Abstract
The avian brainstem serves as a useful model system to address the question of how afferent activity influences viability of target neurons. Approximately 20–30% of neurons in the avian cochlear nucleus, nucleus magnocellularis (NM) die following deafferentation (i.e., deafness produced by cochlea removal). Previous studies have identified cellular events that occur within hours following cochlea removal, which are thought to lead to the ultimate death of NM neurons. We have recently shown that chronic lithium treatment increases neuronal survival following deafferentation. To assess where in the cell death cascade lithium is having its effect, we evaluated some of the early deafferentation-induced cellular changes in NM neurons. Lithium did not affect deafferentation-induced changes that occur across the entire population of NM neurons. There were still deafferentation-induced increases in intracellular calcium concentrations and early changes in the ribosomes, as indicated by Y10b immunolabeling. Lithium did, however, affect changes that are believed to be indicative of the subpopulation of NM neurons that will eventually die. Ribosomes recovered in all of the deafferented NM neurons (as assessed by Y10b labeling) by 10 hours following cochlea removal in subjects pretreated with lithium, while a subpopulation of the NM neurons in saline-treated subjects showed dramatic reduction in Y10b labeling at that time. Lithium treatment also prevented the robust upregulation of Bcl-2 mRNA that is observed in a subpopulation of deafferented NM neurons 6 hours following cochlea removal.
Keywords: Auditory system, Cell death, Bcl-2, Fura-2, Neuroprotection, Nucleus magnocellularis
It is generally accepted that sensory experience plays an important role in the development of the brain. This idea is supported by studies showing changes in innervation patterns, or even cell death, following the loss of sensory experience in young animals (e.g. Catsicas et al., 1992; Pope and Wilson, 2007; Meisami and Safari, 1981). The effects of sensory deprivation have been extensively studied in the brainstem auditory system of the chick (Rubel et al., 1990). Loss of sensory input, produced by cochlea removal, results in the death of approximately 20–30% of the neurons in the ipsilateral cochlear nucleus, nucleus magnocellularis (NM) (Born and Rubel, 1985).
The brainstem auditory system of the chick has proven to be a fruitful model system for examining the effects of sensory deprivation, in part, because of its relatively simple organization. In this system, the ipsilateral auditory nerve provides NM neurons with their sole excitatory input (Parks and Rubel, 1978; Rubel 1978; Born et al. 1991). Consequently, a unilateral manipulation of the auditory periphery affects input to ipsilateral NM, yet leaves input to contralateral NM intact. This allows for within-subject comparisons of deprived and intact NM neurons following unilateral manipulations of auditory nerve activity. The most common procedure for examining the effects of auditory deprivation in this system has been to induce deafness by removing one basilar papilla (cochlea) and then compare the NM neurons on the deafened and intact sides of the brain.
A second advantage of studying the chick brainstem is that the effects of altering sensory input are relatively rapid. This allows one to track the sequence of events that occurs between the onset of deafness and the ultimate death of the neurons. As noted, approximately 20–30% of NM neurons die within two days following deafness (Born and Rubel, 1985). The remaining neurons show decreased soma size and reduced metabolism, but survive. Cellular changes in NM are observed, however, much earlier following deafferentation. As early as one hour following cochlea removal, all deafferented NM neurons show a rise in intracellular calcium (Zirpel et al., 1995; 1996) and a reduction in overall protein synthesis (Steward and Rubel, 1985). At later time points (6–12 hours post cochlea removal), deafferented NM neurons divide into two populations based on changes in ribosomal structure and function. The majority of neurons show some recovery of protein synthesis, but 20–30% of the neurons appear to show a complete disruption of protein synthesis (Steward and Rubel, 1985) that appears to be attributable to a disassociation of their polyribosomes (Rubel et al., 1991; Garden et al., 1994). The cells that show a complete disruption of their ribosomes are believed to be those that will eventually die within the next 48 hours. The rapid changes in ribosomal function that are observed after cochlea removal are mirrored by changes in antigenicity for a ribosomal epitope that is recognized by the antibody Y10b (Garden et al., 1994; Hyson and Rubel, 1995; Hyson, 1997; 1998).
Analyses of molecular changes that may regulate the death or survival of NM neurons following deafferentation revealed that some molecules known to be involved in the control of traditional apoptotic cell death (cytochrome-c and caspase-9) are also regulated by deafferentation (Wilkinson et al., 2003). Unexpectedly, however, Wilkinson et al. (2002) found an increase in mRNA expression for the neuroprotective protein Bcl-2 in the, presumably, dying population of deafferented NM neurons. They suggested that although mRNA for this neuroprotective molecule increased, the protein synthesis machinery had broken down by the time the mRNA was expressed. Consequently, it was too late for Bcl-2 to have its known neuroprotective influence.
Bush and Hyson (2006) hypothesized that if Bcl-2 protein was upregulated prior to cochlea removal, then NM neurons might be protected from deafferentation-induced cell death. To test this hypothesis, they chronically administered lithium to posthatch chicks prior to cochlea removal. Chronic lithium has been shown to increase expression of Bcl-2 (Chen et al., 1999) and to increase neuronal survival in a variety of animal models of disease and stroke/ischemia (Chuang et al., 2002; Leegwater-Kim and Cha, 2004; Manji et al., 2002; Martin et al., 1998). Additionally, lithium has been shown to be effective in preventing toxic increases in intracellular calcium (Okamoto et al., 1994). Bush and Hyson (2006) found that chronic lithium administration was effective at increasing Bcl-2 protein expression in NM neurons and that this treatment protected the neurons from deafferentation-induced cell death.
Although upregulation of Bcl-2 protein following lithium administration is correlated with cell survival in NM neurons, it is possible that lithium could be influencing other aspects of the cell death cascade. Lithium’s neuroprotective effect could be working by preventing the triggers for cell death, or by preventing cell death cascades that are downstream of the initial trigger. The present series of experiments examines different events in the cell death cascade in NM neurons. Early occurring (calcium levels), mid-occurring (Y10b immunolabeling), and late-occurring (Y10b immunolabeling and Bcl-2 mRNA expression) events are investigated in an attempt to determine which aspects of the cell death cascade are being influenced by lithium treatment.
EXPERIMENTAL PROCEDURES
Subjects
All subjects were Ross × Ross chickens. Eggs, obtained from a local supplier, were incubated and hatched at the Florida State University.
Calcium imaging
Saline or 0.15M lithium chloride (LiCl) was administered onto the chorioallantoic membrane through a hole drilled into the shell. A single injection was given at 60 hours after the beginning of incubation. The method was identical to that used by Ikonomov et al., 2000, with the dose based on their dose-response studies. Since lithium is not metabolized and cannot be excreted in ovo, a single administration results in a chronic lithium environment. The egg is a closed system, so any lithium that is “excreted” is continuously reabsorbed. The lithium dose was based on egg weight, assuming 80% water content, to bring the final concentration to 0.3mM LiCl (see Ikonomov et al., 2000). Incubation was continued until E18.
Tissue from eight subjects (5 lithium and 3 saline-treated) was prepared for Fura-2 ratiometric imaging as described by Zirpel et al. (1995; 1996). The embryo was anesthetized by cooling and decapitated. A 300 µm thick coronal section that was midway between the rostral and caudal extent of NM was obtained as previously described (Hyson and Rubel, 1995; Hyson, 1997; 1998). This procedure results in bilateral deafferentation of NM neurons and changes in these deafferented neurons can then be tracked over time in vitro. NM neurons were loaded with Fura-2 by incubating slices in oxygenated artificial cerebral spinal fluid (ACSF) containing 6 µM of Fura-2 AM (Molecular Probes, Eugene, OR), 1.7% anhydrous demethylsulfoxide (DMSO), and 0.03% Pluronic (Molecular Probes, Eugene, OR) for 20–30 minutes at room temperature. ACSF contained (in mM) sodium chloride, 130; sodium bicarbonate, 26; potassium chloride, 3; calcium chloride, 2; magnesium chloride, 2; sodium phosphate, 1.25; and D-glucose, 10. ACSF was oxygenated using a 95% oxygen/5% carbon dioxide gas mixture. Slices were then placed in an imaging chamber where they were superfused with oxygenated ACSF for 5 minutes before data acquisition. Fura-2 loaded neurons were alternately excited with 340 nm and 380 nm wavelengths of light from a xenon source using a computer controlled shutter and filter wheel. Paired 340/380 excitation images were acquired every 5 minutes for 1 hour and analyzed with Metafluor software. At the end of this test period, ACSF containing 75 mM KCl was applied to the slice. Only cells showing a reliable response to KCl were included in the final analyses (see Zirpel et al, 1995). All cells for a given slice were averaged and that average measure was used to pool data across different slices for statistical analyses. Data was analyzed as the change in ratio over time by ANOVA using SPSS software.
Daily injections
All procedures were in accordance with Animal Care and Use Committee guidelines. Post-hatch chicks (P0) of either sex, received daily sub-cutaneous injections of either LiCl or saline for 17 days, prior to unilateral cochlea removal surgery. The dose of LiCl was gradually increased across age, beginning at 1.5 mEq/kg for the first four days, followed by 2.3 mEq/kg for seven days, and finally, 3.0 mEq/kg for the last six days. This schedule was identical to that used by Bush and Hyson (2006) and adapted from the protocols of both Wei et al. (2001) and Nonaka and Chuang (1998). The volume of each injection was 1 ml/kg. Control subjects received daily 1 ml/kg injections of saline.
Cochlea removal surgery
One hour after the final injection, subjects were anesthetized with Halothane. A small incision was made to widen the ear canal and the tympanic membrane was punctured. The columella was removed, followed by the extraction of the basilar papilla (cochlea) through the oval window using forceps. The extracted tissue was viewed under a dissection microscope to ensure complete cochlea removal. The middle ear cavity was packed with Gelfoam and the external incision sealed with surgical adhesive. Subjects were then allowed to survive for 3, 6, or 10 hours prior to perfusion and subsequent tissue processing (n per drug treatment group = 5, 3, and 5, respectively). Subjects processed for in situ hybridization survived 6 hours post cochlea removal (lithium n = 5 and saline n = 3).
Tissue preparation
At the predetermined survival period following cochlea removal, subjects were deeply anesthetized with pentobarbital and perfused with 0.9% saline followed by ice cold 4% paraformaldehyde. Brainstems were blocked and post-fixed in 4% paraformaldehyde for 1–2 hours followed by overnight cryoprotection at 4°C in phosphate buffered saline (PBS) containing 20% sucrose. The tissue was rapidly frozen in 2-methylbutane on dry ice and embedded in TBS tissue freezing medium for cryosectioning using a Leica CM 1850 cryostat.
Immunohistochemistry
Sections for Y10b immunoreactivity were cut at 20–25 µm and were collected into ice cold PBS. Every section containing NM was collected. Pairs of subjects, one treated with lithium and one treated with saline, were processed simultaneously. Puncture marks were made in the ventral portion of the brain stem to identify treatment condition following the simultaneous processing. Sections containing NM were placed in a vial containing PBS. Sections were then washed 2 × 10 minutes in PBS and endogenous peroxidase activity was quenched by incubating in 0.03% H2O2 in methanol for 20 minutes. Following 3 10-minute rinses in PBS, sections were placed in a blocking solution containing 1% Normal Horse Serum (NHS) in PBS for 1 hour. Sections were then transferred to a 1:500 concentration of the primary antibody, Y10b, in blocking solution. The Y10b antibody recognizes a ribosomal epitope (Garden et al., 1994; Hyson, 1998). A series of sections were run without the application of the primary antibody in order to ensure specificity of the reaction. Following overnight incubation on a rotator, sections were washed 3 × 10 minutes in PBS. Sections were then incubated in a 1:200 concentration of horse anti-mouse antibody in blocking solution for 1 hour. Following 3 10-minute washes with PBS, sections were incubated in avidin-biotin-peroxidase complex (ABC; Vector Laboratories, Burlingame, CA, USA) for 1 hour. After another round of washes with PBS, sections were reacted with diaminobenzidine (DAB) tetrahydrochloride and H2O2 for visualization. Following a final round of washes, sections were mounted on slides and allowed to dry overnight. The following day, slides were dehydrated in a graded series of alcohols and cleared in xylenes for 10 minutes. Slides were coverslipped in DPX mounting medium and allowed to dry overnight.
In situ hybridization
The in situ hybridization methods were identical to those used by Wilkinson et al. (2002). Briefly, cryosections (30 µm) were collected in ice-cold diethyl-polycarbonate (DEPC)-treated, 2× sodium chloride, sodium citrate buffer (SSC) in autoclaved scintillation vials. After the buffer was removed, the tissue was covered with prehybridization buffer containing 3.25 ml formamide, 2× SSC, 10% dextran, 1× Denhardts, 50ml DTT and 250µl of salmon sperm DNA (about 1ml/40 sections). The Bcl-2 oligonucleotide probe (Invitrogen) was complementary to nucleotides 1147–1191 of the chicken Bcl-2 sequence. The oligonucleotide was end-labeled with 35S, and was added to the prehybridization buffer after 1 hour and incubated at 39°C overnight. The following day, sections were washed with diluted serial concentrations of SSC (2×, 1×, and 0.5× for 15 minutes at 39°C). Sections were then mounted onto slides with gelatin and dried. Sections were exposed to x-ray film (Amersham) for 2 days and then dipped in photographic emulsion (Kodak NTB2) and stored in light-tight boxes for a period ranging from 2–4 weeks. The slides were then developed (Kodak D-19) and fixed (Kodak fixer), counterstained with thionin, and coverslipped.
Objective analysis of anatomical tissue
Immunolabeling of NM neurons was objectively analyzed using densitometry (NIH Image J). For some of the experiments, the staining densities on the intact side of the brainstem were compared to those on the deafferented side of the same tissue section. When the comparison was between lithium- and saline-treated subjects, the tissue was processed simultaneously in the same reagents to prevent processing variables from affecting the results. The light levels and contrast settings remained the same for all tissue sections analyzed. On average, 40–50 neurons in each nucleus (intact and deafferented; saline and lithium) were measured. All neurons with a clear intact visible cell membrane were included in the analysis. Neurons were measured starting from the most medial edge of the nucleus and proceeding laterally until either no other neurons were present in NM or the criterion number of cells was reached. One to 4 sections were analyzed per brain. To prevent bias, the identity of whether the subject was treated with lithium or saline was not revealed until all measurements were completed.
For analysis of in situ hybridization, emulsion dipped slides were analyzed using NIH Image J. A gray scale threshold was visually set to identify developed silver grains. This threshold remained constant for all measurements. The grain density, defined as the percentage of soma area that was covered by silver grains, was measured for approximately 40 cells within each NM in a given tissue section. The data were analyzed using Microsoft EXCEL and SPSS.
RESULTS
Calcium ratiometric imaging
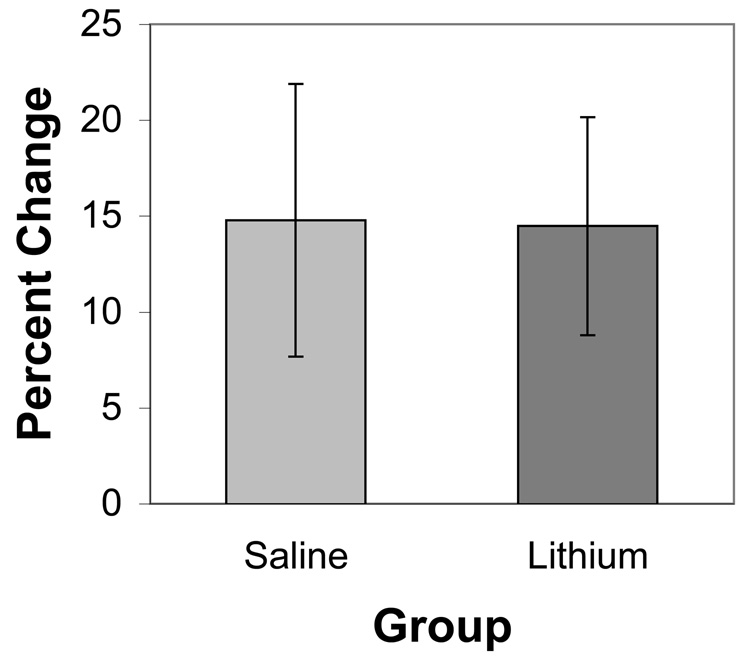
Intracellular calcium levels increased over time in the deafferented NM neurons maintained in vitro (see also Zirpel et al., 1995). This was true for both lithium- and saline-treated subjects. For statistical analysis, ratios during a baseline 10 minute period were compared to those after 60 minutes in vitro (Figure 1). A mixed ANOVA, using time as the within-subject variable and drug treatment as the between-subjects variable, revealed a reliable effect of time (F(1,7) = 14.6, p < 0.01), but no reliable effect of treatment, nor a treatment × time interaction (F(1,7) = 2.5 and 0.7, respectively, p > 0.1). Although there was a trend for lithium-treated subjects to have a higher baseline ratio, this effect was not statistically reliable. Regardless of starting ratio, however, subjects in both groups showed increased ratios over time (paired t(4) = 3.0 and 2.96, for saline and lithium groups, respectively, p < 0.05).
Figure 1.
Percent change of average 340/380 ratios over Fura-2 loaded NM neurons from saline- and lithium-treated subjects. Both groups showed an increase of intracellular calcium over time (p < 0.01), but there was no reliable difference between lithium- and saline-treated groups. Error bars represent standard error in the mean.
Immunohistochemistry
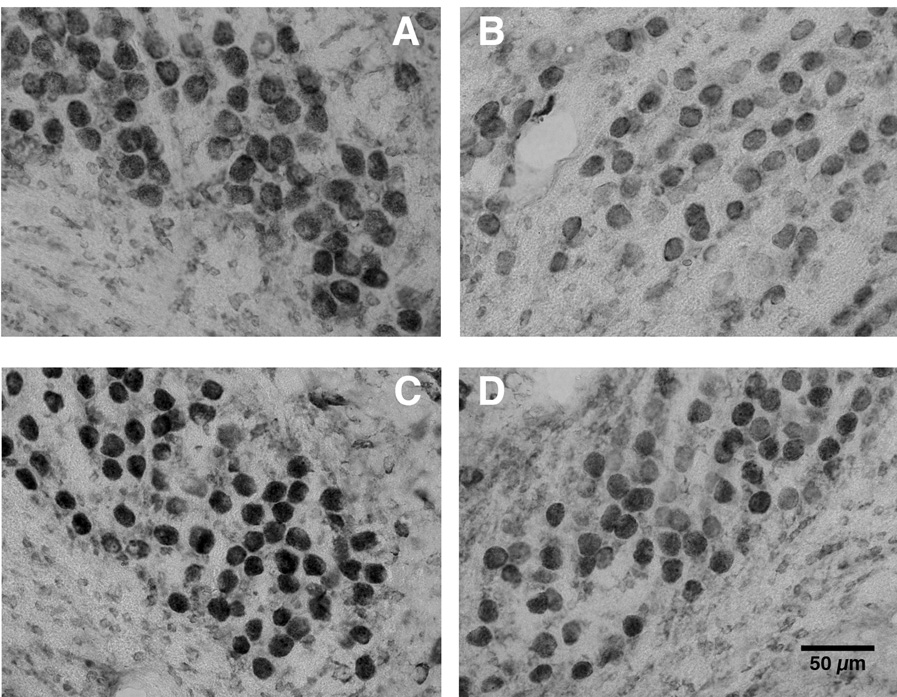
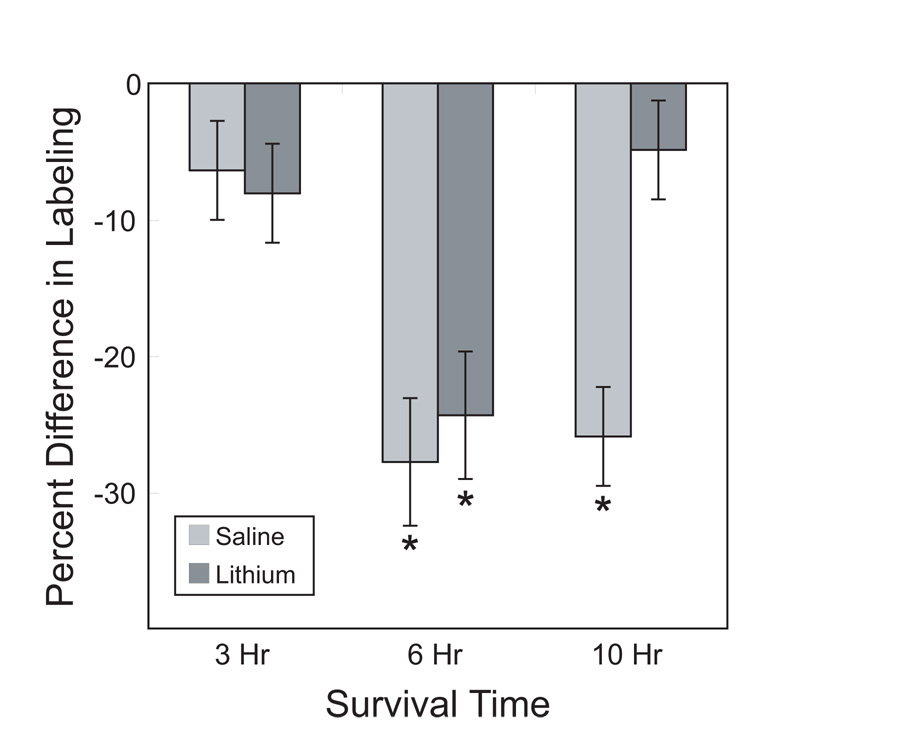
As expected, saline-treated control subjects showed lighter immunolabeling for the ribosomal epitope recognized by the Y10b antibody on the deafferented side of the brain. Although chronic lithium had a tendency to increase the overall antigenicity for Y10b, it did not prevent the initial deafferentation-induced reduction in immunolabeling (Figure 2). At later time points, however, the effects of lithium became apparent such that by 10 hours after cochlea removal, there was no longer a difference in immunolabeling between the intact and deafferented sides of the brain. Objective analyses of the gray scale values confirmed these visual impressions (Figure 3). A 3-way mixed ANOVA, using drug treatment and survival time as between-subjects variables and side of the brain as a within-subject variable, showed that there was a reliable difference between sides (F(1, 20) = 118.5, p < 0.001), a marginal overall effect of survival time (F(2,20) = 3.3, p = 0.058) and no main effect of drug treatment (F (1,20) = 2.6, p = 0.12). Importantly, however, there was a reliable side × time interaction (F (2,20) = 14.7, p < 0.001) and a reliable 3-way interaction of side × time × drug (F(2,20) = 5.9, p < 0.01). This means that the effect of cochlea removal depended on both survival time and drug treatment. Post-hoc Newman-Keuls pairwise comparisons revealed that there were reliable effects of cochlea removal only in the 6 hour survival time for both drug treatment groups and in the saline treated group at the 10 hour survival time. More powerful individual paired t-tests between the intact and deafferented sides of the brain showed reliable differences for each group (p < 0.05) except for the 10 hour survival time, lithium-treated group.
Figure 2.
Representative photomicrographs of intact and deafferented sides of saline- (top) and lithium-treated (bottom) subjects 6 hours after cochlea removal. Tissue sections were immunolabeled with the antibody Y10b, which recognizes a ribosomal epitope. A.) saline intact side. B.) saline deafferented side. C.) lithium intact side. D.) lithium deafferented side. There was greater labeling in the lithium-treated subjects compared to saline-treated subjects (Panel C compared to Panel A), but cochlea removal decreased labeling in both groups (Panel B and D compared to A and C, respectively).
Figure 3.
Percent change of average gray scale density measured over individual intact and deafferented NM neurons immunolabeled for Y10b either 3, 6, or 10 hours post cochlea removal (100*(mean deafferented-mean intact)/mean intact). Negative numbers indicate that the labeling was lighter on the deafferented side of the section. There was a robust difference in labeling densities between deafferented and intact sides of the section at 6 hours following cochlea removal in both saline- and lithium-treated subjects, but this difference was no longer apparent in lithium-treated subjects at 10 hours following cochlea removal. Asterisks indicate reliable (p < 0.05) differences between sides (Student Newman-Keuls following 3-way ANOVA). Error bars represent standard error of the mean.
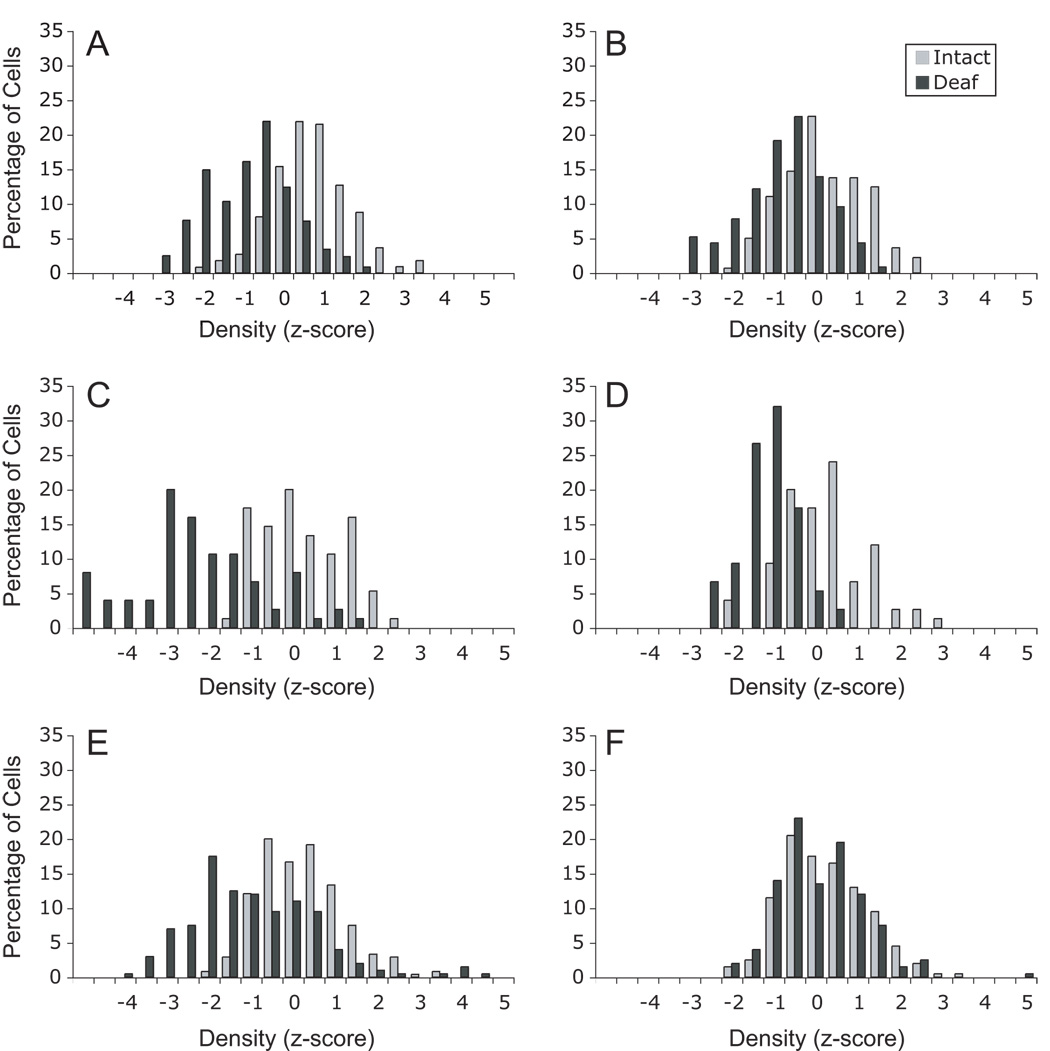
To compare the effects across the population of NM neurons, each density measurement score was converted to a z-score based on the mean and standard deviation of labeling density measured on the intact side of each individual section. These z-scores were then compiled across brains and are presented in Figure 4 as the percent of cells on each side falling within the 0.5 bins of z-score values. By definition, the distribution of z-score values for the intact side are relatively normal with a mean of zero and a standard deviation of 1. As can be seen in Figure 4, the distributions of labeling densities for cells on the deafferented sides of the sections shifted towards lighter labeling with the greatest shift observed at 6 hours following cochlea removal. Note that in the lithium treated groups, there was a shift in the distributions at the early time points but that there was no apparent difference in the distributions by 10 hours following cochlea removal.
Figure 4.
Frequency distributions for labeling densities of individual cells in subjects 3 hours (A, B), 6 hours (C, D), or 10 hours (E, F) following cochlea removal. Labeling densities are expressed as z-scores based on the mean and standard deviation of gray scale labeling densities measured over NM neurons on the intact sides of the tissue. Distributions on the left (A, C, E) are data from saline-treated control subjects and distributions on the right (B, D, F) are data from the lithium-treated subjects. Note that for saline-treated subjects, the distribution of labeling densities for deafferented neurons shifts towards lighter labeling and becomes broader. The distributions for labeling densities for the deafferented NM neurons in the lithium-treated subjects, however, shows an initial shift towards lighter labeling, but it does not appear to get broader. This shift in labeling density is no longer apparent by 10 hours following cochlea removal in the lithium-treated subjects.
In situ hybridization
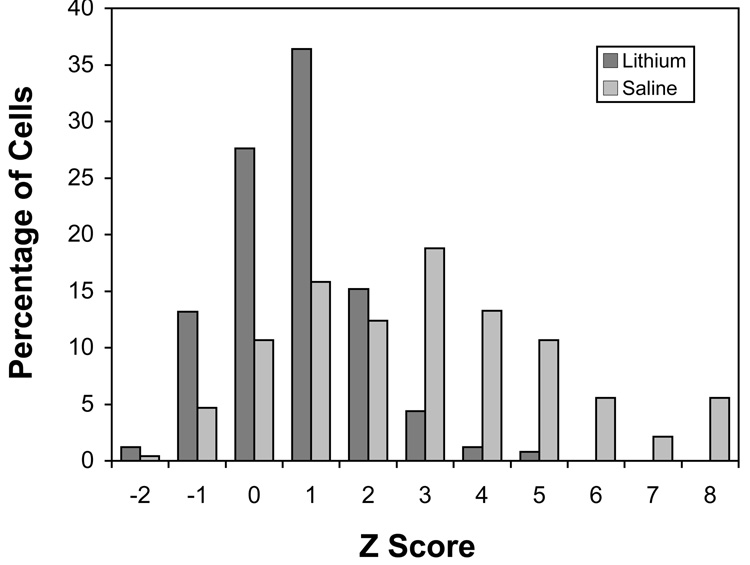
As reported previously (Wilkinson et al., 2002), there was an increase in labeling for Bcl-2 mRNA in deafferented neurons in the saline-treated subjects (Figure 5). This deafferentation-induced difference appeared to be blocked by chronic lithium treatment. Objective analyses of grain densities measured over individual NM neurons confirmed these visual impressions. A two-way mixed ANOVA on the grain density measurements, using side of the brain as a within-subject variable and drug treatment as a between-subjects variable, revealed a reliable effect of side (F (1, 6) = 15.5, p < 0.01), a marginal effect of drug treatment (F (1, 6) = 5.26, p < 0.062) and importantly, a side × drug treatment interaction (F (1, 6) = 9.9, p < 0.05). Post-hoc pairwise comparisons (Newman-Keuls, p < 0.05) revealed that the grain densities on the deafferented side of the saline-treated group were reliably higher than the grain densities of the intact side on the saline-treated group and higher than either side of the lithium-treated group. No other comparisons showed reliable differences. Since processing variables dramatically influence overall levels of labeling, the raw grain density scores for each brain were converted to z-scores based on the mean and standard deviation of the distribution of grain densities measured on the intact side of the tissue section. The pooled distributions of these z-scores for the deafferented sides of the saline- and lithium-treated groups are shown in Figure 6. As can be seen, the distribution for the saline-treated group is broader and many cells fall several standard deviations (z) away from the mean labeling density of cells on the intact side of the brain. Using the criterion of 4 standard deviations above the mean (see Wilkinson et al., 2002), 23.9% of NM neurons would be considered “heavily-labeled” in the saline-treated subjects. This same criterion would account for only 0.8% of deafferented neurons in the lithium-treated subjects.
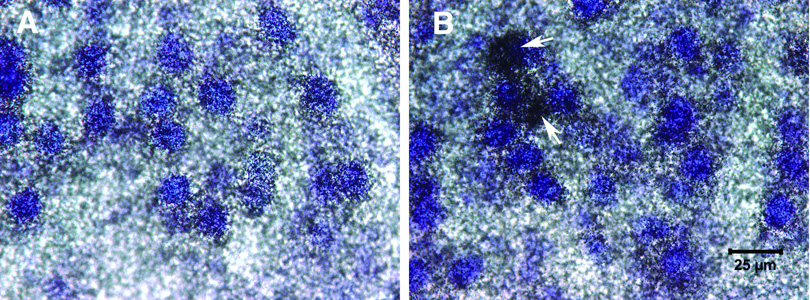
Figure 5.
Photomicrographs of intact (A) and deafferented (B) sides of the same tissue section processed for Bcl-2 in situ hybridization. Slides were emulsion-coated and counterstained with thionin. In saline-treated subjects, there was an upregulation of Bcl-2 mRNA on the deafferented side of the brain, with some cells (arrows) showing dramatic upregulation.
Figure 6.
Grain density distribution of deafferented NM neurons from saline- and lithium-treated subjects processed for Bcl-2 in situ hybridization 6 hours following cochlea removal. Grain density measurements were converted to z-scores based on the mean and standard deviation of grain densities measured over NM neurons on the intact sides of the tissue. There were higher silver grain densities on the deafferented side of the saline-treated subjects (p < 0.01), with many cells showing a robust upregulation of Bcl-2 mRNA. There was no reliable difference between intact and deafferented sides in the lithium-treated subjects.
DISCUSSION
Previous work (Bush and Hyson, 2006) showed that chronic lithium administration protected NM neurons from deafferentation-induced cell death, but did not prevent the deafferentation-induced reduction in soma size. The present sets of experiments examined different events that occur in the cell death cascade in deafferented NM neurons to determine which of these events are influenced by chronic lithium administration. This work focused on a subset of the known array of effects that occurs following cochlea removal (Rubel et al., 1990). Chronic lithium did not appear to block the rise in intracellular calcium levels that occurs within one hour following cochlea removal, nor did it appear to block the early changes in ribosomes (decreased Y10b immunolabeling) that are observed across the entire population of NM neurons in the first few hours following cochlea removal. At later times, however, the affects of lithium became apparent. Deafferented NM neurons in lithium-treated subjects appeared to recover their Y10b antigenicity. In addition, lithium appeared to prevent changes in gene expression (increased Bcl-2 mRNA) that are observed predominately in a subpopulation of cells 6–12 hours following cochlea removal.
Intracellular calcium
Zirpel and colleagues (Zirpel et al., 1995) have shown that intracellular calcium levels in NM neurons rise within an hour following cochlea removal. This rise can be observed over time in vitro and is not observed if the auditory nerve is electrically stimulated (Zirpel et al., 1996). The rapid change in intracellular calcium levels following cochlea removal suggests that calcium could be an early trigger in the cell death cascade, as observed in other systems (Brauchi et al., 2006; Doonan and Cotter, 2004). One difficulty with this simple hypothesis, however, is that calcium levels rise in all deafferented NM neurons, but only a subset (20–30%) of the neurons die. Thus, although it is possible that increases in calcium play a role in cell death following deafferentation, it does not appear that an increase in calcium commits the cell to die. The present studies replicated the work of Zirpel et al. (1995) in showing a rise in intracellular calcium in deafferented NM neurons in vitro. Although it is possible that lithium is having subtle effects on calcium levels that were not detected in these experiments, the main point is that there was still a rise in calcium in deafferented NM neurons even when the subject was pretreated with lithium. If the rise in calcium is important for initiating cell death, then lithium is not preventing this cell death “trigger”, and therefore must be affecting a later downstream consequence of this trigger.
Conclusions based on the present results must also be qualified to some extent by one technical limitation. It appears that only embryonic NM neurons reliably load with the Fura-2 AM calcium indicator dye. This required that lithium be administered in ovo for the calcium imaging studies, while all other experiments examining the effects of lithium in this system have been following chronic injections in post-hatch birds. Although the concentration of lithium used in ovo is known to have effects in other systems (Ikonomov et al., 2000), one cannot be certain that embryonic and post-hatch NM neurons respond identically to lithium treatment.
Y10b immunolabeling
One of the first changes in the cellular functions observed in NM neurons following cochlea removal is a decrease in protein synthesis (Steward and Rubel, 1985). This decrease in ribosomal activity appears to correspond with decreases in antigenicity to the antibody Y10b, which recognizes a ribosomal epitope (Garden et al., 1994). The present studies replicated previous reports showing a decrease in Y10b labeling within the first few hours following cochlea removal. Chronic administration of lithium did not affect this early difference between deafferented and intact NM neurons. Once again, it appears that deafferentation-induced changes that occur across the entire population of NM neurons are not affected by lithium pretreatment.
Garden et al. (1994) reported that two populations of neurons can be distinguished based on Y10b immunolabeling at 6–12 hours following cochlea removal; one population that is within the normal distribution of immunlabeling density of intact NM neurons, and one population that shows a substantial loss of antigenicity. It has been suggested that the population of neurons that show the substantial loss of antigenicity, the so-called “ghost cells”, are those that will eventually die. While our data did not show a clear bimodality in antigenicity, it was clear that the distribution of gray-scale densities was broader for NM neurons on the cochlea removal side of the brain in saline treated animals at both 6 and 10 hours following deafferentation (see Figure 4). Many of these cells were 2–5 standard deviations below the average labeling density observed in cells on the intact side of the section. In lithium-treated subjects, however, the labeling distribution at 6 hours following cochlea removal was shifted towards lighter labeling, but the distribution was not broader than that on the intact side of the section. There were very few cells that showed substantial decreases in antigenicity in the lithium-treated group. By 10 hours following cochlea removal, the distributions on the intact and deafferented sides of the section overlapped in the lithium-treated group. Together, this pattern of results suggests that lithium is having its greatest effect by preventing the dramatic changes in the subpopulation of NM neurons that will eventually die.
Bcl-2 mRNA
Approximately 20–30% of the deafferented NM neurons die within 2 days following cochlea removal. Indicators of which cells will die and which will survive, however, can be observed as early as 6–12 hours following deafferentation. At this time, a subpopulation of neurons appear to have a complete cessation of protein synthesis (Steward and Rubel, 1985) and substantial loss of Y10b antigenicity (Garden et al., 1994). In addition, changes in gene expression also appear to dissociate NM neurons into two populations 6–12 hours after cochlea removal. Wilkinson et al. (2002) reported that approximately 20–30% of the NM neurons show a dramatic upregulation of Bcl-2 mRNA expression. They suggested that signaling mechanisms in the dying subpopulation of neurons leads to an upregulation of this potentially neuroprotective molecule, but that all these cells go on to die anyway, partly because they are unable to translate the message into protein by this time. The present experiments replicated the upregulation of Bcl-2 mRNA in deafferented neurons in saline-treated subjects. Wilkinson et al. (2002) used the criterion that a “labeled” cell was one with a grain density that was greater than 4 standard deviations above the mean grain density measured over neurons on the intact side of the brain section. They reported that, on average, 26.4% of the deafferented NM neurons would be considered “labeled” by this criterion in tissue processed 6 hours following cochlea removal. The present data set did not visually appear as distinctly bimodal as that of Wilkinson et al. (2002), but using the same criterion, 23.9% of the deafferented cells would be considered “labeled” in the saline-treated subjects. Importantly, chronic administration of lithium prevented this upregulation in Bcl-2 mRNA expression. Only 0.8% of the deafferented NM neurons would be considered labeled in brains from lithium-treated subjects. The 0.8% of labeled cells may seem somewhat low since Bush and Hyson (2006) did report some cell death in the lithium-treated subjects. One possible explanation of this discrepancy is that the effects of lithium in the present experiment were evaluated on the same day as cochlea removal. In the Bush and Hyson (2006) report, lithium treatment was discontinued in most of the animals at the time of cochlea removal. Thus it is possible that cells showed a delayed response to deafferentation, resulting in some cell death over the 5 day survival period as lithium levels decreased over time. Indeed, Bush and Hyson (2006) reported only 5% cell death in a subset of animals that were treated with lithium throughout the survival period. The correspondence between the effects of lithium on cell death (Bush and Hyson, 2006) and Bcl-2 expression supports the hypothesis that upregulation of Bcl-2 mRNA is an indicator of cells that are likely to die.
Synthesis
The present results blend well with previous work showing that chronic lithium administration protects NM neurons from deafferentation-induced cell death, but does not prevent the deafferentation-induced reduction in soma size. The rapid deafferentation-induced changes that occur in all NM neurons, including increased calcium levels and changes in Y10b antigenicity, were still observed in lithium-treated subjects. On the other hand, the substantial loss of Y10b antigenicity at 6–12 hours following cochlea removal, and the upregulation of Bcl-2 mRNA, a change that is observed when cells begin to divide into surviving and dying populations, were both prevented by chronic lithium administration. Chronic lithium did not prevent the presumed initial trigger (influx of calcium) that occurs following cochlea removal. Perhaps the changes in gene expression produced by chronic lithium administration results in higher levels of neuroprotective molecules (e.g., Bcl-2) in the NM neurons at the time of cochlea removal. This might allow more of the neurons to survive challenges, such as a rise in intracellular calcium levels. Changes in the expression of proteins that are known to influence apoptosis may also account for the neuroprotective effects of lithium in other systems (Chen et al., 1999; Rowe and Chuang, 2004). Lithium can result in changes in the activity of various transcription factors, thereby providing a wide range of possible mechanisms for neuroprotection (Bush and Hyson, 2008; Grimes and Jope, 2001). It has been suggested that this neuroprotective influence may be part of the mechanism underlying the therapeutic effects of lithium on bipolar disorder (Chuang et al., 2002). Such changes in gene expression would take time to be effective and this may explain why lithium is therapeutic only following chronic administration.
Acknowledgements
This work was supported by PHS grant DC000858.
Abbreviations
- ABC
avidin biotin complex
- ACSF
artificial cerebral spinal fluid
- ANOVA
analysis of variance
- Bcl-2
b cell leukemia/lymphoma 2
- BSA
bovine serum albumin
- DAB
diaminobenzidine
- DEPC
diethyl pyrocarbonate
- DMSO
dimethyl sulfoxide
- DTT
dithiothrietol
- IHC
immunohistochemistry
- mGluR
metabotropic glutamate receptor
- NA
nucleus angularis
- NHS
normal horse serum
- NL
nucleus laminaris
- NM
nucleus magnocellularis
- PBS
phosphate buffered saline
- SSC
saline-sodium citrate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Born DE, Rubel EW. Afferent influences on brainstem auditory nuclei of the chicken, neuron number and size following cochlea removal. J Comp Neurol. 1985;231:435–445. doi: 10.1002/cne.902310403. [DOI] [PubMed] [Google Scholar]
- Born DE, Durham D, Rubel EW. Afferent influences on brainstem auditory nuclei of the chick: nucleus magnocellularis neuronal activity following cochlea removal. Brain Res. 1991;557:37–47. doi: 10.1016/0006-8993(91)90113-a. [DOI] [PubMed] [Google Scholar]
- Brauchi S, Cea C, Farias J, Bacigualpo J, Reyes J. Apoptosis produced by prolonged exposure to odorants in cultured cells from rat olfactory epithelium. Brain Res. 2006;1101:114–122. doi: 10.1016/j.brainres.2006.05.072. [DOI] [PubMed] [Google Scholar]
- Bush AL, Hyson RL. Lithium increases Bcl-2 expression in chick cochlear nucleus and protects against deafferentation-induced cell death. Neuroscience. 2006;138:1341–1349. doi: 10.1016/j.neuroscience.2005.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush AL, Hyson RL. Effects of lithium and deafferentation on expression of glycogen synthase kinase-3beta, NFkappaB, beta-catenin and pCreb in the chick cochlear nucleus. Brain Res. 2008;1203:18–25. doi: 10.1016/j.brainres.2008.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catsicas M, Pequignot Y, Clarke PG. Rapid onset of neuronal death induced by blockade of either axoplasmic transport or action potentials in afferent fibers during brain development. J Neurosci. 1992;12:4642–4650. doi: 10.1523/JNEUROSCI.12-12-04642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang D, Chen R, Chalecka-Franaszek E, Ren M, Hashimoto R, Senatorov V, Kanai H, Hough C, Hiroi T, Leeds P. Neuroprotective effects of lithium in cultured cells and animal models of diseases. Bipolar Disorders. 2002;4:129–136. doi: 10.1034/j.1399-5618.2002.01179.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Zeng WZ, Yuan PX, Huang LD, Jiang YM, Zhao ZH, Manji HK. The mood stabilizing agents lithium and valproate robustly increases the levels of the neuroprotective protein Bcl-2 in the CNS. J Neurochem. 1999;72:879–882. doi: 10.1046/j.1471-4159.1999.720879.x. [DOI] [PubMed] [Google Scholar]
- Doonan F, Cotter TG. Apoptosis: a potential therapeutic target for retinal degenerations. Curr Neurovasc Res. 2004;1:41–53. doi: 10.2174/1567202043480215. [DOI] [PubMed] [Google Scholar]
- Garden GA, Canady KS, Lurie DI, Bothwell M, Rubel EW. A biphasic change in ribosomal conformation during transneuronal degeneration is altered by inhibition of mitochondrial, but not cytoplasmic protein synthesis. J Neurosci. 1994;14:1994–2008. doi: 10.1523/JNEUROSCI.14-04-01994.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Hyson RL, Rubel EW. Activity-dependent regulation of a ribososomal RNA epitope in the chick cochlear nucleus. Brain Res. 1995;672:196–204. doi: 10.1016/0006-8993(94)01390-4. [DOI] [PubMed] [Google Scholar]
- Hyson RL. Transneuronal regulation of ribosomes after blockade of ionotropic excitatory amino acid receptors. Brain Res. 1997;749:61–70. doi: 10.1016/s0006-8993(96)01160-2. [DOI] [PubMed] [Google Scholar]
- Hyson RL. Activation of metabotropic glutamate receptors is necessary for transneuronal regulation of ribosomes in chick auditory neurons. Brain Res. 1998;809:214–220. doi: 10.1016/s0006-8993(98)00873-7. [DOI] [PubMed] [Google Scholar]
- Ikonomov O, Petrov T, Soden K, Shisheva A, Manji HK. Lithium treatment in ovo: effects on embryonic heart rate, natural death of ciliary ganglion neurons, and brain expression of a highly conserved chicken homolog of human MTG8/ETO. Dev Brain Res. 2000;123:13–24. doi: 10.1016/s0165-3806(00)00074-2. [DOI] [PubMed] [Google Scholar]
- Leegwater-Kim J, Cha JJ. The paradigm of Huntington’s disease: Therapeutic opportunities in neurodegeneration. J Am Soc Exper NeuroTher. 2004;1:128–138. doi: 10.1602/neurorx.1.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji HK, Chen G. PKC and MAP kinases and the bcl-2 family of proteins as long term targets for mood stabilizers. Mol Psychiatry. 2002;7:546–556. doi: 10.1038/sj.mp.4001018. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Al-Abdulla NA, Brambrink AM, Kirsch JR, Sieber FE, Portera-Cailliau C. Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: A perspective on the contributions of apoptosis and necrosis. Brain Res Rev. 1998;46:281–309. doi: 10.1016/s0361-9230(98)00024-0. [DOI] [PubMed] [Google Scholar]
- Meisami E, Safari L. A quantitative study of the effects of early unilateral olfactory deprivation on the number and distribution of mitral and tufted cells and of glomeruli in the rat olfactory bulb. Brain Res. 1981;221:81–107. doi: 10.1016/0006-8993(81)91065-9. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Chuang DM. Neuroprotective effects of chronic lithium on focal cerebral ischemia in rats. Neuroreport. 1998;9:2081–2084. doi: 10.1097/00001756-199806220-00031. [DOI] [PubMed] [Google Scholar]
- Okamato Y, Kagaya A, Motohashi N, Yamawaki S. Inhibitory effects of lithium ion on intracellular calcium mobilization in the rat hippocampal slices. Neurochem Intl. 1994;26:233–238. doi: 10.1016/0197-0186(94)00130-m. [DOI] [PubMed] [Google Scholar]
- Parks TN, Rubel EW. Organization and development of the brainstem auditory nuclei of the chicken: primary afferent projections. J Comp Neurol. 1978;180:439–448. doi: 10.1002/cne.901800303. [DOI] [PubMed] [Google Scholar]
- Pope K, Wilson DA. Olfactory system modulation of hippocampal cell death. Neurosci Lett. 2007;422:13–17. doi: 10.1016/j.neulet.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe MK, Chuang DM. Lithium neuroprotection: molecular mechanisms and clinical implications. Expert Rev Mol Med. 2004;21:1–18. doi: 10.1017/S1462399404008385. [DOI] [PubMed] [Google Scholar]
- Rubel EW. Ontogeny of structure and function in the vertebrate auditory system. In: Jacobson M, editor. Handbook of Sensory Physiology, Vol. IX: Development of Sensory Systems. New York: Springer-Verlag; 1978. pp. 135–237. [Google Scholar]
- Rubel EW, Hyson RL, Durham D. Afferent regulation of neurons in the brain stem auditory system. J Neurobiol. 1990;21:169–196. doi: 10.1002/neu.480210112. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Falk PM, Canady KS, Steward O. A cellular mechanism underlying activity-dependent transneuronal degeneration: Rapid but reversible destruction of neuronal ribosomes. Brain Dysfunct. 1991;4:55–74. [Google Scholar]
- Steward O, Rubel EW. Afferent influences on brain stem auditory nuclei of the chicken: presynaptic action potentials regulate protein synthesis in nucleus magnocellularis neurons. J Neurosci. 1985;231:385–395. doi: 10.1523/JNEUROSCI.08-03-00901.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Qin ZH, Senatorov VV, Wei W, Wang Y, Qian Y, Chuang DM. Lithium suppresses excitotoxicity-induced striatal lesions in a rat model of Huntington’s disease. Neuroscience. 2001;106:603–612. doi: 10.1016/s0306-4522(01)00311-6. [DOI] [PubMed] [Google Scholar]
- Wilkinson BL, Sadler KA, Hyson RL. Rapid deafferentation-induced upregulation of Bcl-2 mRNA in the chick cochlear nucleus. Mol Brian Res. 2002;99:67–74. doi: 10.1016/s0169-328x(02)00113-4. [DOI] [PubMed] [Google Scholar]
- Wilkinson BL, Elam JS, Fadool DA, Hyson RL. Afferent regulation of cytochrome c and active caspase-9 in the avian cochlear nucleus. Neuroscience. 2003;120:1071–1079. doi: 10.1016/S0306-4522(03)00387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirpel L, Lachicha EA, Lippe WR. Deafferentation increases the intracellular calcium of cochlear nucleus neurons in the embryonic chick. J Neurophysiol. 1995;74:1355–1357. doi: 10.1152/jn.1995.74.3.1355. [DOI] [PubMed] [Google Scholar]
- Zirpel L, Rubel EW. Eighth nerve activity regulates intracellular calcium concentration of avian cochlear nucleus neurons via a metabotropic glutamate receptor. J Neurophysiol. 1996;76:4127–4137. doi: 10.1152/jn.1996.76.6.4127. [DOI] [PubMed] [Google Scholar]