Finally, blots were visualized via chemiluminescence using the SuperSignal West Pico kit (Pierce, Rockford, IL). Expression of each band was normalized to its corresponding β-actin band.
Computerized morphometry
Computerized morphometry was determined as described by Farivar et al.(13) Images of H&E-stained tracheal sections were taken with a high-resolution digital camera attached to a microscope. Images analyzed using NIH Image software. The percentage of luminal obstruction was derived by outlining the inner surface of the cartilage. A line was drawn by connecting the 2 ends of the tracheal cartilage. The cursor was used to trace the inner surface of the actual residual lumen. The cross-sectional area within the actual residual lumen was then subtracted from the entire area contained within the cartilage. The percentage airway obstruction was then calculated using the following formula: (area within cartilage-area within residual lumen)/area within carriage × 100%.
CXCR4 antagonist
The CXCR4 antagonist TN14003 (14) was synthesized by the Microchemical Core Facility at Emory University. TN14003 was designed based on a specific CXCR4 inhibitor T140, a 14-residue peptide. TN14003 was generated by amidating the COOH-terminal of T140 and by substituting basic residues with non-basic polar amino acids to reduce the total-positive charges of the molecule.(15) TN14003 is less cytotoxic and more stable in serum compared with T140. The concentrations of T140 and TN14003 required for 50% protection of HIV-induced cytopathogenicity in MT-4 cells (EC50) are 3.3 and 0.6 nM, respectively. The concentrations of T140 and TN14003 that induce a 50% reduction of the viability of MT-4 cells [50% cytotoxic concentration (CC50)] are 59 and 410 μM, respectively. These results reflect the improved therapeutic index for TN14003 over T140 (SITN14003 = 680,000; SIT140 = 17,879; selective index (SI) = CC50/EC50).
Histology, immunohistochemistry and immunofluorescence
Five grafts from both isograft and allograft groups were used for immunohistochemistry and immunofluorescence analysis. To determine the SDF-1 and CXCR4 positive cells in the grafts, frozen sections were stained with a rabbit anti-SDF-1 antibody (e-Bioscience, San Diego, CA) and a rabbit anti-CXCR4 antibody (Abcam, Cambridge, MA). Slides were then treated with a FITC or rhodamine-conjugated donkey anti-rabbit antibody. Nuclei were detected by DAPI staining. For experiments to determine obstruction, sections were stained with H&E for routine histologic examination and Masson’s trichrome staining to delineate collagen. To determine the CD4 and CD8 infiltration, tracheas were stained with an anti-CD4 (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-CD8 (eBioscience) antibodies. DAB was used as the chromagen (Vector Laboratories, Burlingame, CA). In human tissue, immunohistochemistry was performed with an antibody for human SDF-1 and an antibody for human CXCR4 (R & D Systems, Minneapolis, MN). DAB was used as the chromogen.
Patient population
With Institutional Review Board (IRB) approval (Emory University), four pairs of archived lung samples with the diagnosis of OB and normal control were enrolled into the study.
Statistical Methods
For comparisons between groups, paired or unpaired t-test and one-way analysis of variance (ANOVA) tests were used (p values <0.05 were considered significant). We used GraphPad Prism and GraphPad InStat to calculate the statistics.
Results
SDF-1 and CXCR4 expression in a mouse OB model
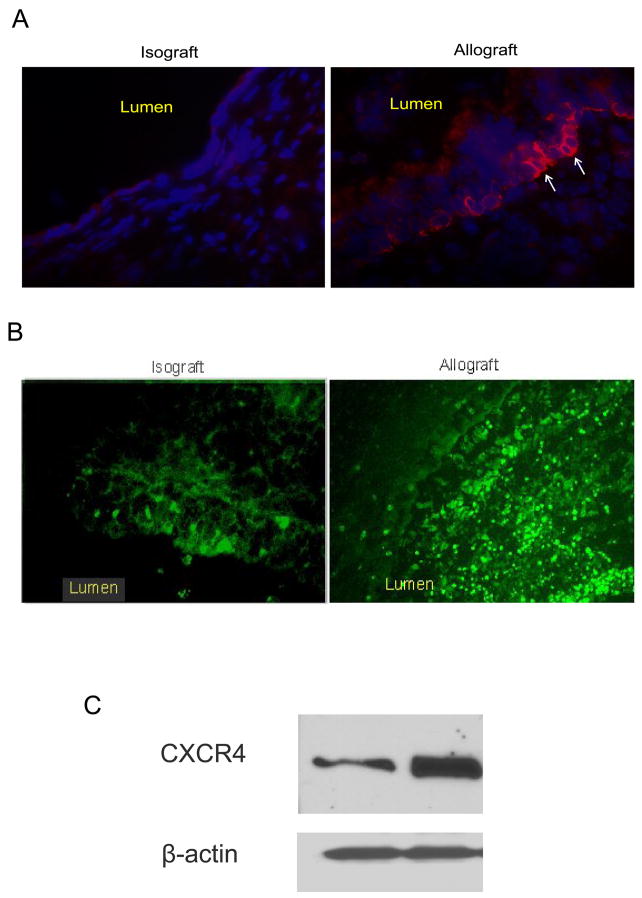
To determine whether SDF-1 expressing cells were increased in the transplants, a mouse heterotopic allogeneic airway transplant model was employed in the study. Donor tracheal segments from BALB/c (allografts) and C57BL/6 (isografts) were heterotopically transplanted into C57BL/6 recipients. Sections from isografts and allografts harvested 10 days after transplant were stained with antibodies specific for SDF-1 and CXCR4. As illustrated in the photomicrographs in figure 1, staining for SDF-1 positive cells in allografts was dramatically increased as compared with isografts. The SDF-1 positive cells were located at subepithelium layer. Subepithelial inflammation and injury is a characteristic of OB.(16) These results indicate that SDF-1 was produced at the site of injury and inflammation. On the other hand, CXCR4 positive cells filled the thickened trachea wall at day 10 after transplant as shown in immunofluorescence staining (Figure 1B). Increased levels of CXCR4 were also revealed by Western blot analysis using cell lysates from transplants (Figure 1C). These results indicate that SDF-1/CXCR4 may be involved in the pathogenesis of OB.
Figure 1.
SDF-1/CXCR4 expression at day 10 post-transplant. Mice were sacrificed day 10 post-transplant. (A) Frozen trachea sections from isograft and allograft groups were stained with a rabbit anti-SDF-1 antibody (red fluorescence). Photographs were taken using a fluorescence microscope at 20x magnification. (B) Frozen sections from isografts and allografts were stained with a rabbit anti-CXCR4 antibody. (C) CXCR4 protein expression as shown in western blot.
A CXCR4 antagonist attenuates the occlusion of lumen in allografts
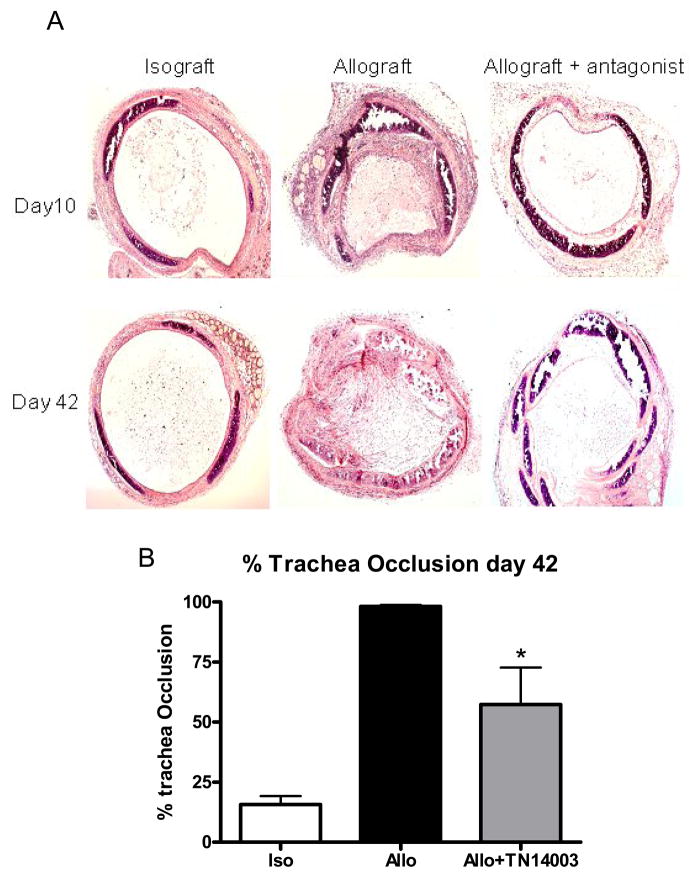
To determine the underling mechanism of OB, we then examined whether loss of epithelium and lumen occlusion would be blocked by a CXCR4 antagonist TN14003, which has been shown to alleviate lung fibrosis induced by bleomycin in a mouse model.(6) Mice were divided into three groups, isograft, allograft and allograft+TN14003 which was given daily intraperitoneally at the dose of 160ng/g. When allografts were examined on day 10 post-transplant, epithelium was lost in allograft (Figure 2A). However, epiethelium was still intact in both isograft and TN14003 treatment group. When allografts were analyzed at 42 days after transplantation, the obliterative process was already fully developed (Figure 2A). Luminal obstruction was 98.21% in the transplanted tracheas. Transplanted tracheas from mice treated with TN14003 were found to exhibit only 57.40% occlusion. This reduction in luminal obstruction was statistically significant at p < 0.01 (Figure 2B).
Figure 2.
CXCR4 antagonist reduces epithelial loss and trachea occlusion. C57BL/6 mice were transplanted heterotopically with C57BL/6 (isograft) or BALB/c (allograft) trachea. In allograft group, half of the mice were injected daily with 160ng/g of CXCR4 antagonist TN14003, a 14 amino acid peptide with loop structure. Tracheal grafts were harvested on days 10 and 42 post-transplant, stained with H&E (figure 2A, 2B) and percentage of trachea occlusion determined on day 42 (figure 2C). Values represent mean ± standard error. n=4, *, p<0.05.
TN14003 reduces CD45+CXCR4+ cell infiltration
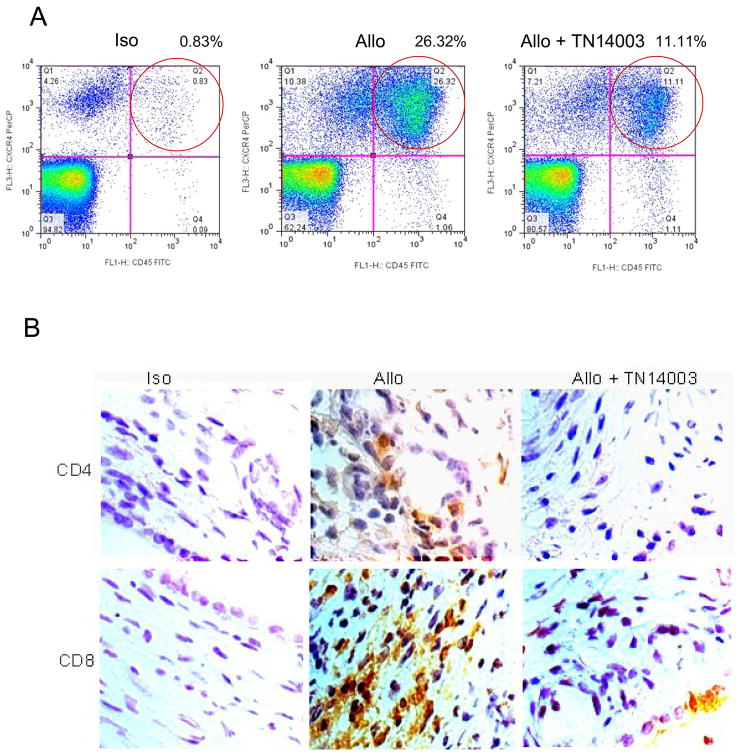
We next examined whether there was an alteration in total leukocyte infiltration into trachea transplants and, if so, which subsets were affected. Using flow cytometry analysis, we noticed that there was a significant increase in the accumulation of CXCR4+CD45+ in the allografts day 10 post-transplant. Furthermore, the accumulation was blocked by the CXCR4 antagonist TN14003 (Figure 3A). To determine the identity of the accumulated inflammatory cells, immunohistochemistry was used to detect CD4 T cells, CD8 T cells, and neutrophils in the transplants. The results revealed that the majority of the cells were CD4+ and CD8+ T cells (Figure 3B) and very few neutrophils. Furthermore, infiltration of CD4+ and CD8+ T cells were both reduced with TN14003 treatment.
Figure 3. TN14003 reduces CD45+CXCR4+ cell infiltration.
(A) Trachea cells from day 10 post-transplant in isograft (Iso), allograft (Allo) and allograft plus TN14003 groups were labeled with anti-mouse CXCR4 and CD45 and subjected to FACS analysis. (B) Paraffin sections from Isografts, allografts and allografts plus TN14003 groups were stained with an anti-CD4 or an anti-CD8 antibody. Cells with dark brown staining represent positive cells for CD4 or CD8.
Effect of TN14003 on cytokine levels
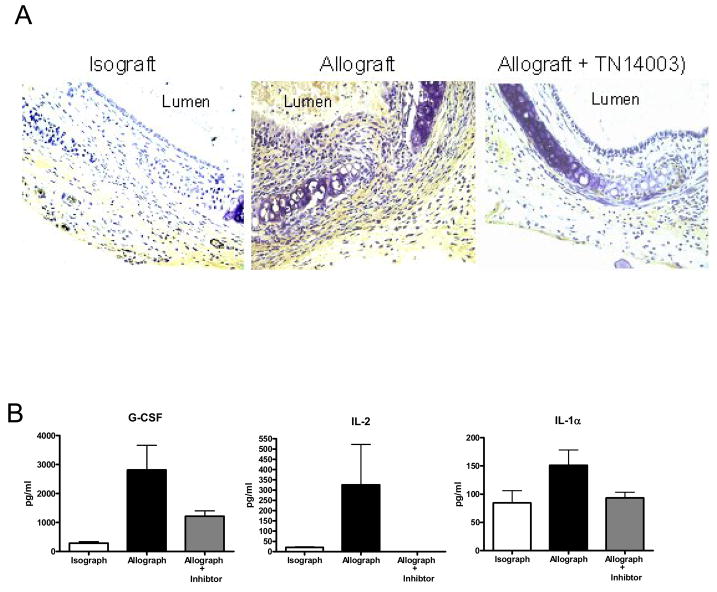
Increased expression of TGF- β1 has been shown as key factors in OB (17–19). We determined the TGF-β1 expression in trachea transplants by immunohistochemistry (Figure 4A). At day 10 after transplantion, TGF-β1 staining was quite extensive in the allograft section as compared with that of isografts. In comparion, sections from allografts treated with TN14003 showed only scattered positivity. We also determined cytokine levels in the serum of recipient mice at day 10 after transplant using Luminex. G-CSF, IL-2 and IL-1α levels were significantly increased in allografts and the increase was blocked by TN14003 (Figure 4B). These results revealed that G-CSF, IL-2, IL-1α and TGF-β1 levels were increased in allografts and reduced by treatment with CXCR4 antagonist.
Figure 4. Effect of TN14003 on cytokine levels.
(A) TGF-β1 expression in trachea 10 days post-transplant was determined by immunohistochemistry. (B) Cytokine levels in the serum of recipient mice at day 10 post-transplant were determined using Luminex. Values represent mean ± standard error. *, **, p<0.05, n=4.
SDF-1 and CXCR4 are increased in lungs of humans with OB
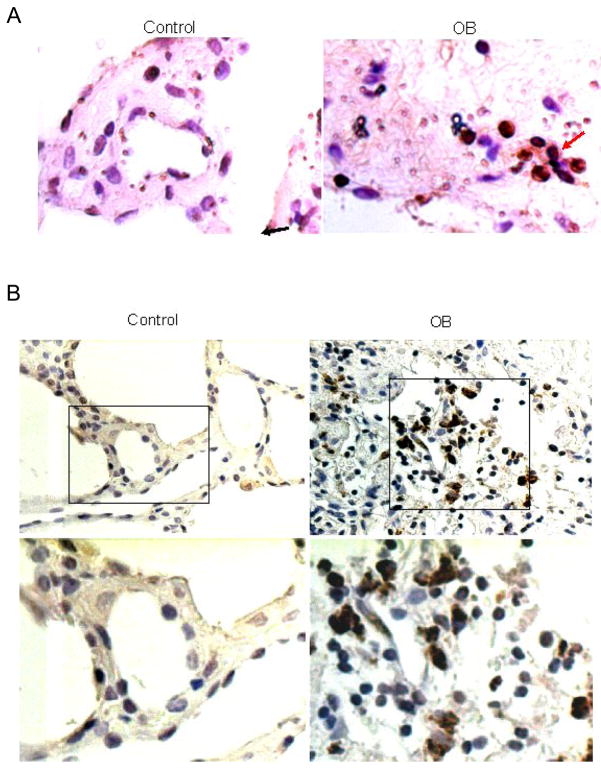
The above data implicate the SDF-1/CXCR4 axis in the pathogenesis of trachea transplants in mice, but whether similar pathogenetic processes are operative in OB in humans remains to be shown. We analyzed tissue samples of normal human lungs and samples of human lungs from patients with the clinical picture of OB. Figure 5 shows photomicrographs of sections from normal and OB lungs stained with antibodies specific for either SDF-1 or CXCR4. SDF-1 and CXCR4 positive cells were clearly identifiable in lungs from OB patients, but very few were observed in normal lungs. In both cases, positively stained cells were more frequent in the OB lungs. These results are in concordance to the data from the rodent OB model and suggest that recruitment of CXCR4+ cells by SDF-1, to the lungs is important in the pathogenesis of OB.
Figure 5.
Increased SDF-1 and CXCR-4 in OB lung samples compared to normal human lungs. Lung tissues from OB patients (right) and normal control (left) were paraffin embedded and sectioned. Immunohistochemistry was performed for SDF-1 (A) and CXCR-4 (B). Cells stained dark brown were SDF-1 or CXCX4 expressing cells (see arrows).
Discussion
In this study, we sought to investigate the mechanism by which cells are recruited to the transplants in a mouse heterotopic airway transplant model and to determine the consequences of inhibiting the recruitment. Based on studies in the literature, we hypothesized that the SDF-1/CXCR4 axis is a primary system for inflammatory cell recruitment to the allografts and therefore could be important in the fibrotic response.
SDF-1 is produced by stromal cells. Chemokine receptor CXCR4 expression has been found in leukocytes, including peripheral blood lymphocytes, neutrophils and monocytes.(20) CXCR4 is by far the most widely expressed of the functional chemokine receptor in nonhematopoietic cells (21). High transcript levels were demonstrable in several tissues, including heart, brain, liver and colon (22). At basal state, tissue fibroblasts and leukocytes contribute to the production of SDF1 and CXCR4 respectively (23).
To demonstrate the role of SDF-1 in the pathogenesis of OB, we used the HTT model described by Hertz et al (11) in which a transplantation of a trachea from a genetically discordant donor mouse into a subcutaneous pocket in the back of a recipient mouse resulted in rejection of the tracheal airway similar to those seen in human OB. Airway rejection in the HTT model, is preceded by substantial periairway/subepithelial mononuclear cell infiltration similar to lymphocytic bronchiolitis that peaks between Days 10 and 14, followed by lumen obliteration comparable to human OB by Days 21 to 28. In contrast to the rejection pathology observed in tracheal airway allografts, genetically identical airway isografts have normal-appearing tracheal histology at Day 28, with evidence of neovascularization (1). Despite a high level of reproducibility in the HTT model, and the similarities to human OB, there are limitations to the model. First, the tracheal allograft is not a functional airway. A second limitation to the HTT model, and commonly cited is that the transplanted airway is not primarily vascularized, different to the human lung transplant. Although limitations in the murine HTT experimental model certainly exist, similarities to human OB pathology make it very useful to study immune mechanisms of airway rejection(1).
Our results showed that SDF-1 producing cells were increased and located at subepithelial layer in allografts, which is a characteristic site for inflammation and injury in this model. CXCR4 expressing cells and levels in the allografts were significantly increased as determined by Western blot. Flow cytometry analysis revealed that there was a significant increase in CD45+CXCR4+ cells in allografts. A big proportion of the accumulated cells were CD4 and CD8 T cells as determined by immunohistochemical analysis. A CXCR4 antagonist, TN14003, blocked CD4 and CD8 cell accumulation and alleviated epithelial loss and lumen occlusion. In lungs from patients with OB, we found increased positive staining cells for SDF-1 and CXCR4 compared to normal human lungs. Taken together these data are consistent with the hypothesis that SDF-1/CXCR4 axis played a role in the pathogenesis of OB. OB may involve continued expression of SDF-1 in the bronchiole and consequent continued recruitment of inflammatory cells.
SDF-1 is chemotactic for human lymphoid, myeloid and CD34 positive cells. Lymphopoiesis and myelopoiesis are markedly reduced in CXCR4 and SDF-1 deficient mice.(24) A variety of stem cells express CXCR4,(5, 25, 26) including hematopoietic stem cells(27), as well as progenitor cells committed to neural,(24) myocardial,(28) and endothelial(29, 30) differentiation pathways. Inflammatory cells, such as lymphocytes, eosinophils, and neutrophils, also express CXCR4 which contributes to the chemotaxis, activation, and homeostasis of these cells.(31–33) SDF-1/CXCR4-induced chemotaxis of T (34). SDF-1/CXCR4 interactions play a central role in the CD4+ T cell accumulation in rheumatoid arthritis synovium.(35) Rheumatoid fibroblast-like synoviocytes overexpress SDF-1, which supports distinct patterns and rates of CD4+ and CD8+ T cell migration within synovial tissue.(36)
Studies from our lab demonstrated that TN14003 reduces lung injury and fibrosis induced by bleomycin.(6) Watanabe et al. showed AMD3100, a CXCR4 antagonist in clinical trial, have the similar effect. The CXCR4 antagonist TN14003 we used is a peptide with specificity for the receptor that, like other similar agents, has anti-HIV-1 activity(15, 37, 38) and inhibits metastases of breast cancer in animal models.(14, 39, 40) Treatment of mice with either the CXCR4 antagonist that we used in the present study or with a neutralizing antibody to SDF-1 alleviates OB, although the fibrosis was not completely prevented in either case. It is possible CXCR4 independent pathways may also be involved in the process.
CD4+ and CD8+ T cells are important for the immunological rejection of most tissue allografts. CD4+ T cell ‘helper’ activity promotes the differentiation and continued Ag responsiveness of graft-reactive precursor cytolytic CD8+ T cells, as a consequence of both direct cell-to-cell contact as well as cooperation through the activation of APCs (41). APCs serves as a platform for CD8+ T cells to benefit from IL-2 produced by neighboring CD4 T cells (42). CD4 T cells can also act primarily on dendritic cells (DCs) to increase their ability to stimulate CD8 T cells (40).
The studies we report here implicate the SDF-1/CXCR4 axis in the pathogenesis of OB in mice. The fact that the lungs of patients with OB contain increased numbers of cells expressing SDF-1 and CXCR4 is consistent with the idea that chronic active injury in the lungs is accompanied by continued expression of SDF-1 and continued recruitment of CXCR4+ lymphocytes that lead to the rejection.
Nonstandard abbreviations used
- (OB)
obliterative bronchiolitis
- (IPF)
idiopathic pulmonary fibrosis
- (BM)
bone marrow
- (PBS)
phosphate buffered saline
- (SDF-1)
stromal cell-derived factor-1
- (FACS)
fluorescence-activated cell sorting
- (APC)
antigen-presenting cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McDyer JF. Human and murine obliterative bronchiolitis in transplant. Proceedings of the American Thoracic Society. 2007;4:37–43. doi: 10.1513/pats.200605-107JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicod LP. Mechanisms of airway obliteration after lung transplantation. Proceedings of the American Thoracic Society. 2006;3:444–9. doi: 10.1513/pats.200601-007AW. [DOI] [PubMed] [Google Scholar]
- 3.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 4.Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–37. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petit I, Goichberg P, Spiegel A, et al. Atypical PKC-zeta regulates SDF-1-mediated migration and development of human CD34+ progenitor cells. J Clin Invest. 2005;115:168–76. doi: 10.1172/JCI21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J, Mora A, Shim H, Stecenko A, Brigham KL, Rojas M. Role of the SDF-1/CXCR4 Axis in the Pathogenesis of Lung Injury and Fibrosis. Am J Respir Cell Mol Biol. 2007;37:291–9. doi: 10.1165/rcmb.2006-0187OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–46. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lukacs NW, Berlin A, Schols D, Skerlj RT, Bridger GJ. AMD3100, a CxCR4 antagonist, attenuates allergic lung inflammation and airway hyperreactivity. The American journal of pathology. 2002;160:1353–60. doi: 10.1016/S0002-9440(10)62562-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthys P, Hatse S, Vermeire K, et al. AMD3100, a potent and specific antagonist of the stromal cell-derived factor-1 chemokine receptor CXCR4, inhibits autoimmune joint inflammation in IFN-gamma receptor-deficient mice. J Immunol. 2001;167:4686–92. doi: 10.4049/jimmunol.167.8.4686. [DOI] [PubMed] [Google Scholar]
- 10.Petty JM, Sueblinvong V, Lenox CC, et al. Pulmonary stromal-derived factor-1 expression and effect on neutrophil recruitment during acute lung injury. J Immunol. 2007;178:8148–57. doi: 10.4049/jimmunol.178.12.8148. [DOI] [PubMed] [Google Scholar]
- 11.Hertz MI, Jessurun J, King MB, Savik SK, Murray JJ. Reproduction of the obliterative bronchiolitis lesion after heterotopic transplantation of mouse airways. The American journal of pathology. 1993;142:1945–51. [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly KE, Hertz MI, Mueller DL. T-cell and major histocompatibility complex requirements for obliterative airway disease in heterotopically transplanted murine tracheas. Transplantation. 1998;66:764–71. doi: 10.1097/00007890-199809270-00011. [DOI] [PubMed] [Google Scholar]
- 13.Farivar AS, Woolley SM, Naidu BV, et al. Poly (ADP) ribose synthetase inhibition reduces obliterative airway disease in rat tracheal allografts. J Heart Lung Transplant. 2004;23:993–1002. doi: 10.1016/j.healun.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Liang Z, Wu T, Lou H, et al. Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4. Cancer Res. 2004;64:4302–8. doi: 10.1158/0008-5472.CAN-03-3958. [DOI] [PubMed] [Google Scholar]
- 15.Tamamura H, Fujisawa M, Hiramatsu K, et al. Identification of a CXCR4 antagonist, a T140 analog, as an anti-rheumatoid arthritis agent. FEBS Lett. 2004;569:99–104. doi: 10.1016/j.febslet.2004.05.056. [DOI] [PubMed] [Google Scholar]
- 16.Markopoulo KD, Cool CD, Elliot TL, et al. Obliterative bronchiolitis: varying presentations and clinicopathological correlation. Eur Respir J. 2002;19:20–30. doi: 10.1183/09031936.02.00282001. [DOI] [PubMed] [Google Scholar]
- 17.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–76. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramirez AM, Takagawa S, Sekosan M, Jaffe HA, Varga J, Roman J. Smad3 deficiency ameliorates experimental obliterative bronchiolitis in a heterotopic tracheal transplantation model. The American journal of pathology. 2004;165:1223–32. doi: 10.1016/S0002-9440(10)63382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou H, Latham CW, Zander DS, Margolin SB, Visner GA. Pirfenidone inhibits obliterative airway disease in mouse tracheal allografts. J Heart Lung Transplant. 2005;24:1577–85. doi: 10.1016/j.healun.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Loetscher M, Geiser T, O’Reilly T, Zwahlen R, Baggiolini M, Moser B. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. The Journal of biological chemistry. 1994;269:232–7. [PubMed] [Google Scholar]
- 21.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annual review of immunology. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 22.Federsppiel B, Melhado IG, Duncan AM, et al. Molecular cloning of the cDNA and chromosomal localization of the gene for a putative seven-transmembrane segment (7-TMS) receptor isolated from human spleen. Genomics. 1993;16:707–12. doi: 10.1006/geno.1993.1251. [DOI] [PubMed] [Google Scholar]
- 23.Eitner F, Cui Y, Hudkins KL, Alpers CE. Chemokine receptor (CXCR4) mRNA-expressing leukocytes are increased in human renal allograft rejection. Transplantation. 1998;66:1551–7. doi: 10.1097/00007890-199812150-00021. [DOI] [PubMed] [Google Scholar]
- 24.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 25.Rosu-Myles M, Gallacher L, Murdoch B, et al. The human hematopoietic stem cell compartment is heterogeneous for CXCR4 expression. Proc Natl Acad Sci U S A. 2000;97:14626–31. doi: 10.1073/pnas.97.26.14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 2002;16:1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- 27.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–20. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damas JK, Eiken HG, Oie E, et al. Myocardial expression of CC- and CXC-chemokines and their receptors in human end-stage heart failure. Cardiovasc Res. 2000;47:778–87. doi: 10.1016/s0008-6363(00)00142-5. [DOI] [PubMed] [Google Scholar]
- 29.Dar A, Goichberg P, Shinder V, et al. Chemokine receptor CXCR4-dependent internalization and resecretion of functional chemokine SDF-1 by bone marrow endothelial and stromal cells. Nat Immunol. 2005;6:1038–46. doi: 10.1038/ni1251. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi J, Kusano KF, Masuo O, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–8. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 31.Nagase H, Miyamasu M, Yamaguchi M, et al. Expression of CXCR4 in eosinophils: functional analyses and cytokine-mediated regulation. J Immunol. 2000;164:5935–43. doi: 10.4049/jimmunol.164.11.5935. [DOI] [PubMed] [Google Scholar]
- 32.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–9. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yousefi S, Cooper PR, Potter SL, Mueck B, Jarai G. Cloning and expression analysis of a novel G-protein-coupled receptor selectively expressed on granulocytes. J Leukoc Biol. 2001;69:1045–52. [PubMed] [Google Scholar]
- 34.Okabe S, Tauchi T, Nakajima A, et al. Depsipeptide (FK228) preferentially induces apoptosis in BCR/ABL-expressing cell lines and cells from patients with chronic myelogenous leukemia in blast crisis. Stem Cells Dev. 2007;16:503–14. doi: 10.1089/scd.2007.9994. [DOI] [PubMed] [Google Scholar]
- 35.Nanki T, Hayashida K, El-Gabalawy HS, et al. Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium. J Immunol. 2000;165:6590–8. doi: 10.4049/jimmunol.165.11.6590. [DOI] [PubMed] [Google Scholar]
- 36.Bradfield PF, Amft N, Vernon-Wilson E, et al. Rheumatoid fibroblast-like synoviocytes overexpress the chemokine stromal cell-derived factor 1 (CXCL12), which supports distinct patterns and rates of CD4+ and CD8+ T cell migration within synovial tissue. Arthritis Rheum. 2003;48:2472–82. doi: 10.1002/art.11219. [DOI] [PubMed] [Google Scholar]
- 37.Murakami T, Nakajima T, Koyanagi Y, et al. A small molecule CXCR4 inhibitor that blocks T cell line-tropic HIV-1 infection. J Exp Med. 1997;186:1389–93. doi: 10.1084/jem.186.8.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schols D, Struyf S, Van Damme J, Este JA, Henson G, De Clercq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med. 1997;186:1383–8. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kucia M, Reca R, Miekus K, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–94. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 40.Smith CM, Wilson NS, Waithman J, et al. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol. 2004;5:1143–8. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 41.Richards DM, Zhang N, Dalheimer SL, Mueller DL. Allopeptide-specific CD4(+) T cells facilitate the differentiation of directly alloreactive graft-infiltrating CD8(+) T Cells. Am J Transplant. 2007;7:2269–78. doi: 10.1111/j.1600-6143.2007.01934.x. [DOI] [PubMed] [Google Scholar]
- 42.Cassell D, Forman J. Linked recognition of helper and cytotoxic antigenic determinants for the generation of cytotoxic T lymphocytes. Ann N Y Acad Sci. 1988;532:51–60. doi: 10.1111/j.1749-6632.1988.tb36325.x. [DOI] [PubMed] [Google Scholar]