Abstract
In this chapter, roles of bioactive sphingolipids in the regulation of cancer pathogenesis and therapy will be reviewed. Sphingolipids have emerged as bioeffector molecules, which control various aspects of cell growth, proliferation, and anti-cancer therapeutics. Ceramide, the central molecule of sphingolipid metabolism, generally mediates anti-proliferative responses such as inhibition of cell growth, induction of apoptosis, and/or modulation of senescence. On the other hand, sphingosine 1-phosphate (S1P) plays opposing roles, and induces transformation, cancer cell growth, or angiogenesis. A network of metabolic enzymes regulates the generation of ceramide and S1P, and these enzymes serve as transducers of sphingolipid-mediated responses that are coupled to various exogenous or endogenous cellular signals. Consistent with their key roles in the regulation of cancer growth and therapy, attenuation of ceramide generation and/or increased S1P levels are implicated in the development of resistance to drug-induced apoptosis, and escape from cell death. These data strongly suggest that advances in the molecular and biochemical understanding of sphingolipid metabolism and function will lead to the development of novel therapeutic strategies against human cancers, which may also help overcome drug resistance.
Keywords: Apoptosis, ceramide, drug resistanc, cancer therapeutic, sphingolipids
16.1 Introduction
Sphingolipids are a family of membrane lipids that contribute to the regulation of the fluidity and the sub-domain structure of the lipid bilayers (Futerman and Hannun, 2004). In addition, bioactive sphingolipids such as ceramide, sphingosine 1-phophate (S1P), sphingosine, and glucosylceramide (GlcCer), function as bioeffector molecules, which are involved in the regulation of various aspects of cancer pathogenesis and therapy, including apoptosis, cell proliferation, cell migration, senescence, or inflammation (Futerman and Hannun, 2004; Kok and Sietsma, 2004; Ogretmen and Hannun, 2004; Reynolds et al., 2004; Fox et al., 2006; Modrak et al., 2006).
16.1.1 Structure and Metabolism of Ceramide
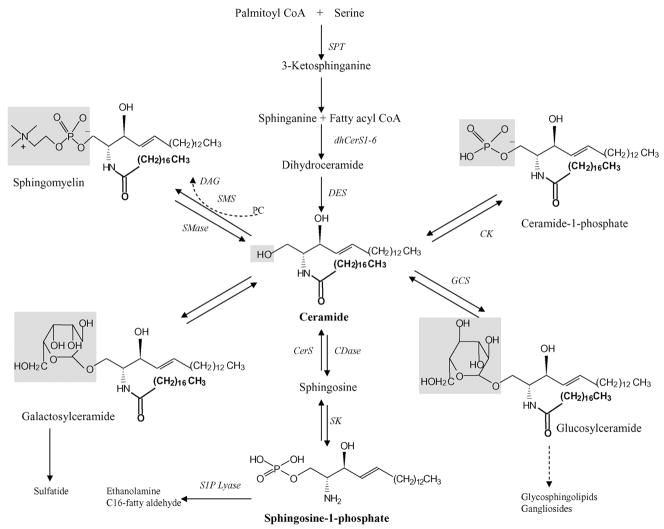
Ceramide is composed of sphingosine, which is an amide-linked to a fatty acyl chain, varying in length from C14 to C26 (Fig. 16.1). Ceramide then serves as the metabolic and structural precursor for complex sphingolipids, which are composed of hydrophilic head groups, such as sphingomyelin (SM), ceramide 1-phosphate (C1P), and GlcCer (Gouaze-Andersson and Cabot, 2006). The synthesis of GlcCer is the precursor for the generation of complex glycosphingolipids and gangliosides (Fig. 16.1) (Futerman and Hannun, 2004; Futerman and Riezman, 2005). Endogenous ceramide levels are regulated by complex and integrated metabolic pathways, and each of these pathways involves a number of specialized enzymes (Fig. 16.1) (Futerman and Hannun, 2004; Futerman and Riezman, 2005). In addition to the activation of sphingomyelinases (SMases) (Andrieu-Abadie and Levade, 2002; Clarke and Hannun, 2006; Clarke et al., 2006) that hydrolyze SM to yield ceramide, endogenous ceramide can be generated via the de novo pathway (Dolgachev et al., 2004; Reynolds et al., 2004). In the de novo pathway, serine and palmitoyl CoA condense to form 3-ketosphinganine by serine palmitoyl transferase (SPT) (Merrill et al., 1988; Nagiec et al., 1996), leading to the synthesis of dihydroceramide by dihydroceramide synthases (dhCerS1-6) (Bose et al., 1995; Venkataraman et al., 2002). Then, a double bond is inserted between carbons 4–5 in the sphingosine backbone of dihydroceramide to generate ceramide (Michel et al., 1997; Kraveka et al., 2007). Ceramide can then be utilized as a substrate by ceramidases (CDases) to liberate sphingosine (Park and Schuchman, 2006), which is phosphorylated to generate S1P. Ceramide is also metabolized by the functions of ceramide kinase (CK), or SM synthase (Sugiura et al., 2002; Van der Luit et al., 2007) (Fig. 16.1), which requires the transport of ceramide from the endoplasmic reticulum (ER) to the Golgi apparatus by ceramide transporter protein, CERT, via non-vesicular transport (Hanada et al., 2003; Kumagai et al., 2005; Rao et al., 2007; Kudo et al., 2008). Ceramide can also be converted into glucosylceramide (GlcCer) in the Golgi, however this process is CERT independent (D’Angelo et al., 2007). Importantly, non-vesicular transport of GlcCer from its site of synthesis (early Golgi) to distal Golgi compartments is carried out by FAPP2, four-phosphate adaptor protein, controlling the synthesis of glycosphingolipids, which might essentially play crucial roles in determining the lipid composition of the plasma membrane (D’Angelo et al., 2007).
Fig. 16.1.
Sphingolipid metabolism. Sphingolipids are comprised of three main components: a sphingosine backbone, a fatty-acid chain, and a head group. Characteristics of sphingolipids change based on the head group, and recently, it was shown that the fatty-acid chain length could influence sphingolipid function. Ceramide is central in sphingolipid metabolism. Ceramide can be generated through several pathways, in particular it can be synthesized de novo from palmitoyl CoA and serine. Also, interestingly, ceramide, which is a pro-apoptotic sphingolipid, can proceed on to form S1P, a pro-survival sphingolipid. Thus, sphingolipid metabolic enzymes play a crucial role in determining the fate of cancer cells
16.1.2 Ceramide Synthases and the de novo Generation of Ceramide
Recently, biochemical and clinical studies indicate that different fatty-acid chain lengths of ceramide may have different functions within the cell, high-lighting the importance of ceramide synthase (CerS) in sphingolipid metabolism (Pewzner-Jung et al., 2006). CerS, identified as the yeast longevity assurance gene 1 (LAG1), is known to regulate life-span/longevity in Saccharomyces cerevisiae, and its deletion prolongs the replicative life-span of yeast (Jazwinski and Conzelmann, 2002; Obeid and Hannun, 2003). Additionally, an LAG1 homologue, LAC1 was determined as a key component of CerS (Jazwinski and Conzelmann, 2002; Obeid and Hannun, 2003; Pewzner-Jung et al., 2006). The discovery of the mouse homologue of LAG1, also known as LASS1, or the upstream of growth and differentiation factor 1 (UOG1) (Lee, 1991; Venkataraman et al., 2002), demonstrated that it specifically regulates the synthesis of C18-ceramide with a high degree of fatty-acid chain length specificity (Venkataraman et al., 2002; Riebeling et al., 2003; Mizutani et al., 2005; Kageyama-Yahara and Riezman, 2006). Further studies showed that there are six LASS proteins (LASS1–6), which were recently renamed as ceramide synthases 1–6 (CerS1–6). CerS1–6 are associated with the ER membrane and contain a crucial TRAM-Lag1p-CLN8 (TLC) domain. The TLC domain constitutes the catalytic activity of CerS, and is required for the generation of ceramide (Schulz et al., 2006; Spassieva et al., 2006). All of the CerS proteins, except CerS1, contain a homeobox transcription factor HOX domain found at the N-terminus (Mesika et al., 2007) except for CerS1, which might be important for the enzymatic activity of CerS2–6. However, the physiological roles of the HOX domain of CerS2–5 are still unclear.
Importantly, as mentioned above, each CerS exerts specificity for the generation of endogenous ceramides with distinct fatty-acid chain lengths (Pewzner-Jung et al., 2006). For example, CerS1 specifically generates ceramide with an 18-carbon containing fatty-acid chain (C18-ceramide), whereas CerS5–6 mainly generate C16-ceramide, and to a lesser extent C12- and C14-ceramides (Fig. 16.2) (Pewzner-Jung et al., 2006; Cerantola et al., 2007). Indeed, CerS5 was shown to be the bona fide ceramide synthase for the generation of C16-ceramide (Lahiri and Futerman, 2005), and CerS2 generates very long chain ceramides, particularly C24-ceramide (Laviad et al., 2007).
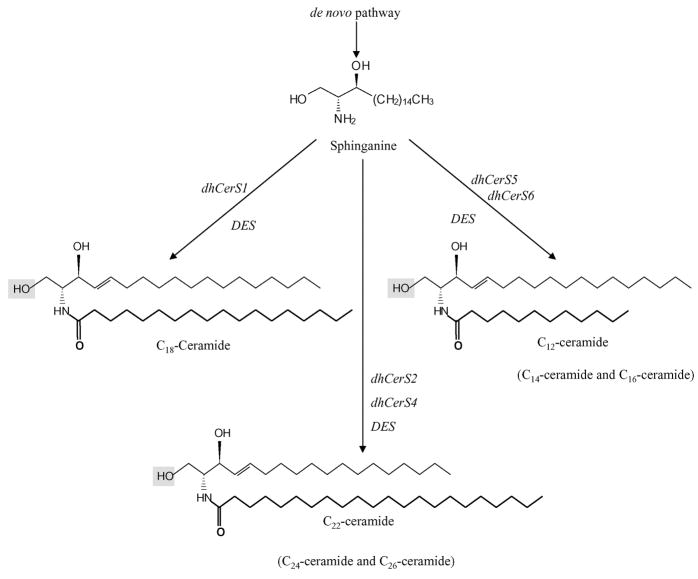
Fig. 16.2. De novo.
generation of ceramide via the function of dhCerS. Recently identified dhCerS1–6 are responsible for the generation and determining the fatty-acid chain length of ceramide in the de novo pathway. For example, dhCerS1, previously known as LASS1, is responsible for generating dihydro-C18-ceramide, whereas CersS2 and CerS4 synthesize dihydro-C22-, C24-, and C26-ceramides. In addition, dihydro-C12-, C14- and C16-ceramides are generated by CerS5 and CerS6. These dihydro-ceramides are then desaturated to form ceramides by DES
Interestingly, recent data (Koybasi et al., 2004; Karahatay et al., 2007) suggest while the levels of C18-ceramide are generally lower, C16-ceramide is significantly up-regulated in the majority (about 80%) of tumor tissues of head and neck squamous cell carcinoma (HNSCC) patients when compared to their adjacent normal tissues (Karahatay et al., 2007). Decreased C18-ceramide and increased C16-ceramide in HNSCC tumor tissues were associated with decreased and increased expression of CerS1 and 6, respectively. Remarkably, clinical analyses revealed that lower levels of C18-ceramide in HNSCC tumor tissues are significantly associated with higher incidences of lymphovascular invasion and nodal metastasis in HNSCC patients, indicating the clinical relevance of LASS1/C18-ceramide metabolism and signaling in HNSCC pathogenesis and progression (Karahatay et al., 2007). More importantly, these data were also supported by studies which demonstrated that defects in the LASS1-dependent generation of C18-ceramide play important roles in HNSCC growth (Koybasi et al., 2004), and/or response to therapy in human HNSCC cells in situ and in vivo (Senkal et al., 2007). Recently, a role for LASS1 in the regulation of sensitivity to various chemotherapeutic agents has been further confirmed in an independent study using human cancer cell lines in situ (Min et al., 2007).
16.1.3 Down-Stream Targets of Ceramide Signaling in Cancer
Ceramide mediates the regulation of growth arrest, senescence, and/or apoptosis (Ogretmen and Hannun, 2004). Some of these biological functions might be controlled through novel sphingolipid-protein interactions (Snook et al., 2006). Most frequently, these direct targets of ceramide constitute protein phosphatases and kinases that regulate important signaling pathways in cancer, such as Akt, protein kinase C (PKC), MAP kinases, or phospholipase D (Hannun and Obeid, 2002; Ogretmen and Hannun, 2004) (Fig. 16.3). The regulation of protein phosphatase-1 and -2 (PP1 and PP2A)-family enzymes, also referred to as ceramide activated protein phosphatases (CAPPs), by ceramide has been well documented previously (Dobrowsky and Hannun, 1992; Dobrowsky et al., 1993; Fishbein et al., 1993; Wolff et al., 1994). Upon ceramide-mediated activation of CAPPs, various down-stream targets, such as Bcl-2-family proteins, cyclin dependent kinases, Rb, and c-Myc onco-protein, are regulated (Fig. 16.3) (Ogretmen and Hannun, 2004). Another important ceramide binding protein is cathepsin D, which is activated via ceramide interaction, leading to induction of apoptosis (Fig. 16.3) (Heinrich et al., 2004). Similarly, ceramide was shown to associate with protein kinase zeta (PKC-zeta) (Fig. 16.3), which seems to be important for its activation and the formation of a pro-apoptotic complex between PKC-zeta and prostate apoptotic response-4 (PAR-4) in differentiating stem cells (Wang et al., 2005). Further studies also indicated that the activation of PKC-zeta by ceramide is involved in the inactivation of Akt within the structured membrane micro-domains, leading to growth arrest in vascular smooth muscle cells (Fox et al., 2007). Recently, one of the best characterized ceramide-binding proteins, CERT, was implicated in cancer biology, and the data showed that down-regulation of CERT results in increased sensitivity against chemotherapeutic agents (Swanton et al., 2007), suggesting that alterations of sphingolipid metabolism by CERT might confer a survival advantage to cancer cells. Thus, these data underscore the importance of identification of novel ceramide-protein interactions as immediate and direct mechanisms involved in the regulation of ceramide-mediated biological responses in various patho-physiological conditions, including cancer.
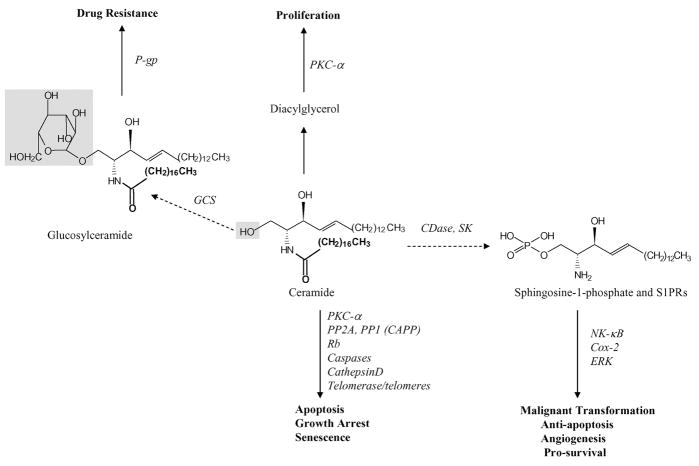
Fig. 16.3.
Ceramide and S1P signaling in cancer cells. Ceramide signaling mainly occurs through the regulation of its immediate and direct targets, leading to apoptosis, growth arrest, and senescence. Conversely, when ceramide is metabolized into S1P, cells will undergo cellular transformation, anti-apoptosis, or induction of angiogenesis. Conversion of ceramide to SM leads to the liberation of DAG from PC, and DAG is a known activator of PKC, which is involved in promoting cellular proliferation. Also, ceramide can be further metabolized into glucosylceramide, which leads to drug resistance. Phosphorylation of ceramide by CK to generate C1P also associated with induction of cell growth. Thus, these data support the hypothesis that while ceramide induces anti-proliferation, alterations in its generation and/or accumulation might result in pro-survival and anti-apoptosis
16.1.4 Sub-Cellular Functions of Ceramide
The biological roles of ceramide might also be controlled by its subcellular localization. For example, when ceramide is generated in the plasma membrane via the hydrolysis of SM by SMases, it activates pathways associated with growth inhibition, oxidative stress-mediated cell death, and lipid raft functions (Segui et al., 2001; Testai et al., 2004). Cell signaling pathways activated by mitochondrial ceramide proceeds through ceramide-activated protein phosphatases, PP1 and PP2A, involved in the regulation of Bcl-2 family proteins, cytochrome C release, and loss of mitochondrial membrane potential, leading to intrinsic cell death (Smyth et al., 1996; Thon et al., 2005). Ceramide can also be generated in the lysosomes by the function of acid SMase, which interacts with cathepsin D, leading to the cleavage of BID and subsequent apoptotic cell death in cancer cells (Heinrich et al., 2004). Lysosomal ceramides can also be metabolized by the function of acid CDase, which is over-expressed in majority of human cancer cells, and may result in resistance to apoptosis (Saad et al., 2007; Liu et al., 2008).
Ceramides generated in the ER might be topologically associated with the nucleus, since the nuclear membrane is a continuous structure of the ER membranes. In the nucleus, ceramide activates protein phosphatase-1, which then dephosphorylates serine/arginine-rich proteins (SR-proteins), which induce the alternative splicing of pro-apoptotic proteins Bcl-XS or caspase-9 (Chalfant et al., 2001, 2002). Another recently identified nuclear target of ceramide includes a pro-survival protein telomerase, which catalyzes the elongation/maintenance of telomeres at the end of chromosomes (Blackburn, 2005). Core telomerase contains two main subunits, telomerase reverse transcriptase (hTERT), and the RNA component (hTR), which acts as an intrinsic template (Blackburn, 2005). Ceramide mediates the repression of the hTERT promoter, and mediates the inhibition of telomerase activity (Ogretmen et al., 2001a). Specifically, in addition to inhibition of c-Myc-dependent activation of hTERT promoter (Ogretmen et al., 2001b), exogenous C6-ceramide, or the C18-ceramide generated by CerS1 induces the deacetylation of Sp3 transcription factor by histone deacetylase 1 (HDAC1) (Wooten and Ogretmen, 2005), and deacetylated Sp3 then helps the recruitment of HDAC1 to the promoter of hTERT, which causes local histone deacetylation and repression of the hTERT promoter in human lung cancer cells (Wooten-Blanks et al., 2007). Interestingly, overexpression of CerS6, which generates C16-ceramide did not inhibit hTERT expression (Wooten-Blanks et al., 2007), supporting the novel view that endogenous ceramides with different fatty-acid chain lengths might have distinct biological roles.
16.2 Anti-Proliferative Roles of Ceramide
16.2.1 Ceramide and Apoptosis
Apoptosis can be induced by various factors including chemotherapeutic agents, CD95, tumor necrosis factor-1, growth factor withdrawal, hypoxia, or DNA damage. Many of these mediators of apoptosis are regulators of ceramide generation, suggesting a role for ceramide in apoptosis (Pettus et al., 2002). There are a myriad of studies which indicate that the changes in endogenous levels of ceramide in response to these agents occur before triggering an apoptotic cascade (Dbaibo et al., 1997). Additionally, increasing the endogenous levels of ceramide with inhibitors of ceramide metabolism enzymes or by over-expression of ceramide-generating enzymes results in apoptosis and/or growth arrest (Abe et al., 1995; Bielawska et al., 1996). For example, in leukemia cells, expression of bacterial SMase, which generates ceramide from intracellular pools of SM, has been shown to cause a significant increase in ceramide levels and to induce apoptosis (Zhang et al., 1997). In addition, ionizing radiation activates acid SMase for ceramide generation. Importantly, human lympho-blasts and mice deficient in SMase are resistant to high doses of irradiation, suggesting an active role of ceramide in the regulation of apoptosis (Santana et al., 1996). On the other hand, inhibitors of the de novo pathway, such as fumonisin B1 (Plo et al., 1999) prevents apoptosis in response to these agents, further providing evidence for the role of ceramide generation in mediating apoptosis.
One of the mechanisms by which ceramide regulates apoptosis is via the induction of Fas capping, which involves the lateral segregation of cross-linked Fas ligand with its surface receptor at the SM-enriched plasma membrane of Jurkat T lymphocytes, necessary for its optimal function in cell killing (Cremesti et al., 2001). On the other hand, cells that are resistant to ceramide and CD95/Fas-induced apoptosis have defective mitochondrial apoptosis (Raisova et al., 2000), indicating that perturbations of ceramide-CD95/Fas signaling can result in the development of resistance to cell death in human cancer cells.
In conclusion, these studies show that ceramide is closely associated with apoptosis, and that it plays an important role in how cells respond to various stress stimuli for induction of apoptosis in various cancer models.
16.2.2 Ceramide in Growth Inhibition and Differentiation
One of the well characterized functions of ceramide is its capability to induce a G0/G1 cell cycle arrest, which can be linked to the activation of the retinoblastoma gene product (Rb) (Dbaibo et al., 1995). In addition, ceramide specifically inactivates the cyclin-dependent kinase cdk2, but not cdk4, through activation of a phosphatase (Lee et al., 2000). An important in vivo example of ceramide-mediated growth arrest was observed with the use of ceramide-coated balloon catheters, which caused growth arrest of vascular smooth muscle-cells (VSMC) after stretch injury in vivo (Charles et al., 2000). Mechanistically, this growth arrest of VSMC was linked to ceramide-induced Akt inhibition, which was mediated through PKC-zeta (Bourbon et al., 2002).
The idea that ceramide is a regulator of cell differentiation has been recognized since the discovery that vitamin D3-induced differentiation of HL-60 and U037 human leukemia cells resulted in a progressive increase in the hydrolysis of SM by neutral-SMase (N-SMase), resulting in the elevation of ceramide, which induced monocytic, but not neutrophilic or macrophage-type, differentiation of these cells (Okazaki et al., 1989). In neuronal cell lines, ceramide mimics nerve growth factor function, and induces differentiation in T9 glioma cells, Purkinje cells, and hippocampal neurons (Dobrowsky et al., 1994).
16.2.3 Ceramide and Senescence
A breakthrough in understanding the relationship between ceramide and senescence came with the observation that ceramide increased significantly as human fibroblasts entered the senescent phase (Venable et al., 1995). This was supported by the fact that fibroblasts that were treated with ceramide recapitulated the morphologic and biochemical changes of senescence such as activation of Rb, regulation of cdk’s, or inhibition of growth factor signaling (Venable et al., 1995). Mechanistically, these changes induced by ceramide occur through inhibition of phospholipase D, which leads to the reduction of diacylglycerol (DAG) generation, and results in the failure to translocate and activate PKC to the membrane, a critical response in transducing mitogenic stimuli (Venable et al., 1995). Additionally, the fact that yeast aging genes, lac1 and lag1 are known to be essential components of ceramide synthase (Guillas et al., 2001) provides a genetic link between ceramide and aging.
Senescence is also regulated by alterations in telomere length, which is maintained by telomerase, one of the down-stream targets of ceramide signaling. The telomerase regulation by ceramide involves two distinct mechanisms; the inactivation of c-Myc transcription factor via increased ubiquitin/proteasome function for its rapid proteolysis, which otherwise activates the hTERT promoter, and the recruitment of Sp3/HDAC1 repressor complex into the hTERT promoter for repression in the A549 human lung adenocarcinoma cell line (Ogretmen et al., 2001a, b; Wooten and Ogretmen, 2005; Wooten-Blanks et al., 2007). In addition, ceramide also mediates telomerase-independent rapid shortening of telomere length in A549 cells (Sundararaj et al., 2004). Ceramide-mediated telomere shortening was linked to the inhibition of an unexpected role of a nuclear form of glyceraldehydes 3-phosphate dehydrogenase (GAPDH) in telomere/binding and protection function (Sundararaj et al., 2004). Taken together, these results support that ceramide and sphingo-lipids play important roles in the regulation of senescence and aging.
16.3 S1P and S1P Receptor Signaling in Cancer Biology
S1P, a product of SK (Fig. 16.1), is considered to be a pro-survival lipid, because of its involvement in malignant transformation, cancer proliferation, inflammation, vasculorogenesis, and resistance to apoptotic cell death (Maceyka et al., 2002; Hla, 2004; Taha et al., 2006a). Increased generation of S1P triggers signaling pathways that mediate these pro-survival processes mainly by engaging with S1P receptors 1–5 (S1PR), members of the family of transmembrane hepta-helical, G-protein-coupled receptors, which are encoded by the endothelial differentiation genes (EDG), in paracrine or autocrine modes after being secreted from the cell (Fig. 16.3) (Rosen and Goetzl, 2005). Overexpression of SK1 results in malignant transformation and tumor formation in 3T3 fibroblasts (Xia et al., 2000). Increased S1P levels promote proliferation and survival in human glioma and breast cancer cells (Nava et al., 2002; Van Brocklyn et al., 2002; Sarkar et al., 2005). Additionally, in endothelial and smooth muscle cells, SK1/S1P/S1PR signaling also has several specific effects on the promotion of vascular development and angiogenesis (Argraves et al., 2004; Chae et al., 2004; Mizugishi et al., 2005). Importantly, in ovarian cancer patient samples, elevated S1P levels are found in the serum (Tilly and Kolesnick, 2002), and S1PR1 has been recently discovered to be a requirement for tumor angiogenesis in vivo (Rosen and Goetzl, 2005).
Interestingly, partial inhibition of SK1 expression results in apoptosis in MCF-7 human breast cancer cells (Taha et al., 2006b). An important role for SK1/S1P pathway has also been shown in a colon carcinogenesis model in rats, which was linked to the up-regulation of Cox-2 (Kawamori et al., 2006).
S1P generation can also be catalyzed by SK2, and overexpression of SK2, interestingly, mediates growth inhibitory effects, possibly via the pro-apoptotic functions of its BH3-like domain (Liu et al., 2003). However, recently, it was suggested that endogenous SK2, which is localized mainly to the nucleus, might act similar to SK1, providing a pro-survival characteristics to cancer cells. Specifically, inhibition of the expression of SK2 in the nucleus results in p21-mediated growth inhibition (Sankala et al., 2007). To address these ambiguous data regarding opposing roles of exogenous and endogenous SK2 in the regulation of cancer cell death or survival, it was suggested that overexpressed SK2 might not be effectively targeted to the nucleus, leading to the release of its BH3-like domain into the cytosol, and this might be a reasons why it acts as a pro-apoptotic molecule in studies in which it is exogenously introduced into the cells (Sankala et al., 2007). These interesting data further confirm the importance of subcellular localization of sphingolipid metabolism in the regulation of cancer cell growth and/or apoptosis (Birbes et al., 2001).
16.4 Sphingolipid Signaling in Cancer Therapy
Previous studies suggest that understanding the intrinsic mechanisms of action for ceramide and S1P can open doors to new therapies to battle cancer. It has been well established that increases in intracellular ceramide will promote apoptosis. Thus, finding ways to intrinsically elevate ceramide in cancer cells is desirous. Conversely, S1P has been shown to promote cancer pathogenesis, thus, suppression of its generation/accumulation could suppress tumor growth. Some of these therapeutic approaches are summarized in Table 16.1.
Table 16.1.
Sphingolipid analogues & inhibitors of ceramide metabolism
| Compound | Mode of Action | Cancer Type |
|---|---|---|
| B13 | Acid ceramidase inhibitor | Prostate, Colon and HNSCC |
| D-MAPP | Neutral/Alkaline ceramidase inhibitor | Squamous cell carcinoma |
| Pyridinium ceramide | Mitochondrial targeting | HNSCC, lung, colon and breast |
| 4,6-diene-ceramide | Ceramide analogue | Breast |
| C16-serinol | Ceramide analogue | Neuroblastoma |
| PPMP, PPPP | GCS inhibitors | Solid tumors |
| Dimethylsphingosine | Sphingosine Kinase inhibitor | Leukemia, colon and breast |
| Anti-S1P monoclonal antibody | Binds S1P | Solid tumors |
| Pegylated lyposomes with ceramide | Improved delivery | Breast |
| Vincristine in sphingomyelin- liposomes | Improved delivery | Acute lymphoid leukemia |
| Safingol (L-t-dihydro- sphingosine) | Sphingosine kinase inhibitor | Solid tumors |
| FTY-720 | Myriocine analogue | Bladder, prostate, breast, lymphoma |
D-MAPP, D-erythro-2-(N-myristoylamino)-1-phenyl-1-propanol; HNSCC, Head and neck squamous cell carcinoma; PPMP, 1-phenyl-2-palmitoylamino-3-morpholino-1-propanol; GCS, glucosylceramide synthase.
16.4.1 Ceramide Metabolism in Cancer Therapeutics
Increasing endogenous ceramide has been suggested as an effective method to regulate cancer cell growth. To this end, certain chemotherapeutic agents such as daunorubicin, camptothecin, fludarabine, etoposide, and gemcitabine increase ceramide generation through the de novo pathway, or via activation of SMases (Futerman and Hannun, 2004; Ogretmen and Hannun, 2004). Alternatively, targeting enzymes of ceramide clearance appears to elevate endogenous ceramide, leading to increased anti-proliferative responses in various cancer cells (Fox et al., 2006; Ogretmen, 2006). Moreover, combining the chemotherapeutic agent gemcitabine with SM synergistically inhibits pancreatic tumor growth in vivo (Modrak et al., 2004). The therapeutic effects of doxorubicin were also enhanced when used in combination with SM in various human cancer cell lines (Veldman et al., 2004). Mechanistically, SM was shown to increase the cellular uptake of doxorubicin via altering cell membrane permeability, leading to increased accumulation and bioavailability of the drug in these cells (Veldman et al., 2004). Also,Δ9-tetrahydrocannabinol exerts apoptosis in Jurkat cells via the CB(2) cannabinoid receptor by activation of ceramide generation de novo, which plays a role in the mitochondrial intrinsic pathway (Carracedo et al., 2006; Herrera et al., 2006).
Additionally, small molecule inhibitors of the sphingolipid pathway to induce the accumulation of ceramide have been used in some cancers. For example, B13, an inhibitor of acid CDase was used in a metastatic colon cancer mouse model and a prostate cancer xenograph model (Selzner et al., 2001; Samsel et al., 2004). In both cases, B13 caused the accumulation of ceramide and resulted in prevention of tumor growth. Another effective approach to increase ceramide accumulation in cancer cells has been to inhibit SM synthase, or acid CDase (Meng et al., 2004; Saad et al., 2007).
In addition, the use of ceramide analogues or mimetics could also promote apoptotic pathways in cancer cells. In fact, many studies report that exogenous treatment with ceramides induces cell death, and/or growth arrest (Szulc et al., 2006; Bielawska et al., 2008). These findings were supported with in vivo studies, in which treatment with recently developed exogenous ceramides (ceramidoids) inhibited cancer cell growth, and decreased tumor progression in HNSCC and other cancer models (Senkal et al., 2006; Szulc et al., 2006; Bielawska et al., 2008).
It should also be noted that treatment of cells with exogenous ceramides may result in the generation of endogenous long chain ceramides via the sphingosine recycling pathway, which can be blocked by FB1, and not by myriocin. This alternative pathway for the generation of endogenous ceramide seems to be important for the regulation of telomerase and c-Myc in A549 cells (Ogretmen et al., 2002; Sultan et al., 2006).
In a recent study, treatment of prostate and lung cancer cells with γ–tocopherol (γT), the main dietary form of vitamin E, inhibited cell proliferation and induced apoptosis (Jiang et al., 2004), which were concomitant with the accumulation of dihydroceramides. Importantly, fenretinide, which was initially thought to increase ceramide generation, has been reported by various, independent groups, to elevate dihydroceramides, possibly via the negative regulation of dihydroceramide desaturase (DES) (Schulz et al., 2006; Zheng et al., 2006; Kraveka et al., 2007). In fact, recent data revealed that accumulation of endogenous dihydroceramides via down-regulation of DES results in Rb-dependent growth arrest in human neuroblastoma cells (Kraveka et al., 2007). Although dihydroceramides are thought to be inert or biologically inactive molecules, these data suggest that they, too—at least when generated in cells—might be important in the regulation of cancer cell growth and/or survival.
16.4.2 Targeting the SK1/S1P Pathway as an Anti-Cancer Therapeutic
Down-regulation of S1P biosynthesis provides another therapeutic modality for the treatment of cancers. On the other hand, exogenous S1P treatment exerts a protective role against cell death in normal (non-cancerous) human cells (Tilly and Kolesnick, 2002). Additionally, S1P has suppressive effects against chemotherapy- induced apoptosis in the ovary (Tilly and Kolesnick, 2002), and also blocked male germ cell apoptosis in the human testis (Suomalainen et al., 2005). Thus, one anti-cancer therapeutic strategy has been to use inhibitors of SK1. Preliminary studies in situ and in animal models indicate that SK1 inhibitors prevent cancer cell proliferation and tumor growth (French et al., 2006). There are also novel S1PR1 and S1PR3 antagonists (Davis et al., 2005), and inhibition of these receptors with these compounds may inhibit cancer cell growth. Another novel approach to cancer therapeutics is the use of a monoclonal antibody that binds S1P with high affinity and specificity (Visentin et al., 2006). The anti-S1P monoclonal antibody significantly reduced tumor progression effects in various murine xenograft and allograft models (Visentin et al., 2006). The anti-S1P monoclonal antibody also prevented S1P-induced cell proliferation, release of pro-angiogenic cytokines, and protection of tumor cells from apoptosis by S1P (Visentin et al., 2006). Thus, these data strongly suggest that S1P may present an important target for anti-cancer therapeutics.
Additional experimental evidence supporting the pro-survival roles of S1P/S1PR axis was obtained by employing the potent immunosuppressive agent FTY720, which is known to engage with S1PRs (Brinkmann et al., 2004; Chun and Rosen, 2006). FTY-20 is phosphorylated in vivo to FTY720-P possibly by SK2 (Billich et al., 2003; Paugh et al., 2003), which then induces sequestration of lymphocytes in lymph tissues by engaging S1PRs with high affinity and specificity (Brinkmann et al., 2004; Chun and Rosen, 2006). These data implicate that S1PRs play important roles in immunosuppression. More importantly, treatment with FTY720 inhibits angiogenesis and tumor vascularization, and mediates cell death, suggesting that it might be exploited as an anti-cancer therapeutic agent (LaMontagne et al., 2006).
Thus, these data support the hypothesis that inhibition of SK1 may enhance the treatment of cancer cells, whereas selective elevation of S1P in normal cells may provide protection against toxicity during therapy.
16.5 Sphingolipids in Drug Resistance and Chemoprevention
One of the main obstacles involved in cancer therapy is the development of drug resistance. Interestingly, a relationship between the changes in the sphingolipid metabolism and development of drug resistance in human cancer cells has been documented (Ogretmen and Hannun, 2001; Senchenkov et al., 2001; Radin, 2002; Hinrichs et al., 2005). Therefore, one possible approach to overcome this resistance could be through modulation of the sphingolipid pathway.
16.5.1 Role of Ceramide in Drug Resistance
Recent studies indicate that one mechanism of resistance that cancer cells develop against chemotherapy is the alteration of ceramide accumulation. In fact, ceramide is highly metabolized into GlcCer due to an increase in glucosylceramide synthase (GCS) activity and/or expression in some cancer cells (Gouaze-Andersson and Cabot, 2006). This phenomenon has been implicated in development of drug resistance in various cancer cell types, especially in breast cancer cells (Fig. 16.3) (Senchenkov et al., 2001). Although the role of GCS in the development of drug-resistance has been challenged in some cancer models (Veldman et al., 2003; Norris-Cervetto et al., 2004), a mechanistic link between GCS and P-glycoprotein (P-gp), an ABC transporter implicated in drug resistance, has been recently revealed (Gouaze et al., 2005; Gouaze-Andersson et al., 2007). The data demonstrated that knockdown of GCS expression with small interfering RNA (siRNA) significantly inhibits the expression ofMDR1, a gene that encodes for P-gp, and reverses drug resistance (Gouaze et al., 2005; Gouaze-Andersson et al., 2007). These data are consistent with an earlier study which showed that, SDZ PSC 833, an inhibitor of P-gp, inhibits GCS, and alters GlcCer levels (Goulding et al., 2000). Additionally, increased accumulation of GlcCer is found in cells overexpressing P-gp (Gouaze et al., 2004). Interestingly, several members of the ABC transporter family are implicated in the translocation of phospholipids and sphingolipids across the lipid bilayer, and P-gp has been proposed as a specific transporter for glucosylceramide that translocates this molecule across the Golgi to deliver it for the synthesis of neutral glycosphingolipids (De Rosa et al., 2004). Thus, P-gp and GCS appear to function in the same pathway of ceramide/GlcCer metabolism, and this may provide an important link for the function of GCS in drug resistance.
These results also suggest that inhibitors of GCS may be useful in preventing chemotherapy resistance. For example, combinations of fenretinide (4-HPR), which is known to elevate ceramide (Wang et al., 2001) and (dihydro)ceramide levels (Schulz et al., 2006; Zheng et al., 2006; Kraveka et al., 2007), with the inhibitors of GCS or SK, such as PPMP (1-phenyl-2-palmitoylamino-3-morpholino- 1-propanol) or safingol (L-threo-dihydrosphingosine), were reported to suppress the growth of various human cancer cells, synergistically (Maurer et al., 1999, 2000).
16.5.2 Sphingosine Kinase and S1P in Drug Resistance
SK1/S1PR signaling protects cancer cells from chemotherapy-induced apoptosis; therefore, changes in sphingolipid metabolism play functional roles in conferring drug resistance. For example, in prostate adenocarcinoma SK-1 regulates drug-induced apoptosis and serves as a chemotherapy sensor both in culture and in animal models (Pchejetski et al., 2005). In parallel with these data, increasing the expression of SK-1 reduced the sensitivity of A-375 melanoma cells to Fas- and ceramide-mediated apoptosis that could be reversed by inhibition of SK-1 expression (Bektas et al., 2005). Also, high expression levels of SK1 and S1PRs were detected in camptothecin- (CPT) resistant PC3 prostate cancer cells (Akao et al., 2006). Specifically, inhibition of SK1 expression or S1PR signaling significantly inhibited PC3 cell growth, and treatment of these cells with CPT induced upregulation of SK1/S1PR signaling (Akao et al., 2006). These data are also supported by studies conducted using the model organism Dictyostelium discoideum, in which modulation of SK or S1P-lyase contribute to altered sensitivity to cisplatin (Alexander et al., 2006). The role of S1P-lyase in increased sensitivity to this drug in a p38-, and to lesser extent, c-Jun NH2-terminal kinase- (mitogen activated protein kinases) dependent manner, was confirmed in human A549 lung cancer and HEK293 cells (Min et al., 2005). The role of S1P-lyase in the regulation of apoptosis has been also demonstrated in various human cancer models previously (Oskouian et al., 2006).
Importantly, in a recent study, overexpression of SK1 is linked to the upregulation of Bcr-Abl, leading to alterations of the balance between pro-apoptotic C18-ceramide and pro-survival S1P, leading to resistance to imatinib mesylate in K562 human CML cells (Baran et al., 2007). Importantly, down-regulation of SK1 significantly reversed resistance to drug-induced apoptosis in these cells (Baran et al., 2007).
Thus, taken together, these studies indicate that therapeutic strategies that induce tumor levels of ceramide, particularly C18-ceramide in HNSCC, while decreasing S1P accumulation in the serum would be ideal for improving the therapeutic outcome of some cancers in the clinic (Fig. 16.4).
Fig. 16.4.
Roles of ceramide and S1P in anti-cancer therapeutics. The cellular balance between ceramide in tumor samples and S1P is believed to determine the fate of cancer cells. Often, within a tumor, there are altered levels/accumulation of ceramide (such as low C18-ceramide levels in HNSCC) and high S1P levels, which are often secreted into the serum at relatively high concentrations. This causes the metabolic balance to favor S1P resulting in a pro-survival outcome. However, new therapies are being explored to shift this balance in favor of ceramide. Ultimately, these new treatments are aimed at increasing ceramide levels while inhibiting S1P generation, secretion, or signaling through S1PRs
16.5.3 Chemopreventive Roles of Sphingolipids
The roles of sphingolipids in chemoprevention have also been reported previously (Borek and Merrill, 1993). Administration of SM in the diet prevents the formation of chemically induced colon cancer tumors and aberrant colonic crypts by decreasing the rate of cell proliferation and increasing apoptosis in mice (Schmelz et al., 1996). Diets supplemented with ceramide, SM, glucosylceramide, lactosylceramide, or ganglioside GD3 to C57B1/6 J(Min/+) mice with a truncated APC gene product, reduced the number of tumors in the intestine (Schmelz et al., 1996, 2000).
Moreover, resveratrol, a compound found at high levels in red wine, induces apoptosis via increased generation of de novo-generated ceramide in breast cancer cells in situ (Scarlatti et al., 2003, 2007; Minutolo et al., 2005).
In addition, the role of S1P in the induction of Cox2 expression, and in the production of prostaglandins (Pettus et al., 2003), coupled with findings of increased SK1 in colon cancer and colon carcinogenesis (Kawamori et al., 2006), raises the possibility of utilizing SK1 as a novel target for chemoprevention. Thus, there are strong leads which suggest that sphingolipids may play roles in chemoprevention.
16.6 Sphingolipid-Based Anti-Cancer Therapeutics
One common approach to promote apoptosis in cancer cells is the use of exogenous ceramide analogues or mimetics as therapeutic agents (Table 16.1). There have been tremendous recent improvements in the design and delivery of these ceramides. For example, varied chain pyridinium ceramides (Pyr-Cers) have been synthesized with increased water solubility and cell-membrane permeability (Novgorodov et al., 2005; Rossi et al., 2005; Dindo et al., 2006; Senkal et al., 2006; Szulc et al., 2006; Dahm et al., 2008). The positive charge of the pyridinium ring in these structures allows targeting and accumulation of these ceramide analogues mainly into mitochondria, and to a lesser extent, to the nucleus of cancer cells. There are studies which suggest that cancer cells acquire, in general, a more negative charge in their sub-cellular structures (especially mitochondria) (Modica-Napolitano and Aprille, 2001), therefore, Pyr-Cer can preferentially target cancer cells with minimum toxicity to normal cells. In fact, data indicate that L-t-C6-Pyr-Cer and D-e-C16-Pyr-Cer preferentially accumulates in mitochondria-, and nuclei-enriched fractions in several human cancer cells in vitro (Novgorodov et al., 2005; Rossi et al., 2005; Dindo et al., 2006; Senkal et al., 2006; Dahm et al., 2008), and this was consistent with the higher accumulation of the compound in the HNSCC tumor site, compared to the liver and intestines in vivo (Senkal et al., 2006). The accumulation of Pyr-Cer in the mitochondria dramatically altered the structures and functions of mitochondria, resulting in a decrease of the mitochondrial membrane potential, release of mitochondrial cytochrome C, activation of caspase-3 and caspase-9 and causing apoptotic cell death (Novgorodov et al., 2005; Rossi et al., 2005; Dindo et al., 2006).
Other novel structural analogs of ceramide, such as C16-serinol and (2S, 3R)-(4E, 6E)-2-octanoylamidooctadecadiene-1, 3-diol (4,6-diene-ceramide) induced apoptosis in various human cancer cells (Bieberich et al., 2000; Struckhoff et al., 2004). Other ceramide analogs, 5R-OH-3E-C8-ceramide, adamantyl-ceramide and benzene-C4-ceramide selectively inhibited the growth of drug-resistant human breast cancer cell lines (SKBr3 and MCF-7/Adr) (Crawford et al., 2003).
In another approach, delivery of exogenous ceramide in pegylated liposomes, which are known to be more effective at crossing the cell membrane, increased growth inhibitory effects in human breast cancer cells, via enhanced accumulation of ceramide (Stover and Kester, 2003). The liposomal delivery of exogenous natural ceramide also resulted in the inhibition of phosphorylated Akt and stimulated the activity of caspase-3/7 more effectively than non-liposomal ceramide (Stover and Kester, 2003). In vivo therapeutic efficacy of the pegylated ceramide for the delivery of exogenous ceramide, which resulted in slower tumor growth in murine models of breast cancer, was also demonstrated (Stover et al., 2005). Encapsulated vincristine in SM-liposomes, also called sphingosomes has improved efficacy compared to the conventional drug in animal models of adult acute lymphocytic leukemia (ALL), and sphingosomal vincristine is now in clinical phase II trials for treatment of patients with recurrent and refractory adult ALL (Thomas et al., 2006). Polymeric nano-particle delivery systems can also be used to improve the delivery and efficacy of ceramides for the treatment of cancer cells to overcome resistance (van Vlerken et al., 2007).
These preliminary approaches point to the feasibility of developing more active and perhaps more selective analogs of ceramide, which can be tested in clinical trials for the treatment of patients with cancer. They also raise the possibility that several enzymes of ceramide clearance (SM synthases, CDases, CK, and GCS) may serve as novel therapeutic targets. In fact, several independent studies showed that down-regulation of acid CDase, or inhibition of its activity, induces apoptosis, and inhibits tumor growth (Samsel et al., 2004; Morales et al., 2007; Holman et al., 2008). Similar data were also reported for the down-regulation of CK, which inhibited cellular proliferation and enhanced apoptosis induced by serum starvation in A549 human lung cancer cells (Mitra et al., 2007).
16.7 Summary and Future Perspectives
The emerging roles of bioactive sphingolipids in regulating various facets of cancer pathogenesis and therapeutics have been demonstrated in various models. Experimental evidence suggests that there is altered regulation of ceramide levels, coupled with alterations of expression and/or activity of enzymes of sphingolipid metabolism in several cancers, that is consistent with the potential tumor-suppressor functions of ceramide. On the other hand, the SK/S1P/S1PR axis is increasingly implicated in pro-survival, anti-apoptosis, neovascularization, and inflammation. In addition, in situ and in vivo studies indicate functions of sphingolipids in chemoprevention, especially in colon cancers. Therefore, ceramide analogues/mimetics, and inhibitors of SK or enzymes of ceramide clearance might be exploited for the development of novel strategies for anti-cancer therapeutics (Fig. 16.4 and Table 16.1).
These therapeutic efforts will be improved when the complexities of the networks of sphingolipid metabolism, subcellular compartmentalization, and signaling are better understood by using conventional biochemical and molecular biological techniques integrated with more sophisticated approaches such as lipidomics and bioinformatics. To date, most, if not all, of the enzymes involved in sphingolipid metabolismhave been identified and cloned, providing the molecular tools and insights required to discover novel functions of sphingolipids in cancer pathogenesis and therapy. Thus, in the next few years, significant advances are expected in understanding the mechanistic function of bioactive sphingolipids in the regulation of cancer progression, metastasis, and therapeutics.
Acknowledgments
We thank Drs. Z.M. Szulc and Jennifer Schnellmann for their critical review of this chapter. We also thank the members of the Ogretmen laboratory for their helpful discussions. We apologize to those investigators whose important work was not included in this chapter because of space limitations. The Ogretmen laboratory is supported by research grants from the National Institutes of Health.
References
- Abe A, Radin NS, Shayman JA, Wotring LL, Zipkin RE, Sivakumar R, Ruggieri JM, Carson KG, Ganem B. Structural and stereochemical studies of potent inhibitors of glucosylceramide synthase and tumor cell growth. J Lipid Res. 1995;36:611–621. [PubMed] [Google Scholar]
- Akao Y, Banno Y, Nakagawa Y, Hasegawa N, Kim TJ, Murate T, Igarashi Y, Nozawa Y. High expression of sphingosine kinase 1 and S1P receptors in chemotherapy-resistant prostate cancer PC3 cells and their camptothecin-induced up-regulation. Biochem Biophys Res Commun. 2006;342:1284–1290. doi: 10.1016/j.bbrc.2006.02.070. [DOI] [PubMed] [Google Scholar]
- Alexander S, Min J, Alexander H. Dictyostelium discoideum to human cells: pharmacogenetic studies demonstrate a role for sphingolipids in chemoresistance. Biochim Biophys Acta. 2006;1760:301–309. doi: 10.1016/j.bbagen.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Andrieu-Abadie N, Levade T. Sphingomyelin hydrolysis during apoptosis. Biochim Biophys Acta. 2002;1585:126–134. doi: 10.1016/s1388-1981(02)00332-3. [DOI] [PubMed] [Google Scholar]
- Argraves KM, Wilkerson BA, Argraves WS, Fleming PA, Obeid LM, Drake CJ. Sphingosine-1-phosphate signaling promotes critical migratory events in vasculogenesis. J Biol Chem. 2004;279:50580–50590. doi: 10.1074/jbc.M404432200. [DOI] [PubMed] [Google Scholar]
- Baran Y, Salas A, Senkal CE, Gunduz U, Bielawski J, Obeid LM, Ogretmen B. Alterations of ceramide/sphingosine 1-phosphate rheostat involved in the regulation of resistance to imatinib-induced apoptosis in K562 human chronic myeloid leukemia cells. J Biol Chem. 2007;282:10922–10934. doi: 10.1074/jbc.M610157200. [DOI] [PubMed] [Google Scholar]
- Bektas M, Jolly PS, Muller C, Eberle J, Spiegel S, Geilen CC. Sphingosine kinase activity counteracts ceramide-mediated cell death in human melanoma cells: role of Bcl-2 expression. Oncogene. 2005;24:178–187. doi: 10.1038/sj.onc.1208019. [DOI] [PubMed] [Google Scholar]
- Bieberich E, Kawaguchi T, Yu RK. N-acylated serinol is a novel ceramide mimic inducing apoptosis in neuroblastoma cells. J Biol Chem. 2000;275:177–181. doi: 10.1074/jbc.275.1.177. [DOI] [PubMed] [Google Scholar]
- Bielawska A, Greenberg MS, Perry D, Jayadev S, Shayman JA, McKay C, Hannun YA. (1S, 2R)-D-erythro-2-(N-myristoylamino)-1-phenyl-1-propanol as an inhibitor of ceramidase. J Biol Chem. 1996;271:12646–12654. doi: 10.1074/jbc.271.21.12646. [DOI] [PubMed] [Google Scholar]
- Bielawska A, Bielawski J, Szulc ZM, Mayroo N, Liu X, Bai A, Elojeimy S, Rembiesa B, Pierce J, Norris JS, Hannun YA. Novel analogs of d-e-MAPP and B13. Part 2: Signature effects on bioactive sphingolipids. Bioorg Med Chem. 2008;16:1032–1045. doi: 10.1016/j.bmc.2007.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billich A, Bornancin F, Devay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278:47408–47415. doi: 10.1074/jbc.M307687200. [DOI] [PubMed] [Google Scholar]
- Birbes H, El Bawab S, Hannun YA, Obeid LM. Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis. Faseb J. 2001;15:2669–2679. doi: 10.1096/fj.01-0539com. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579:859–862. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Borek C, Merrill AH., Jr Sphingolipids inhibit multistage carcinogenesis and protein kinase C. Basic Life Sci. 1993;61:367–371. doi: 10.1007/978-1-4615-2984-2_34. [DOI] [PubMed] [Google Scholar]
- Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, Kolesnick R. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995;82:405–414. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- Bourbon NA, Sandirasegarane L, Kester M. Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: implications for growth arrest. J Biol Chem. 2002;277:3286–3292. doi: 10.1074/jbc.M110541200. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;4:1019–1025. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- Carracedo A, Lorente M, Egia A, Blazquez C, Garcia S, Giroux V, Malicet C, Villuendas R, Gironella M, Gonzalez-Feria L, Piris MA, Iovanna JL, Guzman M, Velasco G. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell. 2006;9:301–312. doi: 10.1016/j.ccr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Cerantola V, Vionnet C, Aebischer OF, Jenny T, Knudsen J, Conzelmann A. Yeast sphingolipids do not need to contain very long chain fatty acids. Biochem J. 2007;401:205–216. doi: 10.1042/BJ20061128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae SS, Paik JH, Furneaux H, Hla T. Requirement for sphingosine 1-phosphate receptor-1 in tumor angiogenesis demonstrated by in vivo RNA interference. J Clin Invest. 2004;114:1082–1089. doi: 10.1172/JCI22716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfant CE, Ogretmen B, Galadari S, Kroesen BJ, Pettus BJ, Hannun YA. FAS activation induces dephosphorylation of SR proteins; dependence on the de novo generation of ceramide and activation of protein phosphatase 1. J Biol Chem. 2001;276:44848–44855. doi: 10.1074/jbc.M106291200. [DOI] [PubMed] [Google Scholar]
- Chalfant CE, Rathman K, Pinkerman RL, Wood RE, Obeid LM, Ogretmen B, Hannun YA. De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. J Biol Chem. 2002;277:12587–12595. doi: 10.1074/jbc.M112010200. [DOI] [PubMed] [Google Scholar]
- Charles R, Sandirasegarane L, Yun J, Bourbon N, Wilson R, Rothstein RP, Levison SW, Kester M. Ceramide-coated balloon catheters limit neointimal hyperplasia after stretch injury in carotid arteries. Circ Res. 2000;87:282–288. doi: 10.1161/01.res.87.4.282. [DOI] [PubMed] [Google Scholar]
- Chun J, Rosen H. Lysophospholipid receptors as potential drug targets in tissue transplantation and autoimmune diseases. Curr Pharm Des. 2006;12:161–171. doi: 10.2174/138161206775193109. [DOI] [PubMed] [Google Scholar]
- Clarke CJ, Hannun YA. Neutral sphingomyelinases and nSMase2: bridging the gaps. Biochim Biophys Acta. 2006;1758:1893–1901. doi: 10.1016/j.bbamem.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Clarke CJ, Snook CF, Tani M, Matmati N, Marchesini N, Hannun YA. The extended family of neutral sphingomyelinases. Biochemistry. 2006;45:11247–11256. doi: 10.1021/bi061307z. [DOI] [PubMed] [Google Scholar]
- Crawford KW, Bittman R, Chun J, Byun HS, Bowen WD. Novel ceramide analogues display selective cytotoxicity in drug-resistant breast tumor cell lines compared to normal breast epithelial cells. Cell Mol Biol (Noisy-le-grand) 2003;49:1017–1023. [PubMed] [Google Scholar]
- Cremesti A, Paris F, Grassme H, Holler N, Tschopp J, Fuks Z, Gulbins E, Kolesnick R. Ceramide enables fas to cap and kill. J Biol Chem. 2001;276:23954–23961. doi: 10.1074/jbc.M101866200. [DOI] [PubMed] [Google Scholar]
- D’Angelo G, Polishchuk E, Di Tullio G, Santoro M, Di Campli A, Godi A, West G, Bielawski J, Chuang CC, van der Spoel AC, Platt FM, Hannun YA, Polishchuk R, Mattjus P, De Matteis MA. Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature. 2007;449:62–67. doi: 10.1038/nature06097. [DOI] [PubMed] [Google Scholar]
- Dahm F, Bielawska A, Nocito A, Georgiev P, Szulc ZM, Bielawski J, Jochum W, Dindo D, Hannun YA, Clavien PA. Mitochondrially targeted ceramide LCL-30 inhibits colorectal cancer in mice. Br J Cancer. 2008;98:98–105. doi: 10.1038/sj.bjc.6604099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem. 2005;280:9833–9841. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- Dbaibo GS, Pushkareva MY, Jayadev S, Schwarz JK, Horowitz JM, Obeid LM, Hannun YA. Retinoblastoma gene product as a downstream target for a ceramide-dependent pathway of growth arrest. Proc Natl Acad Sci U S A. 1995;92:1347–1351. doi: 10.1073/pnas.92.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dbaibo GS, Perry DK, Gamard CJ, Platt R, Poirier GG, Obeid LM, Hannun YA. Cytokine response modifier A (CrmA) inhibits ceramide formation in response to tumor necrosis factor (TNF)-alpha: CrmA and Bcl-2 target distinct components in the apoptotic pathway. J Exp Med. 1997;185:481–490. doi: 10.1084/jem.185.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa MF, Sillence D, Ackerley C, Lingwood C. Role of multiple drug resistance protein 1 in neutral but not acidic glycosphingolipid biosynthesis. J Biol Chem. 2004;279:7867–7876. doi: 10.1074/jbc.M305645200. [DOI] [PubMed] [Google Scholar]
- Dindo D, Dahm F, Szulc Z, Bielawska A, Obeid LM, Hannun YA, Graf R, Clavien PA. Cationic long-chain ceramide LCL-30 induces cell death by mitochondrial targeting in SW403 cells. Mol Cancer Ther. 2006;5:1520–1529. doi: 10.1158/1535-7163.MCT-05-0513. [DOI] [PubMed] [Google Scholar]
- Dobrowsky RT, Hannun YA. Ceramide stimulates a cytosolic protein phosphatase. J Biol Chem. 1992;267:5048–5051. [PubMed] [Google Scholar]
- Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993;268:15523–15530. [PubMed] [Google Scholar]
- Dobrowsky RT, Werner MH, Castellino AM, Chao MV, Hannun YA. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265:1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- Dolgachev V, Farooqui MS, Kulaeva OI, Tainsky MA, Nagy B, Hanada K, Separovic D. De novo ceramide accumulation due to inhibition of its conversion to complex sphingolipids in apoptotic photosensitized cells. J Biol Chem. 2004;279:23238–23249. doi: 10.1074/jbc.M311974200. [DOI] [PubMed] [Google Scholar]
- Fishbein JD, Dobrowsky RT, Bielawska A, Garrett S, Hannun YA. Ceramide-mediated growth inhibition and CAPP are conserved in Saccharomyces cerevisiae. J Biol Chem. 1993;268:9255–9261. [PubMed] [Google Scholar]
- Fox TE, Finnegan CM, Blumenthal R, Kester M. The clinical potential of sphingolipid- based therapeutics. Cell Mol Life Sci. 2006;63:1017–1023. doi: 10.1007/s00018-005-5543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox TE, Houck KL, O’Neill SM, Nagarajan M, Stover TC, Pomianowski PT, Unal O, Yun JK, Naides SJ, Kester M. Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. J Biol Chem. 2007;282:12450–12457. doi: 10.1074/jbc.M700082200. [DOI] [PubMed] [Google Scholar]
- French KJ, Upson JJ, Keller SN, Zhuang Y, Yun JK, Smith CD. Antitumor activity of sphingosine kinase inhibitors. J Pharmacol Exp Ther. 2006;318:596–603. doi: 10.1124/jpet.106.101345. [DOI] [PubMed] [Google Scholar]
- Futerman AH, Hannun YA. The complex life of simple sphingolipids. EMBO Rep. 2004;5:777–782. doi: 10.1038/sj.embor.7400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman AH, Riezman H. The ins and outs of sphingolipid synthesis. Trends Cell Biol. 2005;15:312–318. doi: 10.1016/j.tcb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gouaze-Andersson V, Cabot MC. Glycosphingolipids and drug resistance. Biochim Biophys Acta. 2006;1758:2096–2103. doi: 10.1016/j.bbamem.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Gouaze-Andersson V, Yu JY, Kreitenberg AJ, Bielawska A, Giuliano AE, Cabot MC. Ceramide and glucosylceramide upregulate expression of the multidrug resistance gene MDR1 in cancer cells. Biochim Biophys Acta. 2007;1771:1407–1417. doi: 10.1016/j.bbalip.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouaze V, Liu YY, Prickett CS, Yu JY, Giuliano AE, Cabot MC. Glucosylceramide synthase blockade down-regulates P-glycoprotein and resensitizes multidrug-resistant breast cancer cells to anticancer drugs. Cancer Res. 2005;65:3861–3867. doi: 10.1158/0008-5472.CAN-04-2329. [DOI] [PubMed] [Google Scholar]
- Gouaze V, Yu JY, Bleicher RJ, Han TY, Liu YY, Wang H, Gottesman MM, Bitterman A, Giuliano AE, Cabot MC. Overexpression of glucosylceramide synthase and P-glycoprotein in cancer cells selected for resistance to natural product chemotherapy. Mol Cancer Ther. 2004;3:633–639. [PubMed] [Google Scholar]
- Goulding CW, Giuliano AE, Cabot MC. SDZ PSC 833 the drug resistance modulator activates cellular ceramide formation by a pathway independent of P-glycoprotein. Cancer Lett. 2000;149:143–151. doi: 10.1016/s0304-3835(99)00353-5. [DOI] [PubMed] [Google Scholar]
- Guillas I, Kirchman PA, Chuard R, Pfefferli M, Jiang JC, Jazwinski SM, Conzelmann A. C26-CoA-dependent ceramide synthesis of Saccharomyces cerevisiae is operated by Lag1p and Lac1p. Embo J. 2001;20:2655–2665. doi: 10.1093/emboj/20.11.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277:25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- Heinrich M, Neumeyer J, Jakob M, Hallas C, Tchikov V, Winoto-Morbach S, Wickel M, Schneider-Brachert W, Trauzold A, Hethke A, Schutze S. Cathepsin D links TNF-induced acid sphingomyelinase to Bid-mediated caspase-9 and -3 activation. Cell Death Differ. 2004;11:550–563. doi: 10.1038/sj.cdd.4401382. [DOI] [PubMed] [Google Scholar]
- Herrera B, Carracedo A, Diez-Zaera M, Gomez del Pulgar T, Guzman M, Velasco G. The CB2 cannabinoid receptor signals apoptosis via ceramide-dependent activation of the mitochondrial intrinsic pathway. Exp Cell Res. 2006;312:2121–2131. doi: 10.1016/j.yexcr.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Hinrichs JW, Klappe K, Kok JW. Rafts as missing link between multidrug resistance and sphingolipid metabolism. J Membr Biol. 2005;203:57–64. doi: 10.1007/s00232-004-0733-4. [DOI] [PubMed] [Google Scholar]
- Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol. 2004;15:513–520. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Holman DH, Turner LS, El-Zawahry A, Elojeimy S, Liu X, Bielawski J, Szulc ZM, Norris K, Zeidan YH, Hannun YA, Bielawska A, Norris JS. Lysosomotropic acid ceramidase inhibitor induces apoptosis in prostate cancer cells. Cancer Chemother Pharmacol. 2008;61:231–242. doi: 10.1007/s00280-007-0465-0. [DOI] [PubMed] [Google Scholar]
- Jazwinski SM, Conzelmann A. LAG1 puts the focus on ceramide signaling. Int J Biochem Cell Biol. 2002;34:1491–1495. doi: 10.1016/s1357-2725(02)00044-4. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Wong J, Fyrst H, Saba JD, Ames BN. gamma-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc Natl Acad Sci U S A. 2004;101:17825–17830. doi: 10.1073/pnas.0408340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama-Yahara N, Riezman H. Transmembrane topology of ceramide synthase in yeast. Biochem J. 2006;398:585–593. doi: 10.1042/BJ20060697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karahatay S, Thomas K, Koybasi S, Senkal CE, Elojeimy S, Liu X, Bielawski J, Day TA, Gillespie MB, Sinha D, Norris JS, Hannun YA, Ogretmen B. Clinical relevance of ceramide metabolism in the pathogenesis of human head and neck squamous cell carcinoma (HNSCC): attenuation of C(18)-ceramide in HNSCC tumors correlates with lymphovascular invasion and nodal metastasis. Cancer Lett. 2007;256:101–111. doi: 10.1016/j.canlet.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamori T, Osta W, Johnson KR, Pettus BJ, Bielawski J, Tanaka T, Wargovich MJ, Reddy BS, Hannun YA, Obeid LM, Zhou D. Sphingosine kinase 1 is upregulated in colon carcinogenesis. Faseb J. 2006;20:386–388. doi: 10.1096/fj.05-4331fje. [DOI] [PubMed] [Google Scholar]
- Kok JW, Sietsma H. Sphingolipid metabolism enzymes as targets for anticancer therapy. Curr Drug Targets. 2004;5:375–382. doi: 10.2174/1389450043345452. [DOI] [PubMed] [Google Scholar]
- Koybasi S, Senkal CE, Sundararaj K, Spassieva S, Bielawski J, Osta W, Day TA, Jiang JC, Jazwinski SM, Hannun YA, Obeid LM, Ogretmen B. Defects in cell growth regulation by C18:0-ceramide and longevity assurance gene 1 in human head and neck squamous cell carcinomas. J Biol Chem. 2004;279:44311–44319. doi: 10.1074/jbc.M406920200. [DOI] [PubMed] [Google Scholar]
- Kraveka JM, Li L, Szulc ZM, Bielawski J, Ogretmen B, Hannun YA, Obeid LM, Bielawska A. Involvement of dihydroceramide desaturase in cell cycle progression in human neuroblastoma cells. J Biol Chem. 2007;282:16718–16728. doi: 10.1074/jbc.M700647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N, Kumagai K, Tomishige N, Yamaji T, Wakatsuki S, Nishijima M, Hanada K, Kato R. Structural basis for specific lipid recognition by CERT responsible for nonvesicular trafficking of ceramide. Proc Natl Acad Sci U S A. 2008;105:488–493. doi: 10.1073/pnas.0709191105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai K, Yasuda S, Okemoto K, Nishijima M, Kobayashi S, Hanada K. CERT mediates intermembrane transfer of various molecular species of ceramides. J Biol Chem. 2005;280:6488–6495. doi: 10.1074/jbc.M409290200. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Futerman AH. LASS5 is a bona fide dihydroceramide synthase that selectively utilizes palmitoyl-CoA as acyl donor. J Biol Chem. 2005;280:33735–33738. doi: 10.1074/jbc.M506485200. [DOI] [PubMed] [Google Scholar]
- LaMontagne K, Littlewood-Evans A, Schnell C, O’Reilly T, Wyder L, Sanchez T, Probst B, Butler J, Wood A, Liau G, Billy E, Theuer A, Hla T, Wood J. Antagonism of sphingosine-1-phosphate receptors by FTY720 inhibits angiogenesis and tumor vascularization. Cancer Res. 2006;66:221–231. doi: 10.1158/0008-5472.CAN-05-2001. [DOI] [PubMed] [Google Scholar]
- Laviad EL, Albee L, Pankova-Kholmyansky I, Epstein S, Park H, Merrill AH, Jr, Futerman AH. Characterization of ceramide synthase 2: Tissue distribution, substrate specificity and inhibition by sphingosine 1-phosphate. J Biol Chem. 2007 doi: 10.1074/jbc.M707386200. in press. [DOI] [PubMed] [Google Scholar]
- Lee JY, Bielawska AE, Obeid LM. Regulation of cyclin-dependent kinase 2 activity by ceramide. Exp Cell Res. 2000;261:303–311. doi: 10.1006/excr.2000.5028. [DOI] [PubMed] [Google Scholar]
- Lee SJ. Expression of growth/differentiation factor 1 in the nervous system: conservation of a bicistronic structure. Proc Natl Acad Sci U S A. 1991;88:4250–4254. doi: 10.1073/pnas.88.10.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Toman RE, Goparaju SK, Maceyka M, Nava VE, Sankala H, Payne SG, Bektas M, Ishii I, Chun J, Milstien S, Spiegel S. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J Biol Chem. 2003;278:40330–40336. doi: 10.1074/jbc.M304455200. [DOI] [PubMed] [Google Scholar]
- Liu X, Elojeimy S, Turner LS, Mahdy AE, Zeidan YH, Bielawska A, Bielawski J, Dong JY, El-Zawahry AM, Guo GW, Hannun YA, Holman DH, Rubinchik S, Szulc Z, Keane TE, Tavassoli M, Norris JS. Acid ceramidase inhibition: a novel target for cancer therapy. Front Biosci. 2008;13:2293–2298. doi: 10.2741/2843. [DOI] [PubMed] [Google Scholar]
- Maceyka M, Payne SG, Milstien S, Spiegel S. Sphingosine kinase, sphingosine-1- phosphate, and apoptosis. Biochim Biophys Acta. 2002;1585:193–201. doi: 10.1016/s1388-1981(02)00341-4. [DOI] [PubMed] [Google Scholar]
- Maurer BJ, Metelitsa LS, Seeger RC, Cabot MC, Reynolds CP. Increase of ceramide and induction of mixed apoptosis/necrosis by N-(4-hydroxyphenyl)- retinamide in neuroblastoma cell lines. J Natl Cancer Inst. 1999;91:1138–1146. doi: 10.1093/jnci/91.13.1138. [DOI] [PubMed] [Google Scholar]
- Maurer BJ, Melton L, Billups C, Cabot MC, Reynolds CP. Synergistic cytotoxicity in solid tumor cell lines between N-(4-hydroxyphenyl)retinamide and modulators of ceramide metabolism. J Natl Cancer Inst. 2000;92:1897–1909. doi: 10.1093/jnci/92.23.1897. [DOI] [PubMed] [Google Scholar]
- Meng A, Luberto C, Meier P, Bai A, Yang X, Hannun YA, Zhou D. Sphingomyelin synthase as a potential target for D609-induced apoptosis in U937 human monocytic leukemia cells. Exp Cell Res. 2004;292:385–392. doi: 10.1016/j.yexcr.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Merrill AH, Jr, Wang E, Mullins RE. Kinetics of long-chain (sphingoid) base biosynthesis in intact LM cells: effects of varying the extracellular concentrations of serine and fatty acid precursors of this pathway. Biochemistry. 1988;27:340–345. doi: 10.1021/bi00401a051. [DOI] [PubMed] [Google Scholar]
- Mesika A, Ben-Dor S, Laviad EL, Futerman AH. A new functional motif in Hox domain-containing ceramide synthases: identification of a novel region flanking the Hox and TLC domains essential for activity. J Biol Chem. 2007;282:27366–27373. doi: 10.1074/jbc.M703487200. [DOI] [PubMed] [Google Scholar]
- Michel C, van Echten-Deckert G, Rother J, Sandhoff K, Wang E, Merrill AH., Jr Characterization of ceramide synthesis. A dihydroceramide desaturase introduces the 4,5-trans-double bond of sphingosine at the level of dihydroceramide. J Biol Chem. 1997;272:22432–22437. doi: 10.1074/jbc.272.36.22432. [DOI] [PubMed] [Google Scholar]
- Min J, Van Veldhoven PP, Zhang L, Hanigan MH, Alexander H, Alexander S. Sphingosine-1-phosphate lyase regulates sensitivity of human cells to select chemotherapy drugs in a p38-dependent manner. Mol Cancer Res. 2005;3:287–296. doi: 10.1158/1541-7786.MCR-04-0197. [DOI] [PubMed] [Google Scholar]
- Min J, Mesika A, Sivaguru M, Van Veldhoven PP, Alexander H, Futerman AH, Alexander S. (Dihydro)ceramide synthase 1 regulated sensitivity to cisplatin is associated with the activation of p38 mitogen-activated protein kinase and is abrogated by sphingosine kinase 1. Mol Cancer Res. 2007;5:801–812. doi: 10.1158/1541-7786.MCR-07-0100. [DOI] [PubMed] [Google Scholar]
- Minutolo F, Sala G, Bagnacani A, Bertini S, Carboni I, Placanica G, Prota G, Rapposelli S, Sacchi N, Macchia M, Ghidoni R. Synthesis of a resveratrol analogue with high ceramide-mediated proapoptotic activity on human breast cancer cells. J Med Chem. 2005;48:6783–6786. doi: 10.1021/jm050528k. [DOI] [PubMed] [Google Scholar]
- Mitra P, Maceyka M, Payne SG, Lamour N, Milstien S, Chalfant CE, Spiegel S. Ceramide kinase regulates growth and survival of A549 human lung adenocarcinoma cells. FEBS Lett. 2007;581:735–740. doi: 10.1016/j.febslet.2007.01.041. [DOI] [PubMed] [Google Scholar]
- Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani Y, Kihara A, Igarashi Y. Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem J. 2005;390:263–271. doi: 10.1042/BJ20050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modica-Napolitano JS, Aprille JR. Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv Drug Deliv Rev. 2001;49:63–70. doi: 10.1016/s0169-409x(01)00125-9. [DOI] [PubMed] [Google Scholar]
- Modrak DE, Gold DV, Goldenberg DM. Sphingolipid targets in cancer therapy. Mol Cancer Ther. 2006;5:200–208. doi: 10.1158/1535-7163.MCT-05-0420. [DOI] [PubMed] [Google Scholar]
- Modrak DE, Cardillo TM, Newsome GA, Goldenberg DM, Gold DV. Synergistic interaction between sphingomyelin and gemcitabine potentiates ceramide-mediated apoptosis in pancreatic cancer. Cancer Res. 2004;64:8405–8410. doi: 10.1158/0008-5472.CAN-04-2988. [DOI] [PubMed] [Google Scholar]
- Morales A, Paris R, Villanueva A, Llacuna L, Garcia-Ruiz C, Fernandez-Checa JC. Pharmacological inhibition or small interfering RNA targeting acid ceramidase sensitizes hepatoma cells to chemotherapy and reduces tumor growth in vivo. Oncogene. 2007;26:905–916. doi: 10.1038/sj.onc.1209834. [DOI] [PubMed] [Google Scholar]
- Nagiec MM, Lester RL, Dickson RC. Sphingolipid synthesis: identification and characterization of mammalian cDNAs encoding the Lcb2 subunit of serine palmitoyl-transferase. Gene. 1996;177:237–241. doi: 10.1016/0378-1119(96)00309-5. [DOI] [PubMed] [Google Scholar]
- Nava VE, Hobson JP, Murthy S, Milstien S, Spiegel S. Sphingosine kinase type 1 promotes estrogen-dependent tumorigenesis of breast cancer MCF-7 cells. Exp Cell Res. 2002;281:115–127. doi: 10.1006/excr.2002.5658. [DOI] [PubMed] [Google Scholar]
- Norris-Cervetto E, Callaghan R, Platt FM, Dwek RA, Butters TD. Inhibition of glucosylceramide synthase does not reverse drug resistance in cancer cells. J Biol Chem. 2004;279:40412–40418. doi: 10.1074/jbc.M404466200. [DOI] [PubMed] [Google Scholar]
- Novgorodov SA, Szulc ZM, Luberto C, Jones JA, Bielawski J, Bielawska A, Hannun YA, Obeid LM. Positively charged ceramide is a potent inducer of mitochondrial permeabilization. J Biol Chem. 2005;280:16096–16105. doi: 10.1074/jbc.M411707200. [DOI] [PubMed] [Google Scholar]
- Obeid LM, Hannun YA. Ceramide, stress, and a “LAG” in aging. Sci Aging Knowledge Environ. 2003:pe27. doi: 10.1126/sageke.2003.39.pe27. [DOI] [PubMed] [Google Scholar]
- Ogretmen B. Sphingolipids in cancer: regulation of pathogenesis and therapy. FEBS Lett. 2006;580:5467–5476. doi: 10.1016/j.febslet.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- Ogretmen B, Hannun YA. Updates on functions of ceramide in chemotherapy-induced cell death and in multidrug resistance. Drug Resist Updat. 2001;4:368–377. doi: 10.1054/drup.2001.0225. [DOI] [PubMed] [Google Scholar]
- Ogretmen B, Kraveka JM, Schady D, Usta J, Hannun YA, Obeid LM. Molecular mechanisms of ceramide-mediated telomerase inhibition in the A549 human lung adenocarcinoma cell line. J Biol Chem. 2001a;276:32506–32514. doi: 10.1074/jbc.M101350200. [DOI] [PubMed] [Google Scholar]
- Ogretmen B, Schady D, Usta J, Wood R, Kraveka JM, Luberto C, Birbes H, Hannun YA, Obeid LM. Role of ceramide in mediating the inhibition of telomerase activity in A549 human lung adenocarcinoma cells. J Biol Chem. 2001b;276:24901–24910. doi: 10.1074/jbc.M100314200. [DOI] [PubMed] [Google Scholar]
- Ogretmen B, Pettus BJ, Rossi MJ, Wood R, Usta J, Szulc Z, Bielawska A, Obeid LM, Hannun YA. Biochemical mechanisms of the generation of endogenous long chain ceramide in response to exogenous short chain ceramide in the A549 human lung adeno-carcinoma cell line. Role for endogenous ceramide in mediating the action of exogenous ceramide. J Biol Chem. 2002;277:12960–12969. doi: 10.1074/jbc.M110699200. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Bell RM, Hannun YA. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. Role in cell differentiation. J Biol Chem. 1989;264:19076–19080. [PubMed] [Google Scholar]
- Oskouian B, Sooriyakumaran P, Borowsky AD, Crans A, Dillard-Telm L, Tam YY, Bandhuvula P, Saba JD. Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is down-regulated in colon cancer. Proc Natl Acad Sci U S A. 2006;103:17384–17389. doi: 10.1073/pnas.0600050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Schuchman EH. Acid ceramidase and human disease. Biochim Biophys Acta. 2006;1758:2133–2138. doi: 10.1016/j.bbamem.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Paugh SW, Payne SG, Barbour SE, Milstien S, Spiegel S. The immunosuppressant FTY720 is phosphorylated by sphingosine kinase type 2. FEBS Lett. 2003;554:189–193. doi: 10.1016/s0014-5793(03)01168-2. [DOI] [PubMed] [Google Scholar]
- Pchejetski D, Golzio M, Bonhoure E, Calvet C, Doumerc N, Garcia V, Mazerolles C, Rischmann P, Teissie J, Malavaud B, Cuvillier O. Sphingosine kinase-1 as a chemotherapy sensor in prostate adenocarcinoma cell and mouse models. Cancer Res. 2005;65:11667–11675. doi: 10.1158/0008-5472.CAN-05-2702. [DOI] [PubMed] [Google Scholar]
- Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta. 2002;1585:114–125. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, Chalfant CE, Obeid LM, Hannun YA. The sphingosine kinase 1/sphingosine- 1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. Faseb J. 2003;17:1411–1421. doi: 10.1096/fj.02-1038com. [DOI] [PubMed] [Google Scholar]
- Pewzner-Jung Y, Ben-Dor S, Futerman AH. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)?: Insights into the regulation of ceramide synthesis. J Biol Chem. 2006;281:25001–25005. doi: 10.1074/jbc.R600010200. [DOI] [PubMed] [Google Scholar]
- Plo I, Ghandour S, Feutz AC, Clanet M, Laurent G, Bettaieb A. Involvement of de novo ceramide biosynthesis in lymphotoxin-induced oligodendrocyte death. Neuroreport. 1999;10:2373–2376. doi: 10.1097/00001756-199908020-00028. [DOI] [PubMed] [Google Scholar]
- Radin NS. The development of aggressive cancer: a possible role for sphingolipids. Cancer Invest. 2002;20:779–786. doi: 10.1081/cnv-120002495. [DOI] [PubMed] [Google Scholar]
- Raisova M, Bektas M, Wieder T, Daniel P, Eberle J, Orfanos CE, Geilen CC. Resistance to CD95/Fas-induced and ceramide-mediated apoptosis of human melanoma cells is caused by a defective mitochondrial cytochrome c release. FEBS Lett. 2000;473:27–32. doi: 10.1016/s0014-5793(00)01491-5. [DOI] [PubMed] [Google Scholar]
- Rao RP, Yuan C, Allegood JC, Rawat SS, Edwards MB, Wang X, Merrill AH, Jr, Acharya U, Acharya JK. Ceramide transfer protein function is essential for normal oxidative stress response and lifespan. Proc Natl Acad Sci U S A. 2007;104:11364–11369. doi: 10.1073/pnas.0705049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CP, Maurer BJ, Kolesnick RN. Ceramide synthesis and metabolism as a target for cancer therapy. Cancer Lett. 2004;206:169–180. doi: 10.1016/j.canlet.2003.08.034. [DOI] [PubMed] [Google Scholar]
- Riebeling C, Allegood JC, Wang E, Merrill AH, Jr, Futerman AH. Two mammalian longevity assurance gene (LAG1) family members, trh1 and trh4, regulate dihydroceramide synthesis using different fatty acyl-CoA donors. J Biol Chem. 2003;278:43452–43459. doi: 10.1074/jbc.M307104200. [DOI] [PubMed] [Google Scholar]
- Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- Rossi MJ, Sundararaj K, Koybasi S, Phillips MS, Szulc ZM, Bielawska A, Day TA, Obeid LM, Hannun YA, Ogretmen B. Inhibition of growth and telomerase activity by novel cationic ceramide analogs with high solubility in human head and neck squamous cell carcinoma cells. Otolaryngol Head Neck Surg. 2005;132:55–62. doi: 10.1016/j.otohns.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Saad AF, Meacham WD, Bai A, Anelli V, Elojeimy S, Mahdy AE, Turner LS, Cheng J, Bielawska A, Bielawski J, Keane TE, Obeid LM, Hannun YA, Norris JS, Liu X. The functional effects of acid ceramidase overexpression in prostate cancer progression and resistance to chemotherapy. Cancer Biol Ther. 2007;6:1455–1460. doi: 10.4161/cbt.6.9.4623. [DOI] [PubMed] [Google Scholar]
- Samsel L, Zaidel G, Drumgoole HM, Jelovac D, Drachenberg C, Rhee JG, Brodie AM, Bielawska A, Smyth MJ. The ceramide analog, B13, induces apoptosis in prostate cancer cell lines and inhibits tumor growth in prostate cancer xenografts. Prostate. 2004;58:382–393. doi: 10.1002/pros.10350. [DOI] [PubMed] [Google Scholar]
- Sankala HM, Hait NC, Paugh SW, Shida D, Lepine S, Elmore LW, Dent P, Milstien S, Spiegel S. Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Res. 2007;67:10466–10474. doi: 10.1158/0008-5472.CAN-07-2090. [DOI] [PubMed] [Google Scholar]
- Santana P, Pena LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, Cordon-Cardo C, Schuchman EH, Fuks Z, Kolesnick R. Acid sphingomyelinase- deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Maceyka M, Hait NC, Paugh SW, Sankala H, Milstien S, Spiegel S. Sphingosine kinase 1 is required for migration, proliferation and survival of MCF-7 human breast cancer cells. FEBS Lett. 2005;579:5313–5317. doi: 10.1016/j.febslet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Scarlatti F, Sala G, Somenzi G, Signorelli P, Sacchi N, Ghidoni R. Resveratrol induces growth inhibition and apoptosis in metastatic breast cancer cells via de novo ceramide signaling. Faseb J. 2003;17:2339–2341. doi: 10.1096/fj.03-0292fje. [DOI] [PubMed] [Google Scholar]
- Scarlatti F, Sala G, Ricci C, Maioli C, Milani F, Minella M, Botturi M, Ghidoni R. Resveratrol sensitization ofDU145 prostate cancer cells to ionizing radiation is associated to ceramide increase. Cancer Lett. 2007;253:124–130. doi: 10.1016/j.canlet.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Schmelz EM, Sullards MC, Dillehay DL, Merrill AH., Jr Colonic cell proliferation and aberrant crypt foci formation are inhibited by dairy glycosphingolipids in 1, 2- dimethylhydrazine-treated CF1 mice. J Nutr. 2000;130:522–527. doi: 10.1093/jn/130.3.522. [DOI] [PubMed] [Google Scholar]
- Schmelz EM, Dillehay DL, Webb SK, Reiter A, Adams J, Merrill AH., Jr Sphingomyelin consumption suppresses aberrant colonic crypt foci and increases the proportion of adenomas versus adenocarcinomas in CF1 mice treated with 1,2-dimethylhydrazine: implications for dietary sphingolipids and colon carcinogenesis. Cancer Res. 1996;56:4936–4941. [PubMed] [Google Scholar]
- Schulz A, Mousallem T, Venkataramani M, Persaud-Sawin DA, Zucker A, Luberto C, Bielawska A, Bielawski J, Holthuis JC, Jazwinski SM, Kozhaya L, Dbaibo GS, Boustany RM. The CLN9 protein, a regulator of dihydroceramide synthase. J Biol Chem. 2006;281:2784–2794. doi: 10.1074/jbc.M509483200. [DOI] [PubMed] [Google Scholar]
- Segui B, Cuvillier O, Adam-Klages S, Garcia V, Malagarie-Cazenave S, Leveque S, Caspar-Bauguil S, Coudert J, Salvayre R, Kronke M, Levade T. Involvement of FAN in TNF-induced apoptosis. J Clin Invest. 2001;108:143–151. doi: 10.1172/JCI11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzner M, Bielawska A, Morse MA, Rudiger HA, Sindram D, Hannun YA, Clavien PA. Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Res. 2001;61:1233–1240. [PubMed] [Google Scholar]
- Senchenkov A, Litvak DA, Cabot MC. Targeting ceramide metabolism–a strategy for overcoming drug resistance. J Natl Cancer Inst. 2001;93:347–357. doi: 10.1093/jnci/93.5.347. [DOI] [PubMed] [Google Scholar]
- Senkal CE, Ponnusamy S, Rossi MJ, Bialewski J, Sinha D, Jiang JC, Jazwinski SM, Hannun YA, Ogretmen B. Role of human longevity assurance gene 1 and C18- ceramide in chemotherapy-induced cell death in human head and neck squamous cell carcinomas. Mol Cancer Ther. 2007;6:712–722. doi: 10.1158/1535-7163.MCT-06-0558. [DOI] [PubMed] [Google Scholar]
- Senkal CE, Ponnusamy S, Rossi MJ, Sundararaj K, Szulc Z, Bielawski J, Bielawska A, Meyer M, Cobanoglu B, Koybasi S, Sinha D, Day TA, Obeid LM, Hannun YA, Ogretmen B. Potent antitumor activity of a novel cationic pyridinium- ceramide alone or in combination with gemcitabine against human head and neck squamous cell carcinomas in vitro and in vivo. J Pharmacol Exp Ther. 2006;317:1188–1199. doi: 10.1124/jpet.106.101949. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Perry DK, Zhang J, Poirier GG, Hannun YA, Obeid LM. prICE: a downstream target for ceramide-induced apoptosis and for the inhibitory action of Bcl-2. Biochem J. 1996;316(Pt 1):25–28. doi: 10.1042/bj3160025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook CF, Jones JA, Hannun YA. Sphingolipid-binding proteins. Biochim Biophys Acta. 2006;1761:927–946. doi: 10.1016/j.bbalip.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Spassieva S, Seo JG, Jiang JC, Bielawski J, Alvarez-Vasquez F, Jazwinski SM, Hannun YA, Obeid LM. Necessary role for the Lag1p motif in (dihydro)ceramide synthase activity. J Biol Chem. 2006;281:33931–33938. doi: 10.1074/jbc.M608092200. [DOI] [PubMed] [Google Scholar]
- Stover T, Kester M. Liposomal delivery enhances short-chain ceramide-induced apoptosis of breast cancer cells. J Pharmacol Exp Ther. 2003;307:468–475. doi: 10.1124/jpet.103.054056. [DOI] [PubMed] [Google Scholar]
- Stover TC, Sharma A, Robertson GP, Kester M. Systemic delivery of liposomal short-chain ceramide limits solid tumor growth in murine models of breast adenocarcinoma. Clin Cancer Res. 2005;11:3465–3474. doi: 10.1158/1078-0432.CCR-04-1770. [DOI] [PubMed] [Google Scholar]
- Struckhoff AP, Bittman R, Burow ME, Clejan S, Elliott S, Hammond T, Tang Y, Beckman BS. Novel ceramide analogs as potential chemotherapeutic agents in breast cancer. J Pharmacol Exp Ther. 2004;309:523–532. doi: 10.1124/jpet.103.062760. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Kono K, Liu H, Shimizugawa T, Minekura H, Spiegel S, Kohama T. Ceramide kinase, a novel lipid kinase. Molecular cloning and functional characterization. J Biol Chem. 2002;277:23294–23300. doi: 10.1074/jbc.M201535200. [DOI] [PubMed] [Google Scholar]
- Sultan I, Senkal CE, Ponnusamy S, Bielawski J, Szulc Z, Bielawska A, Hannun YA, Ogretmen B. Regulation of the sphingosine-recycling pathway for ceramide generation by oxidative stress, and its role in controlling c-Myc/Max function. Biochem J. 2006;393:513–521. doi: 10.1042/BJ20051083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundararaj KP, Wood RE, Ponnusamy S, Salas AM, Szulc Z, Bielawska A, Obeid LM, Hannun YA, Ogretmen B. Rapid shortening of telomere length in response to ceramide involves the inhibition of telomere binding activity of nuclear glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 2004;279:6152–6162. doi: 10.1074/jbc.M310549200. [DOI] [PubMed] [Google Scholar]
- Suomalainen L, Pentikainen V, Dunkel L. Sphingosine-1-phosphate inhibits nuclear factor kappaB activation and germ cell apoptosis in the human testis independently of its receptors. Am J Pathol. 2005;166:773–781. doi: 10.1016/s0002-9440(10)62298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanton C, Marani M, Pardo O, Warne PH, Kelly G, Sahai E, Elustondo F, Chang J, Temple J, Ahmed AA, Brenton JD, Downward J, Nicke B. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell. 2007;11:498–512. doi: 10.1016/j.ccr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Szulc ZM, Bielawski J, Gracz H, Gustilo M, Mayroo N, Hannun YA, Obeid LM, Bielawska A. Tailoring structure-function and targeting properties of ceramides by site-specific cationization. Bioorg Med Chem. 2006;14:7083–7104. doi: 10.1016/j.bmc.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Taha TA, Hannun YA, Obeid LM. Sphingosine kinase: biochemical and cellular regulation and role in disease. J Biochem Mol Biol. 2006a;39:113–131. doi: 10.5483/bmbrep.2006.39.2.113. [DOI] [PubMed] [Google Scholar]
- Taha TA, Kitatani K, El-Alwani M, Bielawski J, Hannun YA, Obeid LM. Loss of sphingosine kinase-1 activates the intrinsic pathway of programmed cell death: modulation of sphingolipid levels and the induction of apoptosis. Faseb J. 2006b;20:482–484. doi: 10.1096/fj.05-4412fje. [DOI] [PubMed] [Google Scholar]
- Testai FD, Landek MA, Dawson G. Regulation of sphingomyelinases in cells of the oligodendrocyte lineage. J Neurosci Res. 2004;75:66–74. doi: 10.1002/jnr.10816. [DOI] [PubMed] [Google Scholar]
- Thomas DA, Sarris AH, Cortes J, Faderl S, O’Brien S, Giles FJ, Garcia-Manero G, Rodriguez MA, Cabanillas F, Kantarjian H. Phase II study of sphingosomal vincristine in patients with recurrent or refractory adult acute lymphocytic leukemia. Cancer. 2006;106:120–127. doi: 10.1002/cncr.21595. [DOI] [PubMed] [Google Scholar]
- Thon L, Mohlig H, Mathieu S, Lange A, Bulanova E, Winoto-Morbach S, Schutze S, Bulfone-Paus S, Adam D. Ceramide mediates caspase-independent programmed cell death. Faseb J. 2005;19:1945–1956. doi: 10.1096/fj.05-3726com. [DOI] [PubMed] [Google Scholar]
- Tilly JL, Kolesnick RN. Sphingolipids, apoptosis, cancer treatments and the ovary: investigating a crime against female fertility. Biochim Biophys Acta. 2002;1585:135–138. doi: 10.1016/s1388-1981(02)00333-5. [DOI] [PubMed] [Google Scholar]
- Van Brocklyn J, Letterle C, Snyder P, Prior T. Sphingosine-1-phosphate stimulates human glioma cell proliferation through Gi-coupled receptors: role of ERK MAP kinase and phosphatidylinositol 3-kinase beta. Cancer Lett. 2002;181:195–204. doi: 10.1016/s0304-3835(02)00050-2. [DOI] [PubMed] [Google Scholar]
- Van der Luit AH, Budde M, Zerp S, Caan W, Klarenbeek JB, Verheij M, Van Blitterswijk WJ. Resistance to alkyl-lysophospholipid-induced apoptosis due to down-regulated sphingomyelin synthase 1 expression with consequent sphingomyelin- and cholesterol-deficiency in lipid rafts. Biochem J. 2007;401:541–549. doi: 10.1042/BJ20061178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vlerken LE, Duan Z, Seiden MV, Amiji MM. Modulation of intracellular ceramide using polymeric nanoparticles to overcome multidrug resistance in cancer. Cancer Res. 2007;67:4843–4850. doi: 10.1158/0008-5472.CAN-06-1648. [DOI] [PubMed] [Google Scholar]
- Veldman RJ, Zerp S, van Blitterswijk WJ, Verheij M. N-hexanoyl-sphingomyelin potentiates in vitro doxorubicin cytotoxicity by enhancing its cellular influx. Br J Cancer. 2004;90:917–925. doi: 10.1038/sj.bjc.6601581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman RJ, Mita A, Cuvillier O, Garcia V, Klappe K, Medin JA, Campbell JD, Carpentier S, Kok JW, Levade T. The absence of functional glucosylceramide synthase does not sensitize melanoma cells for anticancer drugs. Faseb J. 2003;17:1144–1146. doi: 10.1096/fj.02-1053fje. [DOI] [PubMed] [Google Scholar]
- Venable ME, Lee JY, Smyth MJ, Bielawska A, Obeid LM. Role of ceramide in cellular senescence. J Biol Chem. 1995;270:30701–30708. doi: 10.1074/jbc.270.51.30701. [DOI] [PubMed] [Google Scholar]
- Venkataraman K, Riebeling C, Bodennec J, Riezman H, Allegood JC, Sullards MC, Merrill AH, Jr, Futerman AH. Upstream of growth and differentiation factor 1 (uog1), a mammalian homolog of the yeast longevity assurance gene 1 (LAG1), regulates N-stearoyl-sphinganine (C18-(dihydro)ceramide) synthesis in a fumonisin B1-independent manner in mammalian cells. J Biol Chem. 2002;277:35642–35649. doi: 10.1074/jbc.M205211200. [DOI] [PubMed] [Google Scholar]
- Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, Garland WA, Lu Y, Yu S, Hall HS, Kundra V, Mills GB, Sabbadini RA. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Wang G, Silva J, Krishnamurthy K, Tran E, Condie BG, Bieberich E. Direct binding to ceramide activates protein kinase Czeta before the formation of a pro-apoptotic complex with PAR-4 in differentiating stem cells. J Biol Chem. 2005;280:26415–26424. doi: 10.1074/jbc.M501492200. [DOI] [PubMed] [Google Scholar]
- Wang H, Maurer BJ, Reynolds CP, Cabot MC. N-(4-hydroxyphenyl)retinamide elevates ceramide in neuroblastoma cell lines by coordinate activation of serine palmitoyl-transferase and ceramide synthase. Cancer Res. 2001;61:5102–5105. [PubMed] [Google Scholar]
- Wolff RA, Dobrowsky RT, Bielawska A, Obeid LM, Hannun YA. Role of ceramide-activated protein phosphatase in ceramide-mediated signal transduction. J Biol Chem. 1994;269:19605–19609. [PubMed] [Google Scholar]
- Wooten-Blanks LG, Song P, Senkal CE, Ogretmen B. Mechanisms of ceramide-mediated repression of the human telomerase reverse transcriptase promoter via deacetylation of Sp3 by histone deacetylase 1. Faseb J. 2007;21:3386–3397. doi: 10.1096/fj.07-8621com. [DOI] [PubMed] [Google Scholar]
- Wooten LG, Ogretmen B. Sp1/Sp3-dependent regulation of human telomerase reverse transcriptase promoter activity by the bioactive sphingolipid ceramide. J Biol Chem. 2005;280:28867–28876. doi: 10.1074/jbc.M413444200. [DOI] [PubMed] [Google Scholar]
- Xia P, Gamble JR, Wang L, Pitson SM, Moretti PA, Wattenberg BW, D’Andrea RJ, Vadas MA. An oncogenic role of sphingosine kinase. Curr Biol. 2000;10:1527–1530. doi: 10.1016/s0960-9822(00)00834-4. [DOI] [PubMed] [Google Scholar]
- Zhang P, Liu B, Jenkins GM, Hannun YA, Obeid LM. Expression of neutral sphingomyelinase identifies a distinct pool of sphingomyelin involved in apoptosis. J Biol Chem. 1997;272:9609–9612. doi: 10.1074/jbc.272.15.9609. [DOI] [PubMed] [Google Scholar]
- Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, Kelly S, Allegood JC, Liu Y, Peng Q, Ramaraju H, Sullards MC, Cabot M, Merrill AH., Jr Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta. 2006;1758:1864–1884. doi: 10.1016/j.bbamem.2006.08.009. [DOI] [PubMed] [Google Scholar]