Abstract
Objectives
To image angiogenesis produced by endomyocardial injection of phVEGF165 in a swine model of hibernating myocardium using [123I]Gluco-RGD targeting the αvβ3 integrins.
Background
A non-invasive test to monitor the efficacy of therapy inducing angiogenesis is needed. The interaction between extracellular matrix and endothelial cells in sprouting capillaries is effected primarily by αvβ3 integrins that bind through RGD motifs.
Methods
At 21±4 days after LCx ameroid constrictor (AM) placement, 8 swine received endomyocardial injection of 1.2 mg phVEGF165 divided into 6 sites and 6 swine received saline (S) using non-fluoroscopic 3D endocardial mapping system (Noga) guided delivery. After 20±6 days 13 animals were injected with 6.4±1.7 mCi [123I]Gluco-RGD, one VEGF injected animal with I-123 labeled peptide control and all animals with 2.5±0.4 mCi of Tl-201 and underwent SPECT imaging. Blood flow and echocardiographic measurements were made at both time points and tissue analyzed for fibrosis and capillary density by lectin staining.
Results
Hibernating myocardium in the AM territory at time of injections was documented by reduced wall thickening compared to remote. Ratio of MBF in LCx/LAD territories increased by 15±11% in the VEGF animals and fell 13±12% in S injected (p<0.01). There was a small increase in wall thickening (WT) in AM territory following VEGF (8 ± 17%) while in S injected WT fell by 23 ± 31% (p=0.01vs.VEGF). Lectin staining as % positive tissue staining for ameroid territory was higher in VEGF injected compared to S injected (2.5±0.1.5 vs. 0.87±0.52%, p=0.01). Focal uptake of [123I]Gluco-RGD corresponding to Tl-201 defects was seen in VEGF injected but not in S injected animals. [123I]Gluco-RGD uptake in the ameroid territory as % ID correlated with lectin staining (R2=0.80, p=0.002).
Conclusions
These data suggest that SPECT imaging of radiolabeled RGD peptides may be useful non-invasive method to monitor therapy that induces angiogenesis in the heart.
INTRODUCTION
Approaches to produce therapeutic angiogenesis in patients with chronic ischemic myocardium have included administration of recombinant human vascular endothelial growth factor protein into the coronary arteries (1), administration of naked plasmid DNA encoding for phVEGF2 injected into the myocardium either with a thoracotomy or percutaneously using a catheter-based myocardial injection and mapping system (Biosense-Webster) (2-5), and intracoronary injection of genes encoding for VEGF. (6-8) The results of clinical trials have been mixed. Initial experience with cell-based therapy (CD34+ bone marrow cells derived cells) have shown some efficacy in early trials. In the absence of strong evidence to support transformation of these progenitor cells into myocytes, a mechanism proposed for observed benefits is release by these cells of cytokines including VEGF. (9,10) There is a need to develop a non-invasive test to monitor the efficacy of therapy that directly or indirectly induces angiogenesis.
The interaction between the extracellular matrix (ECM) and endothelial cells is effected by a family of cell adhesion receptors (integrins) that bind to cells in sprouting capillaries (11,12) The αvβ3 integrin receptor has been identified as principle integrin expressed in neovascular growth.(13-16) Binding of cells to the extracellular matrix occurs through RGD motifs on ligands. This observation forms the basis for development of radiolabeled ligands with RGD motifs. (17) Haubner and colleagues improved pharmacokinetics of αvβ3 selective first generation tracers by glycosylation of a modified derivative using sugar amino acid to achieve decreased lipophilicity and decreased hepatic uptake. (18-20) It was the purpose of this study to test the hypothesis that angiogenesis produced by endomyocardial injection of phVEGF165 in a swine model of hibernating myocardium can be imaged using [123I]Gluco-RGD targeting the αvβ3 receptors on smooth muscle cells that bind components of the ECM during capillary sprouting.
METHODS
For all experiments, conditioned castrated male juvenile swine weighing 20-30 kg were used. All experiments were performed within the National Institutes of Health guidelines for the care and use of laboratory animals and with the approval of the Rhode Island Hospital Animal Care Committee. Upon arrival at the Animal Care Facility all study animals were fed a standard pig chow.
Surgery
The left thoracotomy approach was used for placement of single left circumflex coronary (LCx) ameroid. The left atrial appendage was lifted and a one-centimeter segment of the proximal circumflex artery was dissected free from the surrounding myocardial tissue. The arterial segment was carefully lifted using silk suture at the proximal and distal ends and the carefully sized ameroid (Research Instruments SW 1264 Stanley Way, Escondido, CA 92027) placed snugly around the artery but not constricting it. The ameroid size for most of the experiments was 2.75 mm (range 2.50 to 3.00) with a gap size of 0.8 mm. The left atrial appendage was then released and the pericardium loosely closed. A chest tube was placed in the chest cavity, exiting through the skin. After re-expansion of the left lung and re-establishment of negative chest pressure anesthetics were discontinued and the animal allowed to awaken. Animals received daily oral antibiotics for five days.
Noga mapping and intramyocardial injection
After 2-3 weeks animals were anesthetized and a midline neck incision made exposing the right carotid artery and internal jugular vein. A 9F sheath was placed in the artery and a catheter placed into the vein. Coronary angiography was performed through the carotid artery followed by transthoracic echocardiography. An Agilent 5500 Sonos platform (Philips, Andover, MA) with a phased array 2-4 MHz probe was used. Animals were positioned supine and imaged in 4 standard imaging planes: parasternal long axis, parasternal short axis, apical four-chamber and apical two-chamber views.
Endocardial R wave mapping was then performed using a non-fluoroscopic 3D endocardial mapping system (Noga) (Johnson & Johnson, Biosense Webster, Irwindale, CA). A reference was centered over the heart using the graphics workstation. The mapping catheter was passed through the carotid sheath and advanced into the LV cavity. The position of the catheter tip was located in 3 dimensional space. The stability of the catheter-to-wall contact was evaluated at every site. With Noga guided placement, 6 injections (1 ml) of VEGF were made in the two selected areas (3 per area) in 8 animals and saline in 6 animals (controls). A catheter was placed in the left ventricle and one injection of colored microspheres was given to document blood flow. The sheath was removed and the carotid repaired.
Final study
Two weeks post intervention the animal was returned to the Cardiovascular Research Laboratory for the final study. Coronary angiography was first performed followed by electromechanical mapping of the heart to obtain 60-90 points. Echocardiography was performed followed by injection of thallium 201 (2-3 mCi) and αvβ3-selective [123I]Gluco-RGD in 13 animals and 123I-labeled negative control peptide in one. One to two hours later (based on blood pool clearance of [123I]Gluco-RGD) two sequential tomographic scans were performed without moving the animal on an ADAC Arc 3000 camera interfaced with a Nuclear Mac (Nuclear Cardiology Systems, Boulder, CO) over 180° orbit at 30 sec per stop for 32 stops using the 70 keV photopeak and 15% window for Tl-201 and 160 keV photopeak with 15% window for I-123.
At completion of all the imaging procedures, anesthesia was deepened and the animal sacrificed with a bolus of KCl and the heart removed. The heart was “breadloaf sliced” and imaged ex-vivo on the detector for I-123. Tissue samples were removed from the heart for weighing, staining, fibrosis measurement and blood flow analysis.
Tracer preparation, injection, and imaging
The labeling precursor used was αvβ3-selective glycopeptide cyclo(-Arg-Gly-Asp-D-Tyr-Lys(SAA)) (Gluco-RGD) where SAA stands for sugar amino acid, which is in this case is 3-acetamido-2,6-anhydro-3-deoxy-β-D-glycero-D-gulo-heptanoic acid. (18) Gluco-RGD (200-300 μg; MW 850.9 g/mol) was dissolved in phosphate buffered saline (PBS) and transferred to an Eppendorf cap coated with Iodogen. Approximately 25 mCi of carrier added 123I-iodine (>2 × 108 GBq/mol) (MDS Nordion, Ottawa, Ontario Canada) was added. After 30 min at room temperature the solvent was removed from the solid oxidizing agent. The labeled compound was separated from precursor by HPLC. The HPLC conditions were 0-40% acetonitrile in water with 0.1% trifluoro acetic acid with flow of 1 ml/min, UV detection at 220 nm and 20 min gradient, using a Nucleosil C-18 5 μm, 125 × 4.6 mm column (Capital HPLC Ltd). The precursor has a retention time of 13 min and [123I]Gluco-RGD of 16 min. The two peaks were well separated. The specific activity was determined by the specific activity of the radioisotope. The solvent was removed prior to injection. The radiochemical yield was about 50%. Activities of 25 mCi resulted in approximately 13 mCi [I-123]Gluco-RGD. The negative control peptide, cyclo(-Arg-Ala-Asp-D-Tyr-Val-) was labelled using the same protocol as described for Gluco-RGD.
Nuclear data processing
The raw tomographic nuclear data were processed using standard software. The thallium and [123I]Gluco-RGD scans were co-registered in polar map display format to display the relative distributions of the two tracers in each heart. The % ID was calculated from the decay corrected injected activity, I-123 counts in the ameroid territory, tissue weight, and camera efficiency.
Echocardiographic data processing
Analysis of LV global and segmental function was performed by experts blinded to the results of other imaging modalities and to pathology. Regional wall thickening was measured off-line using digital calipers for four equal segments (septal, anterior, lateral, and posterior) from the mid short axis view. Wall thickening for each segment was calculated as percentage change in wall thickness from end diastole (ED) to end systole (ES). Global LVEF was calculated using a Simpson’s rule algorithm.
R Wave Voltage Map Processing
Post processing of the endocardial map was done using the Biosense work station. The final map for each animal was selected and displayed. The apex location was checked for accuracy and the map was played back in cine (motion) mode. Poor points were deleted using established criteria.
Tissue processing and image analysis
The heart was prepared for histology and microsphere measurements in the following manner. Each one cm breadloaf slice was divided into anterior, septal, posterior, and lateral regions. The slices were traced and the tracings annotated for localization of sample sources. Samples were taken from each region for microsphere counting. Weights from the lateral wall samples were used for the injected dose calculations. Two four-micron sections from each of the four anatomical regions (anterior, septal, posterior, and lateral) from each one cm slice were taken for fibrosis measurements. Each four micron section was mounted and stained with trichrome. Each stained section was scanned into a computer and imported into Photoshop. Color channels for each image were split. Image math function in NIH Image was used to quantify the percentage fibrosis and irreversibly damaged myocytes for each region. (21)
To identify capillary sprouting a five micron transmural tissue block was taken from the injection region for lectin staining. DB lectin is a glycoprotein capable of recognizing and binding to carbohydrate moieties on endothelial cell membranes of sprouting capillaries. It was biotinylated to link with streptavidin-HRP which links to DAB (brown chromagen). Sections were stained with DAB and counter-stained with hematoxylin. Ten fields were randomly captured at 20x from 3 different slides. Using Image Pro Plus (Media Cybernetics, Inc, Silver Springs, MD) the brown DAB color (lectin) was defined as was the entire field of tissue (blue). The combined areas for all ten fields positive for lectin staining were expressed as % lectin positive staining over total tissue area. To identify vitronectin receptors in the injection site, αvβ3 staining was performed on sections from one experiment injected with VEGF showing uptake of gluco-RGD and from one experiment injected with saline. For fluorescent staining the αvβ3 -FITC antibody was applied (Vitronectin receptor, clone LM609, Chemicon USA). The tissue was fixed in acetone and blocked with 0.3% of H2O2 in methanol. The primary antibody was applied in a dilution of 1:100 (Vitronectin receptor, clone LM609, Chemicon), in an overnight incubation. Staining was validated with positive control in kidney tissue and negative control with FITC-labeled IgG in porcine myocardium.
Blood flow measurements
Colored microspheres (15 ± 0.43 μm) used for blood flow determination were cross-linked polystyrene-divinylbenzene microspheres in 8 colors: red, blue, orange, green, yellow, coral red, violet, and black (E-Z Trac, Los Angeles, CA). These colored microspheres are chemically stable and exhibit no dye leaching, even in tissue exposed to strong acid and base solutions. Regional myocardial blood flow values were measured by using the methods described by Hale et al (22) and as reported previously for our laboratory (23).
Statistical analysis
Numeric data for the two groups (VEGF and saline injected) were compared using a 2-sample t test with equal variance. All results are expressed as ± SD. The myocardial uptake of [123I]Gluco-RGD for each heart was plotted against the % lectin staining using a simple linear regression.
RESULTS
Ameroid constrictors
Ameroid constrictors were placed on circumflex arteries in 14 swine. The mean time between ameroid placement and VEGF injection was 21 ± 6 days and the mean time between VEGF injection and the final imaging and sacrifice was 20 ± 6 days. The initial weight was 25 ± 5 kg and the final weight was 49 ±9 kg. For the 8 VEGF injected animals 6/8 ameroids were patent at the time of injection and 5/8 were patent at the final study with collaterals present in 2/3 of the closed vessels. For the 6 saline injected animals 4/6 ameroids were patent at time of injection and 3/6 were patent at the final study with collaterals seen in one. Hibernation in the circumflex territory was supported by comparing R-wave amplitude, myocardial blood flow, and wall thickening in the circumflex territory to remote myocardium as shown in figure 1. While wall thickening was reduced in the ameroid territory, R-wave amplitude was within “viable” range, and myocardial blood flow was mildly reduced compared to remote myocardium.
Figure 1. Documentation of hibernating myocardium.
All swine underwent unipolar voltage mapping (left panel), myocardial blood flow measurements with microspheres (middle panel) and transthoracic echocardiography (right panel) prior to myocardial injection of either phVEGF125 or saline. The unipolar voltage scores from the Noga map in the ameroid territory were reduced compared to remote myocardium but in the viable range. MBF in cc/g/min was mildly reduced in the ameroid territory compared to remote myocardium. The % wall thickening in the ameroid territory was reduced compared to the remote myocardium. These findings of abnormally functioning but viable myocardium fulfill the criteria for hibernation.
Blood flow and LV function
Transmural myocardial blood flow in the ameroid territory increased by 0.63 ± 0.35 cc/gm/min between injection and final study in animals injected with VEGF, and fell by 0.14 ± 0.34 cc/gm/min in animals injected with saline (p = 0.004). The ratios of blood flow in the lateral wall (ameroid territory) over the remote myocardium at baseline were not different between VEGF and saline injected animals (0.96 ± 0.15 vs. 1.09 ± 0.02, p = 0.12). Ratios increased after three weeks in the VEGF injected animals (15±11%) and decreased in the saline injected animals (-13±12%) (p = 0.006) (figure 2).
Figure 2. Change in blood flow ratios.
MBF is expressed as ratio of MBF in the ameroid territory to remote territory. The change (± SD) from immediately before intramyocardial injection of either phVEGF165 or saline to 3 weeks after injection for both treatment and control experiments are expressed as %. The blood flow ratio increased in the VEGF injected animals (pink bar) and decreased in the saline injected animals (blue bar).
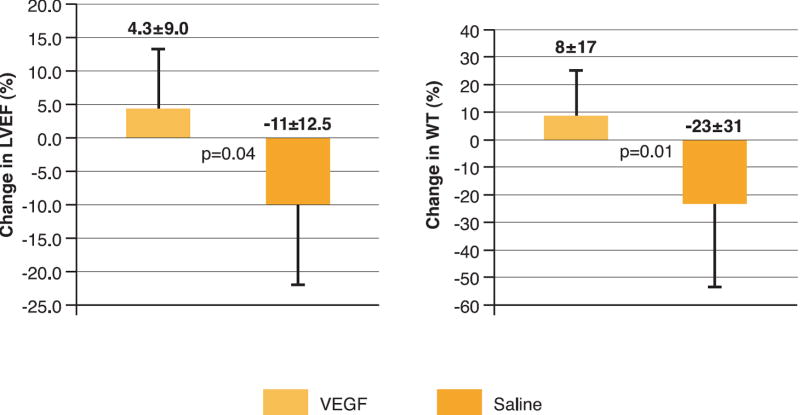
Baseline global and regional LV function was not different between the two groups: LVEF was 59 ± 11% for VEGF group, and 51 ± 15% for saline group (p = 0.28); percentage anterior wall thickening was 40 ±30 % for VEGF group and 55 ± 31% for the saline group (p = 0.38), and percentage lateral wall thickening was 35 ± 18% for the VEGF group and 45 ± 25% for the saline group (p = 0.55). LV function improved slightly between the two studies in the VEGF injected animals but continued to deteriorate in saline injected animals. The percentage change in LVEF in the VEGF injected was +4.3 ± 9.0 % and for the saline injected was -11.4 ± 12.5 %. While this difference was statistically significant (p = 0.04) there was a wide range in values. In the VEGF injected group 5 experiments showed no change in LVEF and 3 increased while in the saline injected group only one showed no change and 5 fell. There was a small increase in wall thickening in the ameroid territory following VEGF (8 ±17%) while in the saline injected animals wall thickening fell by 23 ± 31% (p=0.01vs.VEGF). (figure 3).
Figure 3. Change in LV function.
Global and regional LV function was measured using echocardiography immediately before and three weeks after intramyocardial injection with either phVEGF125 (pink bars) or saline (blue bars). Mean value for LVEF increased slightly but with a large SD (error bar) while mean value for LVEF fell in the saline treated animals but also with a large SD. Wall thickening in the ameroid territory increased slightly in the VEGF treated animals and fell in the saline treated animals.
Fibrosis and angiogenesis
Fibrosis in the ameroid territory (as % of the transmural sections) was higher in the saline injected animals than in the VEGF injected animals (6.2 ± 1.9% vs. 3.9 ± 1.6%) (p = 0.007). Histopathological evidence for neovascularization in the VEGF injected animals compared to the saline injected was documented by lectin positive staining (2.50 ± 1.5% vs. 0.87 ± 0.52%, p = 0.01) (figure 4). VEGF injected tissue stained for vitronectin receptors from several experiments showed positive fluorescence compared to saline injected tissue. (figure 4) Supporting the pathology were the Noga map findings that showed improvement in the R-wave voltage in the VEGF injected territory compared to reduction in the saline injected territory (figure 5).
Figure 4. Immunohistology documenting angiogensis.
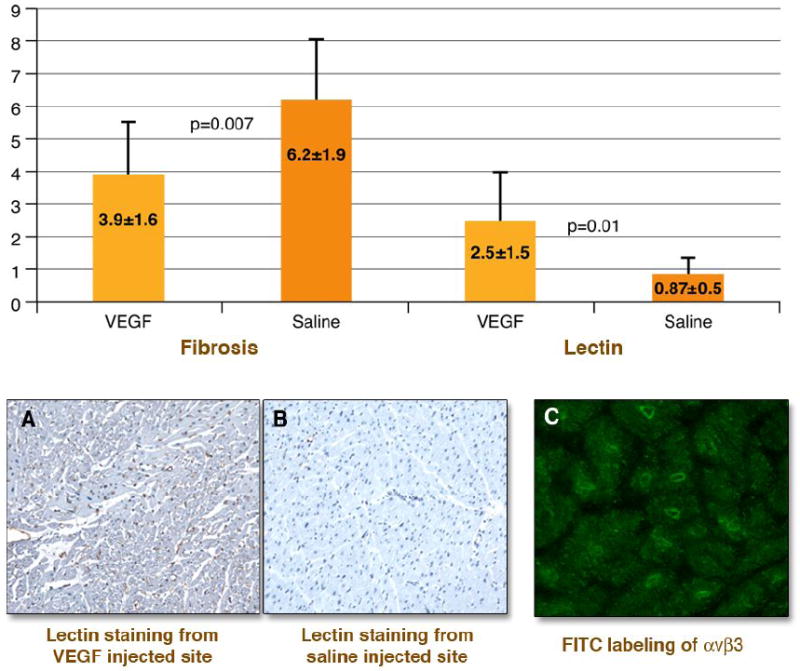
Values from quantitative immunohistology in the ameroid territory for the VEGF injected animals (pink bars) and saline injected animals (blue bars) are shown in the upper panel. The VEGF injected experiments showed less fibrosis and greater capillary sprouting by lectin staining than the saline injected control experiments. The bottom images show representative examples of tissue staining. Panel A shows a myocardial section stained for lectin from the injection territory from one VEGF experiment and panel B shows one saline injected animal. Brown staining of sprouting capillaries is seen in A and is absent in B. Panel C shows green fluorescence identifying integrin expression in capillary sprouts from the injection site of one VEGF injected animal that showed in-vivo uptake of [123I]Gluco-RGD.
Figure 5. Noga maps.

Images shown on the left are unipolar voltage maps at the time of injection with the injection sites marked for one saline injected animal and one VEGF injected animal. The two Noga maps on the right were acquired during the final study on the same two animals. The color bar in the right upper corner of each image corresponds to UPV values from 6 mV (orange) to 15 mV (pink). The saline injected region shows a fall in UPV score while the VEGF injected region shows an increase in UPV score.
Scan results
Blood pool clearance curve of the radiolabeled peptide showed blood levels below 20% of peak by 2 hr after injection. All animals showed mild resting thallium defects in the ameroid territory. The VEGF injected animals showed focal uptake of [123I]Gluco-RGD in the same territory while none of the saline injected animals nor the radiolabled control peptide showed focal uptake of radiotracer in the heart (figure 6). There was no difference in thallium uptake as percentage peak counts in the ameroid territory between VEGF injected and saline injected myocardium (0.78±0.07 vs. 0.70±0.09, p=0.12). The ratio of I-123 counts in the ameroid territory to remote myocardium were 1.71±0.11 for the VEGF injected animals vs. 1.04±0.13 for the saline injected animals (p<0.001). The counts per gram in the injection site were higher for the VEGF injected animals than for the saline injected animals (180±153 vs. 51±14) but the difference was not significant while the %ID also higher in VEGF injected animals than in the saline injected animals (0.26±0.09 vs. 0.15±0.07 × 103) was borderline significant (p = 0.04). The difference in counts per gram between the two groups was not significant and the difference in %ID was borderline significant (p=0.04). When values for the %ID (per gram tissue) for the 11 experiments in which all data for these calculations were available were plotted against quantitative lectin staining in the corresponding myocardial segments there was a significant correlation (R2=0.80, p=0.002) (figure 7). There was however overlap between the two groups. One of the saline injected animals showed higher myocardial background uptake of [123I]Gluco-RGD and one of the VEGF injected animals had a low %ID corresponding to lower lectin staining.
Figure 6. SPECT scans.

On the left are shown reconstructed short axis (SA), vertical (VLA), and horizontal long axis (HLA) SPECT slices from the thallium scans (top rows) and I-123 scans (bottom rows) from one representative animal treated with VEGF and one animal treated with saline. The VEGF injected animal shows a mild anterolateral thallium defect and focal uptake of [123I]Gluco-RGD into the anterolateral wall. The co-localization of the thallium defect and focal hotspot is better displayed on the polar maps. The saline injected animal shows a mild to moderate anterolateral and apical defect without focal uptake of of [123I]Gluco-RGD in the heart.
Figure 7. [123I]Gluco-RGD uptake vs. angiogenesis.

Graph shows %ID per gram of tissue for [123I]Gluco-RGD plotted vs. lectin staining as % area for VEGF injected animals (red triagles) and saline injected (blue squares). Although the relationship is significant there is not a perfect separation between the two groups. One saline injected animal showed no focal hotspot in the myocardium but showed a higher level of background activity for I-123. One of the VEGF injected experiments had low uptake of [123I]Gluco-RGD which corresponded to lower levels of angiogenesis by lectin staining.
DISCUSSION
The present study is the first to image angiogenesis using a radiolabeled cyclo-RGD peptide targeting αvβ3 receptors in VEGF injected hibernating myocardium. Ameroid placement in swine produced myocardial hibernation/stunning based on reduced regional wall thickening with preserved R wave voltage and mildly reduced MBF. Focal uptake of [123I]Gluco-RGD was seen on SPECT imaging corresponding to regions of phVEGF165 injection at approximately 3 weeks after injection in treated animals. No tracer uptake was seen in the ameroid territory of saline injection. Tracer uptake corresponded to documentation of αvβ3 receptors and lectin staining for capillary sprouting in tissue samples from VEGF injected territory. MBF and LV function improved in VEGF injected animals. These data suggest that radiolabeled Gluco-RGD probes may be useful to monitor angiogenesis therapy in hibernating myocardium.
Approaches to stimulate myocardial angiogenesis in patients with intractable angina who are not candidates for PTCA or CABG have included intracoronary infusion or intramyocardial injection of a growth factor gene or protein. (1-8). None of these studies showed unequivocal efficacy and initial enthusiasm for these approaches has been tempered. Subsequent trials of stem cell therapy in myocardial infarction do not support the role of these cells to differentiate into and function as myocytes but do seem to support a paracrine effect of the engrafted cells through secretion of VEGF in response to hypoxia. (10,11) These findings renew interest in developing approaches to non-invasively monitor myocardial angiogenesis by targeting VEGF expression.
Approaches to image angiogenesis in the heart or blood vessel wall have been reported using different imaging platforms. (24-28) Targeting molecular species expressed during angiogenesis with imaging probes has been investigated for cancer and cardiovascular applications. Vascular endothelial growth factors are widely distributed in mammalian tissues. The member of the VEGF family that plays an important role in angiogenesis is VEGF-A These cytokines are expressed in response to up-regulation of hypoxia-inducible factor-1α (HIF-1 α). In response to hypoxia, m-RNA encodes VEGF molecules with 121, 145, 165 amino acid sequences. (29) For capillary sprouting to occur there needs to be an interaction between the endothelial cell growth and the surrounding supporting tissue or extracellular matrix. This interaction necessary for new growth is mediated by the integrins, a family of cell surface receptors that recognize extracellular matrix proteins such as vitronectin, fibrinogen, and fibronectin and are necessary for optimal activation of growth factor proteins. (12-16)
Integrins are heterodimeric transmemebrance glycoproteins consisting of an alpha and beta subunit. (11,12) The integrins aggregate with growth factor receptors and thereby facilitate their activation. Certain integrins are preferentially associated with specific growth factor receptors. The integrin αvβ3 is associated with PDGF and VEGF receptors. (13) This important role of αvβ3 in promoting the binding of growth factor receptor and ligand has made it an important target for development of agents to image angiogenesis. Extracellular matrix proteins like vitronectin, fibrinogen, and fibronectin interact with the integrins via the amino acid sequence arginine-glycine-aspartic acid (RGD). (16) Haubner et al were the first to report the synthesis and biological evaluation of the first glycoslylated RGD-containing peptides. (18) In this study we used Gluco-RGD and labeled it with iodine-123. (19) Direct halogenation involves a multi-step preparation and requires HPLC and in-vivo dehalogenation degrades image quality. Subsequent to the completion of this project additional peptide compounds have been synthesized and tested in-vivo in tumor models. These compounds include versatile chelating moieties that allow linkage with both SPECT and PET tracers. (17) They are based on cyclic pentapeptide conjugated via the ε-amino function of a lysine with chelators or use an αvβ3 binding disulphide-bridged undecapeptide coupled with HYNIC and labeled with 99mTc.
The use of probes containing the RGD motif to image angiogenesis has been reported for nuclear (SPECT and PET) applications and for MRI. (24-28). Myocardial imaging of angiogenesis has been reported in a rat and dog model of chronic myocardial infarction using an In-111-labeled avb3 targeted agent (111In-RP748) for αvβ3 and thallium-201 for perfusion. (26) Dual isotope SPECT imaging showed focal uptake of 111In-RP748 in regions of reduced perfusion corresponding to regions of angiogenesis by histological and immunohistological analysis. (27) A PET approach to myocardial imaging using dual imaging for both gene expression and angiogenesis was reported. (28) In this study a non-replicating adenovirus expressing mutant herpesviral thymidine kinase (HSV1-sr39tk) reporter gene and human VEGF121 gene was constructed and injected into normal pig myocardium. Two days later animals were injected with [18F]fluoro-hydroxymethylbutylguanine (FHBG) probe for the reporter gene and [18F]Galacto-RGD for angiogenesis. This novel approach documented reporter gene expression but there was no significant uptake of the [18F]Galacto-RGD probe. This failure to detect angiogenesis in the normal porcine myocardium was attributed to insufficient time to allow angiogenesis to occur but lack of a hypoxic environment may have contributed. The ameroid model of chronic myocardial ischemia/hibernation may create a substrate for expression of related genes favorable to angiogenesis.
The swine model of hibernating myocardium produced by slow constriction of a coronary was developed by Canty and Fallavollita (30) Swine ameroid models have been used in pre-clinical studies to document angiogenesis. A proof of concept paper by Vale showed that direct injection into the myocardium of phVEGF165 could be performed using nonfluoroscopic electromechanical left ventricular mapping to localize hibernating myocardium and record injection sites. (31-33) They documented VEGF expression by Western blots in the injection sites and in a subsequent paper the same group showed improvement in collateral myocardial blood flow. (33)
Conclusions
In these experiments natural occurring angiogenesis in response to hypoxia was not observed in the saline injected animals based on either lectin staining or detectable uptake of [123I]Gluco-RGD. Failure of the hypoxic stimulus to produce beneficial levels of naturally occurring angiogenesis was also indicated by failure to show improvement in perfusion or LV function. A possible explanation for these findings is that the severity and/or duration of tissue hypoxia were insufficient stimuli to produce “therapeutic levels” of endogenous VEGF. The conditions of these experiments were not designed to address these questions.
Tracer retention in a region might be due to circulating blood levels of compound at the time of imaging combined with differences in intramyocardial blood volume, or interstitial activity associated with changes in vascular permeability, and not active angiogenesis. However, the correlation between lectin staining and percentage injected dose supports actual binding of the [123I]Gluco-RGD to αvβ3 indicating growth factor activity. The study would have been strengthened by evaluation of control hearts without ameroids and treatment, and control hearts with phVEGF treatment. That the correlation between [123I]Gluco-RGD uptake and lectin staining does not show a perfect separation between the VEGF injected and saline injected experiments indicates that at least in one experiment high background activity of 123I probably relating to in vivo de-halogenation affected results. Peptides with more stable linkage to radio-probes including 111In and 99mTc via chelators with have been developed for SPECT imaging. These new tracers eliminate this problem.
Acknowledgments
Sources of funding: Supported by NIH (RO1HL65395)
ABBREVIATIONS
- AM
ameroid
- ECM
extracellular matrix
- ED
end-diastole
- ES
end-systole
- FGF
fibroblastic growth factor
- FS
fractional shortening
- ID
injected dose
- LCX
left circumflex coronary artery
- RGD
arginine-glycine-aspartic acid
- SPECT
single photon computed tomography
- UPV
unipolar voltage
- VEGF
vascular endothelial growth factor
- WT
wall thickening
Footnotes
No conflict of interest for any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lynne L Johnson, Columbia University.
Lorraine Schofield, Rhode Island Hospital.
Tammy Donahay, Rhode Island Hospital.
Mark Bouchard, Rhode Island Hospital.
Athena Poppas, Rhode Island Hospital.
Roland Haubner, Medizinische Universitaet Innsbruck, Austria and Technische Universität München, Munich, Germany.
References
- 1.Henry TD, Annex BH, McKendall GR, et al. The VIVA Trial Vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 2.Symes JF, Losordo DW, Vale PR, et al. Gene therapy with vascular endothelial growth factor for inoperable coronary artery disease. Ann thorac surg. 1999;68:830–837. doi: 10.1016/s0003-4975(99)00807-3. [DOI] [PubMed] [Google Scholar]
- 3.Vale PR, Losordo DW, Milliken CE, et al. Randomized, single-blind, placebo-controlled pilot study of catheter-based myocardial gene transfer for therapeutic angiogenesis using left ventricular electromechanical mapping in patients with chronic myocardial ischemia. Circulation. 2001;101:2138–2143. doi: 10.1161/01.cir.103.17.2138. [DOI] [PubMed] [Google Scholar]
- 4.Losordo DW, Vale PR, Hendel RC, et al. Phase ½ placebo-controlled double-blind, dose-escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia. Circulation. 2002;105:2012–2018. doi: 10.1161/01.cir.0000015982.70785.b7. [DOI] [PubMed] [Google Scholar]
- 5.Kastrup J, Jorgensen E, Ruck A, et al. Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris. J Am coll Cardiol. 2005;45:982–8. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 6.Grines CL, Watkins MW, Helmer G, et al. Gene therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002;105:1291–1297. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- 7.Grines CL, Watkins MW, Mahmarian JJ, et al. A randomized, double-blind, placebo-controlled trial of Ad5FGDF-4 gene therapy and its effect on myocardial perfusion in patients with stable angina. J Am Coll Cardiol. 2003;42:1339–47. doi: 10.1016/s0735-1097(03)00988-4. [DOI] [PubMed] [Google Scholar]
- 8.Stewart DJ, Hilton JD, Arnold JMO, et al. Angiogenic gene therapy in patients with nonrevascularizable ischemic heart disease: a phase 2 randomized, controlled trial of AdVEGF (ADVEFG121) versus maximum medical treatment. Gene Therapy. 2006;13:1503–1511. doi: 10.1038/sj.gt.3302802. [DOI] [PubMed] [Google Scholar]
- 9.Payne TR, Oshima H, Okada M, et al. A Ralationship between vascular endothelial growth factor, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts. J Am Coll Cardiol. 2007;50:1677–85. doi: 10.1016/j.jacc.2007.04.100. [DOI] [PubMed] [Google Scholar]
- 10.Vandervelde S, van Luyn MJA, Harmsen MC. Signaling factors in stem cell-mediated repair of infarcted myocardium. J Mole Cell Cardiol. 2005;39:363–376. doi: 10.1016/j.yjmcc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Xiong JP, Stehle T, Diefenbach B, et al. Crystal structure of the extracellular segment of integrin aVß3. Science. 2001;294:339–346. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 13.Senger DR, Claffey KP, Benes JE, Perruzzi CA, Segiou AP, Detmar M. Angiogenesis promoted by vascular endothelial growth factor: regulation through a1ß1 and a2ß1 integrins. Proc Natl Acad Sci. 1997;94:13612–13617. doi: 10.1073/pnas.94.25.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byzova TV, Goldman CK, Pampori N, et al. A mechanism for modulation of cellurar responses to VEGF: activation of the integrins. Molecular Cell. 2000;6:851–860. [PubMed] [Google Scholar]
- 15.Tsou R, Isik FF. Integrin activation is required for VEGF and FGF receptor protein presence on human microvascular endothelial cells. Mol Cell Biochem. 2001;224:81–89. doi: 10.1023/a:1011947301849. [DOI] [PubMed] [Google Scholar]
- 16.Bayless KJ, Salazar R, Davis GE. RGD-Dependent vacuolation and lumen formation observed during endothelial cell morphogenesis in three-dimensional fibrin matrices involves the avß3 and a5ß1 integrins. Am J Pathol. 2000;156:1673–1683. doi: 10.1016/s0002-9440(10)65038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haubner R. avß3 – integrin imaging: a new approach to characterize angiogenesis? Eur J Nucl Med Mol Imaging. 2006;33:S54–S63. doi: 10.1007/s00259-006-0136-0. [DOI] [PubMed] [Google Scholar]
- 18.Haubner R, Wester HJürge, Burkhart F, et al. Glycosylated RGD-Containing peptides: tracer for tumor targeting and angiogenesis imaging with improved biokinetics. J Nucl Med. 2001;42:326–336. [PubMed] [Google Scholar]
- 19.Haubner R, Kuhnast B, Mang C, Weber WA, Kessler Horst, et al. Wester Hans-Jürgen and Schwaiger Markus. [18F] Galacto-RGD: synthesis, radiolabeling, metabolic stability, and radiation dose estimates. Bioconjugate Chem. 2004;15:61–69. doi: 10.1021/bc034170n. [DOI] [PubMed] [Google Scholar]
- 20.Haubner R, Wester HJ, Weber WA, et al. Noninvasive imaging of avß3 integrin expression using 18F-labeled RGD-containing glycopeptides and positron emission tomography. Cancer Research. 2001;61:1781–1785. [PubMed] [Google Scholar]
- 21.Thambar ST, Schofield L, Poppas A, Bouchard M, Williams DO, Johnson LL. Validation of R wave volgate endomyocardial mapping to assess myocardial fibrosis: Comparison with thallium and dobutamine echocardiography in a swine model. J Interven Cardiol. 2003;16:23–31. doi: 10.1046/j.1540-8183.2003.08011.x. [DOI] [PubMed] [Google Scholar]
- 22.Hale SL, Alker KF, Kloner RA. Evaluation of nonradioactive, colored microspheres for measurement of regional mycardial blood flow in dogs. Circulation. 1988;78:428–34. doi: 10.1161/01.cir.78.2.428. [DOI] [PubMed] [Google Scholar]
- 23.Johnson LL, Schofield L, Mastrofrancesco P, Donahay T, Farb A, Khaw BA. Technetium-99m glucarate uptake in a swine model of limited flow plus increased demand. J Nucl Cardiol. 2000;7:590–8. doi: 10.1067/mnc.2000.108908. [DOI] [PubMed] [Google Scholar]
- 24.Winter PM, Morawski AM, Caruthers SD, et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with avß3-integrin-targeted nanoparticles. Circulation. 2003;108:2270–2274. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 25.Sadeghi MM, Krassilnikova S, Zhang J, et al. Detection of injury –induced vascular remodeling by targeting activated avß3 integrin in vivo. Circulation. 2004;110:84–90. doi: 10.1161/01.CIR.0000133319.84326.70. [DOI] [PubMed] [Google Scholar]
- 26.Hua J, Dobrucki LW, Sadeghi MM, et al. Noninvasive imaging of angiogenesis with a 99m Tc-labeled peptide targeted at avß3 integrin after murine hindlimb ischemia. Circulation. 2005;111:3255–3260. doi: 10.1161/CIRCULATIONAHA.104.485029. [DOI] [PubMed] [Google Scholar]
- 27.Meoli DF, Sadeghi MM, Krassilnikova S, et al. Noinvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J Clin invest. 2004;113:1684–1691. doi: 10.1172/JCI20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner B, Anton M, Nekolla SG, et al. Noninvasive characterization of myocardial molecular interventions by integrated positron emission tomography and computed tomography. J Am Coll Cardiol. 2006;48:2107–15. doi: 10.1016/j.jacc.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 29.Yla-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J. Vascular endothelial growth factors. J Am Coll Cardiol. 2007;49:1015–26. doi: 10.1016/j.jacc.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 30.Fallavollita JA, Logue M, Canty JM. Stability of Hibernating Myocardium in Pigs with a Chronic Left Anterior Descending Coronary Artery Stenosis: Absence of Progressive Fibrosis in the Setting of Stable Reductions in Flow, Function and Coronary Flow Reserve. J Am Coll Cardiol. 2001;37:1989–1995. doi: 10.1016/s0735-1097(01)01250-5. [DOI] [PubMed] [Google Scholar]
- 31.Vale PR, Losordo DW, Tkebuchave T, Chen D, Milliken CE, Isner JM. Catheter-based myocardial gene transfer utilizing nonfluoroscopic electromechanical left ventricular mapping. J Am Coll Cardiol. 1999;34:246–54. doi: 10.1016/s0735-1097(99)00143-6. [DOI] [PubMed] [Google Scholar]
- 32.Vale PR, Losordo DW, Milliken CE, et al. Left ventricular electromechanical mapping to assess e MDfficacy of ph VEGF 165 gene transfer for therapeutic angiogenesis in chronic myocardial ischemia. Circulation. 2000;102:965–974. doi: 10.1161/01.cir.102.9.965. [DOI] [PubMed] [Google Scholar]
- 33.Tio RA, Tkebuchava T, Scheuermann TH, et al. Intramyocardial gene therapy with naked DNA encoding vascular endothelial growth factor improves collateral flow to ischemic myocardium. Human gene therapy. 1999;10:2953–2660. doi: 10.1089/10430349950016366. [DOI] [PubMed] [Google Scholar]