Abstract
The hepatocarcinogen aflatoxin B1 (AFB1) is a potent recombinagen but weak mutagen in the yeast Saccharomyces cerevisiae. AFB1 exposure induces DNA damage-inducible genes, such as RAD51 and those encoding ribonucleotide reductase (RNR), through a MEC1 (ATR homolog)-dependent pathway. Previous studies have indicated that MEC1 is required for both AFB1-associated recombination and mutation, and suggested that AFB1-DNA adducts are common substrates for recombination and mutagenesis. However, little is known about the downstream effectors of MEC1 required for genotoxic events associated with AFB1 exposure. Here we show that AFB1 exposure increases frequencies of RAD51-dependent unequal sister chromatid exchange (SCE) and activates Rad53 (CHK2). We found that MEC1, RAD53, and DUN1 are required for both AFB1-associated mutation and SCE. Deletion of SML1, which encodes an inhibitor of RNR, did not suppress the DUN1-dependent requirement for AFB1-associated genetic events, indicating that higher dNTP levels could not suppress the dun1 phenotype. We identified AFB1-DNA adducts and show that approximately the same number of adducts are obtained in both wild type and rad53 mutants. Since DUN1 is not required for UV-associated mutation and recombination, these studies define a distinct role for DUN1 in AFB1-associated mutagenesis and recombination. We speculate that AFB1-associated DNA adducts stall DNA replication, a consequence of which can either be mutation or recombination.
Keywords: aflatoxin B1, DNA adducts, mutation recombination, cell-cycle checkpoint, Saccharomyces cerevisiae
INTRODUCTION
The incidence of hepatocellular carcinoma (HCC) strongly correlates with exposure to aflatoxin B1 (AFB1) and to hepatitis B and C virus [1]. The correlation of the p53 Ser249 mutation in HCC associated with AFB1 exposure is consistent with the idea that AFB1 is a strong liver carcinogen because it is a mutagen [2-4]. A current hypothesis is that recurrent regeneration after injury renders liver cells more susceptible to carcinogen-associated DNA damage [5]. Thus, active DNA replication may enhance AFB1-associated genotoxic effects.
AFB1-associated mutagenesis is correlated with the number of AFB1-associated DNA adducts. The highly unstable 8,9-dihydro-8-(N7-guanyl)-9-hydroxyaflatoxin B1 (AFB1-N7-Gua) adduct converts to both a formamidopyrimidine (FAPY) and an apurinic site [6]. The FAPY structures are highly stable, stall replication, and are poor substrates for the Escherichia coli polV translesion polymerase [6]. However, introduction of oligonucleotides containing AFB1-associated FAPY structures in E. coli generates mutations, similar to those observed in liver carcinomas, at or near the sties of the AFB1-DNA adducts [6]. These studies suggest that AFB1-associated FAPY structures are indeed mutagenic adducts.
Considering that DNA repair mechanisms are similar in yeast Saccharomyces cerevisiae and higher eukaryotes, yeast is a useful organism to study how genetic factors can increase AFB1-associated genotoxic effects, including mutations and homologous recombination events, such as gene conversion events, and translocations. Observations that rad51 mutants, defective in recombinational repair, exhibit enhanced AFB1-associated mutagenesis implies that a common DNA adduct stimulates both recombination and mutagenesis [7]. These observations suggest that DNA damage tolerance pathways are important in triggering AFB1-associated recombination and mutation; however, how recombinational or mutagenic pathways are selected is unclear.
These AFB1-associated genotoxic responses occur in the context of a global genome response to AFB1-associated DNA damage. The global transcription response to AFB1 includes an induction of recombinational repair, mismatch repair, and nucleotide excision repair genes in nongrowing cells [8], while genes involved in nucleotide metabolism and recombination, such as RNR and RAD51, are induced in growing cells [9]. Cells exposed to AFB1 exhibit an extended S phase and a fivefold decline in histone transcripts, suggesting that AFB1-associated DNA adducts trigger a replication block and a checkpoint response [9]. This is supported by the observation that MEC1 (ataxia telangiectasia and Rad3 related, ATR) is required for both AFB1-associated recombination [8] and mutation [9].
Genes that are involved in the S phase checkpoint include MEC1 (ATR), RAD53, and DUN1. RAD53 is required for replication delay [10]. DUN1 induces RNR (ribonucleotide reductase) transcription by inactivating the Crt1 repressor [11] and activates Rnr enzymatic activities by phosphorylating and thereby inactivating the Sml1 inhibitor [12]. While RAD53 is required for DNA damage-associated homologous recombination [13], DUN1 is neither required for UV or X-ray associated homologous recombination [14].
We determined whether MEC1, RAD53, and DUN1 were involved in either AFB1-associated mutation or sister chromatid exchange (SCE). Based on the identification of AFB1-associated DNA adducts in yeast, we speculate that these DNA adducts initiate the checkpoint activation. We observed that MEC1, RAD53, and DUN1 were required for both AFB1-associated mutation and SCE. However, we observed that DUN1 was not required for UV-associated SCE or mutation. We therefore suggest that distinct genetic requirements for AFB1-associated and UV-associated genotoxic effects.
MATERIALS AND METHODS
Strains and Media
The genotypes of yeast strains used in this study are listed in Table 1. Strains for measuring SCE and mutation are derived from the canavanine sensitive strain YB163, which contains his3 recombination substrates in tandem at TRP1 [15]. Mutants containing sml1:: KanMX mec1-Δ::TRP1, sml1:: KanMX, rad53-Δ::LEU2, and dun1::KanMX were generated by one-step gene disruption [16] using PCR amplified gene fragments and selecting for KanR or the appropriate auxotrophic selection. The primers used for amplifying these gene fragments are listed in the “Yeast Deletion Database” (http://www-deletion.-stanford.edu). The dun1::kanMX sml1::URA3 double mutant was made by genetic cross. Ura- derivatives of the rad51 mutant YB205 [17] and dun1::kanMX sml1::URA3 strains were made by selecting for 5-fluorouracil (FOA) resistance. Plasmid pCS316 [18], containing cytochrome P450 1A2 (CYP1A2) and hOR, was introduced into wild type and checkpoint mutants by selecting for Ura+. pJW730, containing DUN1, was introduced into dun1 mutants by selecting for Trp+ transformants.
Table 1.
Yeast Strains*
| Strain | Genotype | Plasmid | Source |
|---|---|---|---|
| YB353 | MATα ura3-52 his3-Δ200 ade2-n trp1-Δ 1 gal3- leu2-3, 112 GAL1::his3-Δ 5′ trp1::his3-Δ 3′::HOcs Iys2- (leaky) sml1::KanMX rad53::LEU2 |
pCS316 | This laboratory |
| YB163 |
MATa-inc ura3-52his3-Δ200 ade2-101 lys-801 trp1-Δ1 gal3- trp1 ::[his3-Δ3′::HOcs, his3-Δ5′] |
This laboratory | |
| YB384 | MATa rad51 leu2-Δ1 | pCS316 | This laboratory |
| YB368 | MATa-inc mec1-21 leu2 | This laboratory | |
| YB385 | MATa-inc mec1-21 leu2 | pCS316 | pCS316 introduced in YB368 |
| YB327 | MATa-inc mec1-Δ::TRP1 sml1::KanMX | This laboratory | |
| YB386 | MATa-inc mec1-Δ::TRP1 sml1::KanMX | pCS316 | pCS316 introduced in YB327 |
| YB230 | MATa-inc leu2-Δ1 mec2-1(rad53)::LEU2 | This laboratory | |
| YB387 | MATa-inc dun1::KanMX | pCS316 | |
| YB388 | MATa-inc dun1::KanMX | pCS316 + pJW730 | pJW730 i n YB387 |
| YB389 | MATa-inc dun 1::KanMX sml1::URA3 | ||
| YB390 | MATa-inc dun 1::KanMX sml1::ura3 | pCS316 | pCS316 introduced in a FOAR derivative of YB389 |
Strains listed below YB163 have the same genotype as YB163 unless indicated.
Standard media were used for the culture of yeast cells. YP (yeast extract, peptone), YPD (YP, dextrose), SC (synthetic complete, dextrose), SC-LEU (SC lacking leucine), SC-TRP (SC lacking tryptophan), SC-URA (SC lacking uracil), and FOA media are described by Burke et al. [19]. Media to select for canavanine resistance contain SC-ARG (synthetic complete lacking arginine) and 60 μg/mL canavanine (CAN) sulfate.
Measuring DNA Damage-Associated Recombination and Mutation Events
To measure AFB1-associated genotoxic events, log phase yeast cells (A600=0.5-1) were exposed to indicated doses of AFB1, previously dissolved in DMSO. Cells were maintained in nutrient media (SC-URA) during the carcinogen exposure. To measure UV-associated events, log phase cells (A600=0.5-1) were washed and resuspended in sterile H2O and then exposed to indicated doses of UV (260 nM, 2J/m2/s). After the exposure, cells were washed twice in H2O, and then plated on SC-HIS or SC-ARG CAN to measure SCE or mutation frequency, respectively. An appropriate dilution was inoculated on YPD to measure viability.
Detection and Quantification of DNA Adducts
To measure the AFB1-associated DNA adducts in yeast, we used liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI/MS/MS) [20]. Log phase cultures of yeast expressing human CYP1A2 (pCS316) were exposed to 50 μM AFB1 for 4 h. Because standard protocols for isolating yeast DNA involve alkaline buffers, rendering the highly unstable AFB1 N7-Gua DNA adducts labile, we have modified the “smash-and-grab” protocol [21] so that we are using a neutral buffer containing 10 mM Tris-HCl, 1 mM EDTA, 100 mM NaCl, 2% Triton X-100, 1% SDS, pH 7. DNA was then isolated from two independent samples of yeast cells. The DNA adducts were identified and measured by high performance liquid chromatography and tandem mass spectroscopy (LC-ESI/MS/MS) after acid hydrolysis [20].
Determining Rad53 (Chk2) Activation
Activation of Rad53 was determined by Western blots. Cells were inoculated in SC-URA medium. Log phase cells (A600=0.5-1) were concentrated threefold in SC-URA and exposed to 50 μM AFB1 for 4 h. After washing cells twice in H2O, aliquots were plated directly on SC-HIS to measure recombination, appropriately diluted and plated on YPD to measure viability. Protein extracts were prepared as previously described by Foiani et al. [22], separated on 10% acrylamide/0.266% bis-acrylamide gels for Rad53 detection, and transferred to nitrocellulose membranes. Rad53 was detected by Western blotting using goat anti-Rad53 (yC-19, Santa Cruz, Biotechnology, Santa Cruz, CA). The secondary antibody used was anti-goat IgG-HRP (Santa Cruz).
RESULTS
AFB1-Associated SCE Requires RAD51
AFB1 efficiently stimulates homologous recombination in diploid yeast expressing human CYP1A2 and CYP1A1 [8]. Since, compared to wild type, AFB1-associated mutation frequencies increase in rad51 haploid mutants [7], which are defective in homologous recombination, we investigated whether there was a common genetic control for AFB1-associated SCE and mutagenesis in yeast.
We measured mutation and unequal SCE in yeast strains (Table 1) expressing human CYP1A2 by selecting for CanR or His+, respectively (Figure 1). The spontaneous frequency of SCE in wild type and the rad51 mutant was 2×10-5, consistent with previous results [23], while the spontaneous mutation frequency was 10-fold higher (5×10-5/5×10-6) in the rad51 mutant, compared to wild type. Previous experiments indicated 100-fold and 20-fold increase in translocation and gene conversion frequencies, respectively, in wild-type log-phase cells that were maintained in phosphate buffer while exposed to AFB1 [18]. We detected no AFB1-associated SCE in wild-type log-phase cells that were maintained in phosphate buffer while exposed to AFB1 (data not shown). We exposed log phase wild-type and rad51 cells to AFB1 for 4 h in growth (SC-URA) medium, as performed by Guo et al. [7]. While we detected a significant threefold and eightfold stimulation of SCE in wild-type cells after exposure to 15 and 50 μM AFB1, respectively, we detected no significant stimulation of SCE in rad51 cells. However, we did detect a higher net frequency of CanR mutants after exposure to 50 μM AFB1 (35×10-6, n=4) in rad51 cells, compared to wild type (17×10-6, n=4), consistent with a previous study [7]. Thus, AFB1 exposure stimulates RAD51-dependent SCE recombination in haploid cells that are maintained in growth medium at concentrations previously observed to stimulate both gene conversion events and directed translocations in diploid cells.
Figure 1.
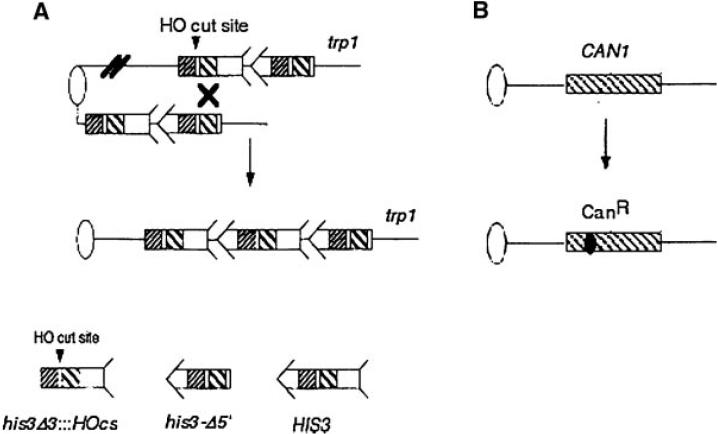
Recombination and mutation assay used in this study. The oval represents the centromere and the single line represents duplex DNA. For simplicity, the left arm of chromosome IV is not shown. (A) Unequal sister chromatid recombination is monitored by selecting for His+ prototrophs that result from recombination between the juxtaposed, truncated his3 fragments. The his3-Δ3' lacks the 3' sequences (arrow head), while the his3-Δ5' lacks to promoter sequences (feathers). Both his3 fragments are located with the amino acid reading frames oriented to the centromere. The his3 fragments share a total of 450 bp sequence homology. (B) The mutation assay is a forward mutation assay by selecting for canavanine resistance.
MEC1-Dependent Rad53 Activation Correlates With AFB1-Associated SCE
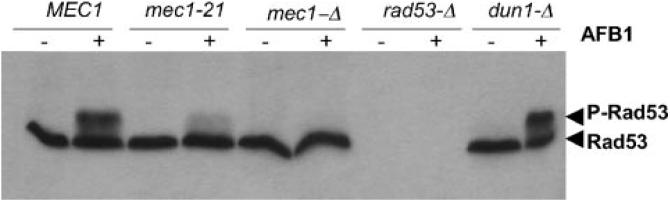
Considering that RNR2 and DUN1 expression is induced in actively growing yeast cells exposed to AFB1 [9], we next determined whether the RAD53-dependent signaling pathway that activates Dun1 was MEC1-dependent by following Rad53 phosphorylation after AFB1 exposure (Figure 2). To obtain sufficient protein and minimize AFB1 amounts, concentrated log phase cells (~5×107 cells/mL) were exposed to AFB1. Similar to previous experiments, we detected greater than fourfold increase in recombination in wild-type cells. By western blots, we measured Rad53 (Chk2) phosphorylation in mec1, dun1, and wild-type cells after 4 h of AFB1 exposure. Rad53 activation occurred in the dun1 mutant, but was reduced or abolished in the mec1-21 (missense) or mec1-Δ (null) mutants, respectively, confirming the requirement for the MEC1 pathway as a response to DNA damaging agents [24]. These studies thus indicate that AFB1 exposure coincidentally stimulates recombination and checkpoint activation.
Figure 2.
AFB1-associated Rad53 activation occurs in wild-type and dun1 cells but not in mec1 null mutants. Cells were exposed to 50 μM AFB1 for 4 h and Rad53 activation was measured in the wild type (YB163), dun1 (YB387), mec1-21 (YB385), and mec1 null (YB386) mutants. The arrow points to phosphorylated Rad53.
AFB1-Associated SCE and Mutation Require MEC1, RAD53, and DUN1
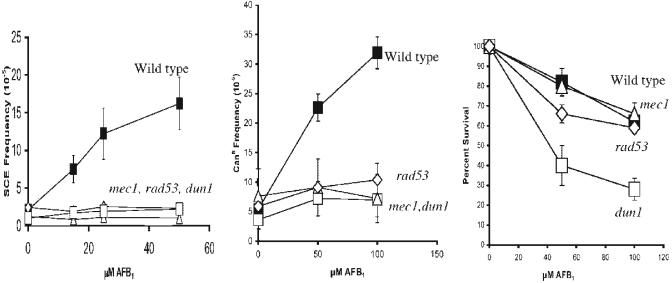
We then determined whether MEC1, RAD53, and DUN1 gene functions were required for both AFB1-associated SCE and mutation. We found no increase in AFB1-associated SCE in mec1, rad53, and dun1 mutants (Figure 3, left). The DUN1 requirement was surprising, considering that UV-associated SCE is DUN1-independent [14]. Interestingly, while rad53 and mec1 mutants exhibit similar viability after AFB1 exposure, we observed that dun1 mutants were the most sensitive to AFB1 (Figure 3, right). These studies indicate that AFB1-associated SCE requires RAD53, DUN1, and MEC1.
Figure 3.
Genetic requirements for AFB1-associated mutagenesis and recombination. Mutation frequency is plotted against AFB1 concentration. The total exposure time is 4 h. Survival is plotted against AFB1 concentration. The solid black square is wild type (YB163), the open black square is dun1(YB387), the open triangle is mec1(YB86), and the open diamond is rad53 (YB353). Genotypes are given next to the symbols.
We then determined whether, similar to SCE recombination, AFB1-associated mutation also required MEC1, RAD53, and DUN1 by measuring CanR mutants. The spontaneous frequency of CanR in wild type was 5×10-6, similar to Guo et al. [7]. After exposure to 100 μM AFB1, we observed approximately sixfold increase in mutation frequency in wild type. However, in dun1, mec1, and rad53 mutants, we observed at most a twofold increase in the frequencies of CanR mutants (Figure 3, center). We observed a 30-fold increase in the frequencies of UV-associated CanR in wild type and the dun1 mutant after cells were exposed to 120 J/m2 (data not shown, n=3). Thus, similar to SCE, we observed that AFB1-associated mutation requires MEC1, RAD53, and DUN1.
To determine that dun1 phenotypes are recessive we introduced the DUN1-containing plasmid (pJW730) in the dun1 strain by selecting for Trp+ transformants. We then measured unequal SCE, mutation, and survival after the Trp+ transformants were exposed to 100 μM AFB1. Compared to spontaneous frequencies, we observed fourfold (22×10-6/5×10-6, n=3) and fivefold (9×10-5/1.9×10-5, n=3) increase in mutation and recombination frequencies, respectively. Compared to the dun1 mutant, we observed an approximately twofold increase in percent survival (51%/28%). These data indicate that extrachromosomal expression of DUN1 suppresses dun1 phenotypes.
DUN1 has multiple functions including induction of RNR, regulation of the G2 checkpoint, and stabilization of stalled replication forks [25]. Since the dun1 DNA damage sensitivity can be suppressed by mutation in the negative regulator of RNR, SML1 [12], we determined whether the dun1 mutation and recombination phenotypes could also be suppressed in a dun1 sml1 mutant. We introduced CYP1A2 (pCS316) into a dun1 sml1 strain and then measured survival, mutation, and recombination frequencies after exposure to 100 μMAFB1. The dun1 sml1 mutant was as resistant (76% survival, n=5) as wild type. Compared to the spontaneous frequencies, we observed less than a twofold increase in the AFB1-associated frequencies of recombination [(8.3±5.4)/(6.5±5.4)×10-5, n=4] and mutation [[(13.7±4.5)/(8.7±4.2)×10-6], n=5], respectively. We conclude that increasing deoxynucleotide triphosphate (dNTP) levels per se is insufficient to suppress the dun1 defect in stimulating AFB1-associated recombination and mutation. However, considering that sml1 mutations do suppress AFB1-associated lethality in dun1, higher dNTP levels increase survival after AFB1 exposure.
AFB1-N7-Gua and AFB1-FAPY DNA Adducts Can be Detected and Measured
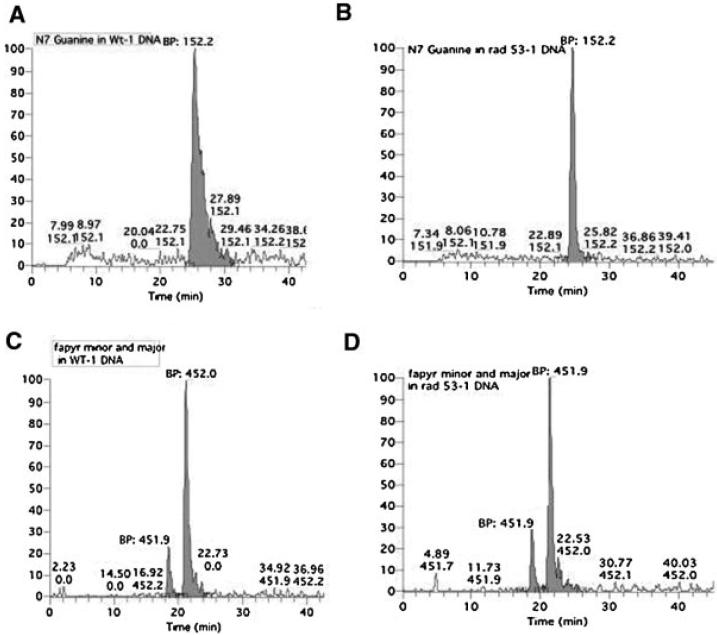
We speculate that the AFB1-DNA adducts, such as the N7-Gua and FAPY-AFB1 adducts, stall DNA replication. We therefore set out to detect AFB1-DNA adducts in both wild type and checkpoint mutants [20]. Log phase cultures of yeast expressing human CYP1A2 were exposed to 50 μM AFB1 for 4 h. DNA was then isolated from two independent samples of yeast cells, and subjected to acid hydrolysis. Both the AFB1-N7-Gua and AFB1-FAPY adducts were identified and measured by LC-ESI/MS/MS (Figure 4). The data indicate that the level of AFB1 adducts are essentially the same for both the wild type and the rad53 mutant (Table 2). We deduced the number of adducts to be ~1-2 for every 1000 kb. Thus, for each yeast genome equivalent, ~15-30 AFB1 adducts are generated under these conditions.
Figure 4.
N7-Gua AFB1 and FAPY AFB1 adducts present in wild type (YB163) and rad53 (YB230) strains. AFB1 adducts were identified by LC-ESI/MS/MS. Relative abundance is plotted against time of detection; adduct quantities were computed from shaded areas under base peaks. DNA from both wild type (A,C) and rad53 (B,D) strains were obtained after cells were exposed to 50 μM AFB1 for 4 h. Base peak (BP) m/z 152.2 (A,B) corresponds with AFB1, while m/z 452 (C,D) corresponds with major and minor FAPY-AFB1 adducts.
Table 2.
Concentrations of AFB1-DNA Adducts in Yeast DNA
| Yeast genotype (strain)a | AFB1-N7-Gua adducts/ mg DNA1b (nmol) |
FAPY adducts/mg DNAc (nmol) |
Ratiod |
|---|---|---|---|
| Wild type (YB163) | 4 × 10-3 | 1.1 × 10-3 | 4:1 |
| rad53 (YB320) | 4.5 × 10-3 | 2.3 × 10-3 | 2:1 |
Relevant genotype respect to RAD53, both strains derived from S288c. See Table 1 for complete genotype.
AFB1-N7 Gua, 8,9-dihydro-8-(N7-guanyl)-9-hydroxyaflatoxin B1; n = 2.
FAPY, formamidopyrimidine; FAPY determined for one sample of each genotype.
Ratio = major AFB1-N7-Gua adduct: FAPY adduct.
DISCUSSION
AFB1 is a strong recombinagen but weak mutagen in yeast [8,18]. Higher AFB1-associated mutation frequencies in a haploid rad51 mutant, compared to wild type [7], suggest that a common DNA lesion could stimulate either SCE or mutation. AFB1 exposure stimulates SCE in lymphocytes and rat hepatoma lines (for review, see [26]); however, no similar studies have been done in yeast. Since AFB1-associated mutation requires MEC1 [7] and RNR induction results from AFB1 exposure [6], we determined whether AFB1-associated genotoxicity requires the MEC1 (ATR), RAD53, DUN1-mediated pathway involved in increasing dNTP levels after DNA damage exposure. The major conclusion of this article is that both recombination and mutation require MEC1, RAD53, and DUN1; however, higher dNTP levels per se do not circumvent the DUN1 requirement, indicating that other checkpoint pathway functions, such as stabilizing replication forks, may be required for AFB1-associated genotoxic effects. We speculate that AFB1-associated mutation and SCE occur when the DNA replication apparatus encounters AFB1-DNA adducts.
The studies thus extended previous work indicating that MEC1 and RAD53 are required for mutagenesis [7] by showing that SCE and mutagenesis are jointly regulated. We observed that AFB1-associated Rad53 activation correlated with higher frequencies of SCE, and detected AFB1-N7-Gua and FAPY DNA adducts that have been previously suggested to impede DNA replication [6]. Indeed although AFB1-N7-guanine adducts are unstable, they were more abundant than FAPY DNA adducts after 4 h AFB1 exposure, indicating that the former is present in growing cells. The protracted presence of these adducts, which are poor templates for DNA polymerases [6] may explain why cells exposed to AFB1 exhibit elongated S phases [9]. The AFB1-associated Rad53 activation could also result from stalled replication forks [27]. We do not know, however, whether recombination precedes mutation, as suggested by observations that Rev1 (error-prone polymerase) accumulates during late S and G2 phases of the cell-cycle [28,29]. Further studies are necessary to determine whether defects in translesion synthesis lead to more AFB1-associated SCE events.
The DUN1-mediated checkpoint pathway for both AFB1 mutation and recombination is not required for UV-associated mutation and SCE [14], but is required for mutagenesis in DNA replication mutants [30]. One possibility is that translesion polymerases bypass UV lesions, such as thymidine dimers [31], more easily compared to AFB1-associated DNA lesions. We speculate that the DUN1 requirement for AFB1-associated events result from DUN1 function in stabilizing DNA replication forks at replication blocks [25]. DUN1 may be involved in repressing translation of RAD5 RNA transcripts, and RAD5 overexpression sensitizes cells to replication blocks [32]. RAD5 is also required for template switching events, indicating a possible function at stalled replication forks [33]. Further experiments are necessary to determine whether AFB1 specific adducts stall DNA polymerase.
The genetic requirements of AFB1-associated SCE are distinct from those for X-ray associated SCE, which is DUN1-independent [14], suggesting that the initiating lesion is not a double-strand break. Standard protocols to measure X-ray associated SCE use log-phase cells incubated in H2O (nutrient-depleted media), not nutrient medium, during the radiation exposure. These observations strengthen the idea that AFB1-associated SCE occurs as a consequence of the DNA replication fork encountering the AFB1-associated DNA lesion.
Although other types of AFB1-associated genotoxic events, such as translocations, require MEC1 [8], we do not know whether the AFB1-DNA adducts that initiate SCE are the same as those that initiate translocations and gene conversion events [8,18]. Fifteen DNA repair genes were induced in cells exposed to AFB1 in phosphate buffer [8], while only RAD51 and RAD54 were induced in cells exposed in nutrient media [9]. It will be important to determine whether different genotoxic events following AFB1 exposure are the consequence of the induction of different DNA repair genes or of different DNA adducts. Further experiments are also necessary to determine whether other AFB1-associated recombination events require RAD53 and DUN1.
In conclusion, we have found that both AFB1-associated recombination and mutation require MEC1, RAD53, and DUN1 and coincide with Rad53 activation. Although AFB1-DNA adducts have been measured in yeast [34], this is the first study to positively identify specific AFB1-DNA adducts in yeast, and indicate that the unstable AFB1-N7-Gua can be detected after a 4 h exposure time. Future studies may reveal how individual adducts are repaired and contribute to AFB1-associated genotoxic effects in yeast. Our results may have some interesting implications for AFB1-associated genotoxicity in humans. For example, liver injury, resulting in increased liver cell proliferation, correlates with a higher incidence of HCC. We speculate that the DNA replication in the presence of AFB1-DNA adducts may correspond to a higher level of AFB1-associated genotoxicity in mammalian cells.
ACKNOWLEDGMENTS
We thank Wei Xiao for pWJ730 containing the DUN1 plasmid. This work was supported by grants ES015954 and PO1ES006052 from the National Institutes of Health.
Abbreviations
- HCC
hepatocellular carcinoma
- AFB1
aflatoxin B1
- AFB1-N7-Gua
8,9-dihydro-8-(N7-guanyl)-9-hydroxyaflatoxin B1
- FAPY
formamidopyrimidine
- RNR
ribonucleotide reductase
- ATR
ataxia telangiectasia and Rad3 related
- SCE
sister chromatid exchange
- CYP1A2
cytochrome P450 1A2
- LC-ESI/MS/MS
liquid chromatography-electrospray ionization tandem mass spectrometry
- dNTP
deoxynucleotide triphosphate
REFERENCES
- 1.McGlynn KA, London WT. Epidemiology and natural history of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2005;19:3–23. doi: 10.1016/j.bpg.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Hsu IC, Metcalf RA, Sun T, Welsh JA, Wang NJ, Harris CC. Mutational hotspot in the p53 gene in human hepatocellular carcinomas. Nature. 1991;350:427–428. doi: 10.1038/350427a0. [DOI] [PubMed] [Google Scholar]
- 3.Shen HM, Ong CN. Mutations of the p53 tumor suppressor gene and ras oncogenes in aflatoxin hepatocarcinogenesis. Mutat Res Rev Genet Toxicol. 1996;366:23–44. doi: 10.1016/s0165-1110(96)90005-6. [DOI] [PubMed] [Google Scholar]
- 4.Wogan GN. Aflatoxin as a human carcinogen. Hepatology. 1999;2:573–575. doi: 10.1002/hep.510300231. [DOI] [PubMed] [Google Scholar]
- 5.Kew MC. Synergistic interaction between aflatoxin B1 and hepatitis B virus in hepatocarcinogenesis. Liver Int. 2003;23:405–409. doi: 10.1111/j.1478-3231.2003.00869.x. [DOI] [PubMed] [Google Scholar]
- 6.Smela ME, Hamm ML, Henderson PT, Harris CM, Harris TM, Essigmann JM. The aflatoxin B(1) formamidopyrimidine adduct plays a major role in causing the types of mutations observed in human hepatocellular carcinoma. Proc Natl Acad Sci USA. 2002;99:6655–6660. doi: 10.1073/pnas.102167699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo Y, Breeden LL, Zarbl H, Preston BD, Eaton DL. Expression of a human cytochrome p450 in yeast permits analysis of pathways for response to and repair of aflatoxin-induced DNA damage. Mol Cell Biol. 2005;25:5823–5833. doi: 10.1128/MCB.25.14.5823-5833.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keller-Seitz M, Certa U, Sengstag C, Wurgler F, Sun M, Fasullo M. Transcriptional response of the yeast to the carcinogen Aflatoxin B1:Recombinational repair involving RAD51 and RAD1. Mol Biol Cell. 2004;15:4321–4336. doi: 10.1091/mbc.E04-05-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo Y, Breeden LL, Fan W, Zhao LP, Eaton DL, Zarbl H. Analysis of cellular responses to aflatoxin B(1) in yeast expressing human cytochrome P450 1A2 using cDNA microarrays. Mutat Res. 2006;593:121–142. doi: 10.1016/j.mrfmmm.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Santocanale C, Diffley JF. A Mec1- and Rad53-dependent checkpoint controls late-firing origins of DNA replication. Nature. 1998;395:615–618. doi: 10.1038/27001. [DOI] [PubMed] [Google Scholar]
- 11.Huang M, Zhou Z, Elledge SJ. The DNA replication and damage checkpoint pathways induce transcription by inhibition of the Crt1 repressor. Cell. 1998;94:595–605. doi: 10.1016/s0092-8674(00)81601-3. [DOI] [PubMed] [Google Scholar]
- 12.Zhao X, Rothstein R. The Dun1 checkpoint kinase phosphorylates and regulates the ribonucleotide reductase inhibitor Sml1. Proc Natl Acad Sci USA. 2002;99:3746–3751. doi: 10.1073/pnas.062502299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fasullo M, Sun M, Dong Z. Saccharomyces cerevisiae RAD53 (CHK2) but not CHK1 is required for double-strand break-initiated SCE and DNA damage-associated SCE after exposure to X rays and chemical agents. DNA Repair. 2005;4:1240–1251. doi: 10.1016/j.dnarep.2005.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fasullo MT, Koudelik J, AhChing P, Giallanza P, Cera C. Radiosensitive and mitotic recombination phenotypes of the Saccharomyces cerevisiae dun1 mutant defective in DNA damage-inducible gene expression. Genetics. 1999;152:909–919. doi: 10.1093/genetics/152.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fasullo MT, Davis RW. Recombination substrates designed to study recombination between unique and repetitive sequences in vivo. Proc Natl Acad Sci USA. 1987;84:6215–6219. doi: 10.1073/pnas.84.17.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothstein R. Targeting, disruption, replacement, and allele rescue: Integrative DNA transformation in yeast. Methods Enzymol. 1991;194:281–301. doi: 10.1016/0076-6879(91)94022-5. [DOI] [PubMed] [Google Scholar]
- 17.Dong Z, Fasullo M. Multiple recombination pathways for sister chromatid exchange in Saccharomyces cerevisiae: Role of RAD1 and the RAD52 epistasis group genes. Nucleic Acids Res. 2003;31:2576–2585. doi: 10.1093/nar/gkg352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sengstag C, Weibel B, Fasullo M. Genotoxicity of aflatoxin B1: Evidence for a recombination-mediated mechanism in Saccharomyces cerevisiae. Cancer Res. 1996;56:5457–5465. [PMC free article] [PubMed] [Google Scholar]
- 19.Burke D, Dawson D, Stearns T. A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor Press; New York: 2000. Methods in yeast genetics. [Google Scholar]
- 20.Egner PA, Groopman JD, Wang JS, Kensler TW, Friesen MD. Quantification of aflatoxin-B1-N7-Guanine in human urine by high-performance liquid chromatography and isotope dilution tandem mass spectrometry. Chem Res Toxicol. 2006;19:1191–1195. doi: 10.1021/tx060108d. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman CS, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 22.Foiani M, Marini F, Gamba D, Lucchini G, Plevani P. The B subunit of the DNA polymerase alpha-primase complex in Saccharomyces cerevisiae executes an essential function at the initial stage of DNA replication. Mol Cell Biol. 1994;14:923–933. doi: 10.1128/mcb.14.2.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fasullo MT, Giallanza P, Bennett T, Cera C, Dong Z. Saccharomyces cerevisiae rad51 mutants are defective in DNA damage-stimulated sister chromatid exchange but exhibit increase rates of homology-directed translocations. Genetics. 2001;158:959–972. doi: 10.1093/genetics/158.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navas TA, Sanchez Y, Elledge SJ. RAD9 and DNA polymerase epsilon form parallel sensory branches for transducing the DNA damage checkpoint signal in Saccharomyces cerevisiae. Genes Dev. 1996;10:2632–2643. doi: 10.1101/gad.10.20.2632. [DOI] [PubMed] [Google Scholar]
- 25.Schollaert KL, Poisson JM, Searle JS, Schwanekamp JA, Tomlinson CR, Sanchez Y. A role for Saccharomyces cerevisiae Chk1p in the response to replication blocks. Mol Biol Cell. 2004;15:4051–4063. doi: 10.1091/mbc.E03-11-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang JS, Groopman JD. DNA damage by mycotoxins. Mutat Res. 1999;424:167–181. doi: 10.1016/s0027-5107(99)00017-2. [DOI] [PubMed] [Google Scholar]
- 27.Alcasabas AA, Osborn AJ, Bachant J, et al. Mrc1 transduces signals of DNA replication stress to activate Rad53. Nat Cell Biol. 2001;3:958–965. doi: 10.1038/ncb1101-958. [DOI] [PubMed] [Google Scholar]
- 28.Waters LS, Walker GC. The critical mutagenic translesion DNA polymerase Rev1 is highly expressed during G(2)/M phase rather than S phase. Proc Natl Acad Sci USA. 2006;103:8971–8976. doi: 10.1073/pnas.0510167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabbioneda S, Bortolomai I, Giannattasio M, Plevani P, Muzi-Falconi M. Yeast Rev1 is cell cycle regulated, phosphorylated in response to DNA damage and its binding to chromosomes is dependent upon MEC1. DNA Repair (Amst) 2007;6:121–127. doi: 10.1016/j.dnarep.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Datta A, Schmeits JL, Amin NS, Lau PJ, Myung K, Kolodner RD. Checkpoint-dependent activation of mutagenic repair in Saccharomyces cerevisiae pol3-01 mutants. Mol Cell. 2000;6:593–603. doi: 10.1016/s1097-2765(00)00058-7. [DOI] [PubMed] [Google Scholar]
- 31.Johnson RE, Prakash L, Prakash S. Distinct mechanisms of cissyn thymine dimer bypass by Dpo4 and DNA polymerase eta. Proc Natl Acad Sci USA. 2005;102:12359–12364. doi: 10.1073/pnas.0504380102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammet A, Pike B, Heierhorst J. Postranscriptional regulation of the RAD5 DNA Repair gene by the Dun1 kinase and the Pan2-Pan3 Poly(A)-Nuclease complex contributes to survival of replication blocks. J Biol Chem. 2002;277:22469–22474. doi: 10.1074/jbc.M202473200. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Lawrence CW. The error-free component of the RAD6/RAD18 DNA damage tolerance pathway of budding yeast employs sister-strand recombination. Proc Natl Acad Sci USA. 2005;102:15954–15959. doi: 10.1073/pnas.0504586102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelkonen P, Lang MA, Negishi M, Wild CP, Juvonen RO. Interaction of aflatoxin B1 with cytochrome P450 2A5 and its mutants: Correlation with metabolic activation and toxicity. Chem Res Toxicol. 1997;10:85–90. doi: 10.1021/tx960078m. [DOI] [PubMed] [Google Scholar]