Abstract
OBJECTIVE
To determine whether any acute effects on evaporative parameters are produced when using a solution containing Hydroxypropyl (HP) (Systane) versus normal saline solution in the eyes of patients with Keratoconjunctivis Sicca (KCS) at 30 and 60 minutes post instillation.
METHODS
Randomized double-blinded placebo-control 2-period cross-over clinical trial. Twelve patients with a clinical diagnosis of KCS were enrolled in this study. Aqueous tear evaporation was measured at baseline, i.e., before the application of drops on the eye, and at 30 and 60 minutes after instillation of one 40μl drop of either the HP-Guar containing drop or normal saline on two separate days. Statistical analysis included descriptive data analysis and paired t test.
RESULTS
HP-Guar resulted in a decrease in aqueous tear evaporation at 30 minutes post instillation under 25–35% relative humidity (RH) (13.2% reduction, p =0.044) and 35–45% RH (10% reduction, p=0.028) conditions. The effect of HP-Guar at 60 minutes post instillation also decreased aqueous tear evaporation but to a lesser degree. Normal saline solution produced no statistically significant increases and decreases of evaporation.
CONCLUSIONS
Aqueous tear evaporation contributes significantly to aqueous tear loss and is humidity dependent. An HP-guar containing solution decreased aqueous tear evaporation 30 and 60 minutes after application. The use of topical medication with known antievaporative effect may be beneficial in dry eye therapy. This effect may also be achieved in normal eyes or sub-clinical dry eyes when in low RH environments.
Keywords: tear evaporation, dry eye, Systane, HP-Guar
INTRODUCTION
Ocular surface tear film evaporation studies provide an opportunity to assess the role of environmental conditions such as low ambient relative humidity (RH), wind, closed working environments, and exposures to occupational and environmental pollutants. These types of laboratory studies indicate that lower RH environments cause an increased rate of evaporation of the ocular surface tear film in both dry eye patients (1) and normal subjects without clinical evidence of dry eyes (2). In a laboratory setting it is possible to more precisely control RH compared to such insults as air movements or pollutant exposures. Since all of these situations have the common feature of increasing ocular surface tear film evaporation, laboratory studies using RH as a stimulus for evaporation serve as good models for studies of ocular surface evaporation and evaluations of the impacts of therapeutic interventions.
These and other types of studies have demonstrated that currently available artificial tear preparations have variable effects on a number of parameters including temporary correction of ocular surface irregularities, contrast sensitivity, glare, and wave-front aberrations seen in symptomatic dry eye subjects (3). In general, the addition of a drop of an artificial tear preparation to the ocular surface of a dry eye thickens the aqueous layer (4). This effect may acutely extend the tear breakup time up to 30 minutes but has no lasting effect (5,6). Others have also shown a decrease in evaporation after 30 days use of an oil-in water emulsion (7).
In this study, we have tested the acute effect of a single drop of a commercial preparation (Systane, Alcon Lab, Fort Worth, Texas) in subjects with the clinical diagnosis of dry eyes. This preparation contains hydroxypropyl (HP) guar, two active demulcents: polyethylene glycol and propylene glycol, and a non-classical preservative: an ionic buffer system based on Borate, AMP, Sorbitol, and Zinc that becomes inactive when in contact with ions present in tears.
In previous in vitro and in vivo studies, Hp-Guar has showed to be beneficial in the treatment of dry eyes by increasing the tear break up time and protecting the cornea from desiccation (5,8,9).
The purpose of this study was to determine whether any acute effect on evaporative parameters are produced when using a solution containing HP-Guar versus normal saline solution in the eyes of patients with Keratoconjunctivis Sicca (KCS) at 30 and 60 minutes post instillation.
MATERIALS AND METHODS
This study was a randomized double-blinded placebo-control 2-period cross-over clinical drug study to determine the effect of single topical applications of HP-Guar in patients with dry eyes. The effect was measured using the determination of tear evaporative parameters in a laboratory setting where RH could be controlled and varied.
The protocol, consent forms, and data accumulation methods used in these studies were previously approved by the University of Texas Southwestern Medical Center Institutional Review Board. Informed consent was obtained from each patient at screening. HIPAA regulations were followed.
Eligibility requirements for the KCS patients included a prior diagnosis of dry eye with symptoms of foreign body sensation or dryness combined with the findings of interpalpebral fissure conjunctival vital dye staining along with a decreased tear film meniscus on slit-lamp biomicroscopy. Patients were excluded if either eye had clinically evident lid or ocular surface inflammation. None of these patients had active systemic disease or were taking medications that could affect tear production. Subjects were excluded if they were participating in any other investigational therapeutic drug or device trial at the time of enrollment or within the previous 30 days. The enrollees were, however, using a variety of tear replacement topical medications prior to the first studies and in the interval between the studies.
Twelve patients, nine women and three men, were enrolled, ages 51 to 83. They were randomly assigned to either treatment sequence: 1 (Saline then Hp-Guar) or 2 (Hp-Guar and then saline at the second study visit). We evaluated the acute effect of single drop applications of each solution on ocular surface evaporation. There was a 2–14 day interval between 1st and 2nd visits. Evaporative rates were measured at baseline with each test agent, i.e., before the application of any test drop on the eye, and also 30 and 60 minutes after the instillation of one 40μl drop of HP-Guar or saline solution.
Following enrollment and in between the study visits, patients were instructed to continue using their usual dry eye therapy except to stop on the day of the evaluation.
Tear evaporation studies were conducted with an evaporometer (Oxdata, Portland, Oregon) that utilized a pump to direct room air through a drying tube into a form-fitting goggle that created a closed environment and contained a humidity/temperature sensor which has been described in detail elsewhere (10) Dry air was pumped into the goggle to reduce RH to 15%, at which time the pump was turned off. The RH within the goggle was allowed to rise. The increase in humidity due to evaporation from skin or evaporating tears was measured. Room temperature was maintained in the range of 22–24 °C. The process was carried out first with the eye closed and then with it open; the difference between these two processes determined the ocular surface tear evaporation rate (11). Using the original formula published by Rolando and Refojo,(12) we calculated the evaporative rates under two different ranges of increasing RH, 25% to 35% and 35% to 45%. The area of the interpalpebral ocular surface was used to calculate evaporation per unit area; the image of the area was captured with the use of a digital camera, and the area was calculated directly with the aid of computer software (Adobe Photoshop, version 6.0.1.2001; Adobe Systems, San Jose, California), (11) expressed as μL/cm2/min. This same sequence of testing was carried out on the day of second testing using a single drop of the opposite test agent.
The data were analyzed in several ways. Within-patient, before and after test agent instillation comparisons in different humidity conditions were conducted using one-sided paired t-tests. Only data from the left eye was used in the calculations. Statistical significance was set to 95% confidence level for all tests. The potential of a therapeutic carry-over effect from the first treatment on the second treatment was evaluated by comparisons between the two different sequence groups using a 2-sided Wilcoxon rank test. Statistical computing was performed using SAS 9.1 (SAS Institute, Cary, NC).
RESULTS
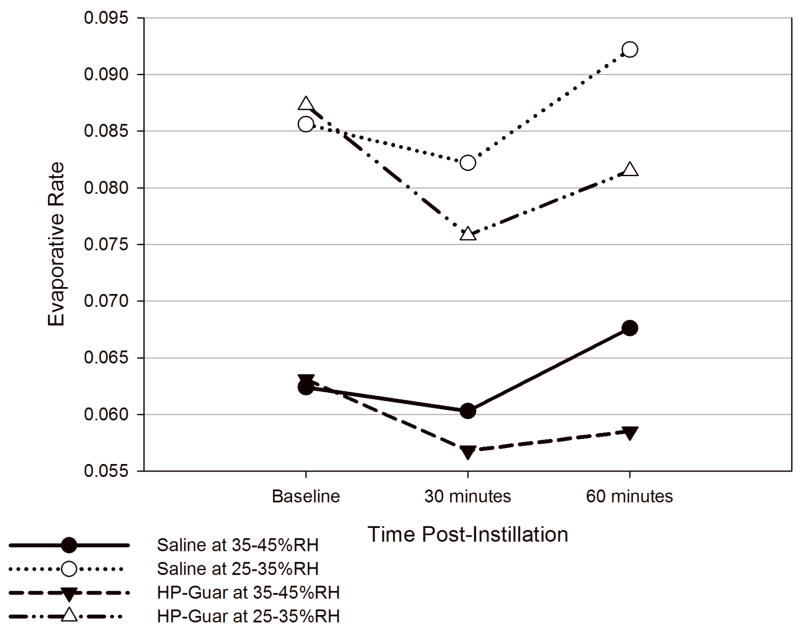
The Table and Figure show several pertinent points and the value of the use of evaporation rate studies utilizing RH as the stimulant. Regardless of which test agent is studied, the rate of evaporation is higher in lower RH environments. In the current studies, the change in RH significantly affected the evaporation rate. When the RH is decreased from 35–45% to 25–35% the average increase in evaporation rate is approximately 40%. Eyes studied following a single instillation of Hp-Guar had a statistically significantly reduction in mean ocular surface evaporation rate at 30 minutes post-instillation compared to that of pre-instillation under both humidity conditions (p = 0.028 and p = 0.023, respectively) (Table). In comparison, a single installation of saline solution did not produce statistically significant changes on the ocular surface evaporative rate after either 30 minutes or 60 minutes post instillation. The effect of HP-Guar 60 minutes post-instillation also showed a trend toward causing decreased evaporation rate but it did not reach statistical significance. Mean evaporation rates for each treatment group at different time points under different relative humidity conditions are presented in the Table.
Table.
Mean evaporative rates following single drop applications of two test agents
| Humidity | Baseline *† | 30 minutes *† | 60 minutes *† |
|---|---|---|---|
| 35–45% | |||
| Saline | 0.0624±0.0312 | 0.0603±0.0248 | 0.0675±0.0381 |
| HP-Guar | 0.0631±0.0332 | 0.0568±0.0330 | 0.0585±0.0255 |
| 25–35% | |||
| Saline | 0.0856±0.0374 | 0.0822±0.0299 | 0.0922±0.0498 |
| HP-Guar | 0.0873±0.0384 | 0.0758±0.0435 | 0.0815±0.0372 |
| 35–45% humidity | Baseline vs. 30 min | Saline: ↓ 3.3% (p = 0.357) | HP-Guar: ↓ 10% (p = 0.028) |
| Baseline vs. 60 min | Saline: ↑ 8.2% (p = 0.235) | HP-Guar: ↓ 7.3% (p = 0.192) | |
| 25–35% humidity | Baseline vs. 30 min | Saline: ↓ 3.9% (p = 0.333) | HP-Guar: ↓ 13.2% (p = 0.023) |
| Baseline vs. 60 min | Saline: ↑ 7.7% (p = 0.241) | HP-Guar: ↓ 6.6% (p = 0.178) | |
| %Changes = 100%(Rate at Post-instillation − Rate at Baseline)/Rate at Baseline | |||
Values expressed as mean ± SD
Units: μl/cm2/min
Figure. A comparison of evaporative rates for HP-Guar and saline measured in two different relative humidity ranges.
HP-Guar resulted in a decrease in aqueous tear evaporation at 30 minutes post instillation under 25–35%RH (13.2% reduction, p =0.044) and 35–45% RH (10% reduction, p=0.077) conditions. The effect of HP-Guar at 60 minutes post instillation decreased aqueous tear evaporation but to a lesser degree. Normal saline solution produced not statistically significant increases and decreases of evaporation.
At 35–45% humidity, the mean percentage reduction in evaporative rate from pre instillation in Hp-Guar-treated eyes was 10% at 30 minutes post-instillation and 7.3% at 60 minutes. In comparison, eyes treated with placebo showed a decrease of 3.3% and an increase of 8.3%, respectively, at 30 and 60 minutes post-instillation (Table).
When the evaporative rates were determined at 25–35% humidity, the mean percent reduction in HP-guar-treated eyes was 13.2% at 30 minutes and 6.6% at 60 minutes post-instillation. In contrast, the effect on the saline-treated eyes was similar to those obtained at 35–45% humidity. Thus, the application of a single drop of saline did not result in a stable change on the evaporative rates in either humidity condition. In contrast, a single drop application of Hp-Guar resulted in decreases in evaporative rates in either humidity condition.
Comparisons of the mean evaporative rates between the two treatments at 30 minutes at the lower RH was statistically significant (p = 0.044). Mean % changes from baseline to 30 minutes and 60 minutes post-instillation and the p-values of 1-sided paired t-test for within-patient comparisons between different treatments are presented in the Table.
No significant carry-over effect from the first study agent to the second was detected under either humidity condition (p = 0.351 for 25–35%, p = 0.434 for 35–45%).
DISCUSSION
These studies emphasize that laboratory assessment of tear evaporation rates using RH as a stimulus are useful for analyzing factors involved in dry eye pathogenesis and its treatment.
The increased evaporation rates in lower RH is an important component of aqueous tear loss in dry eye patients (13) and correlates with clinical impressions and supports the validity of these types of studies.
In a single day our tear films are exposed to different ranges of RH, some of which are extremely low, such as, airplanes, deserts, office buildings, etc. An increasing number of people, especially those with a history of dry eye, become rapidly symptomatic when exposed to these environments.
It is important to not assume that all the currently available commercial artificial tear preparations are adequate to protect against tear film evaporation.
The use of an acute type study with a single application of a test agent provides a novel way to gain insight into the potential suitability of an artificial tear solution for sustained application. Although not a component of these experiments, the results suggest that it would be possible to determine the ideal interval between applications that would minimize the damage associated with ocular surface drying with a particular agent.
In comparison to physiologic saline, a single application of HP-Guar solution decreased the tear evaporation rates in the two tested ranges of RH environments for at least 30 minutes. These results suggest that HP-Guar has attributes that improve the integrity of the ocular surface tear film in a manner that lessens the rate of evaporation. Assuming that tear drainage remains constant in all test eyes regardless of test agent, reduction in evaporation rate is achieved with the HP-Guar single applications. By analogy, it would be expected that this effect would also be achieved in normal eyes or sub clinical dry eyes when in low RH environments.
Acknowledgments
This study was supported in part by grants NIH EY12430, EY016664, a grant from Alcon Laboratories, Inc., Fort Worth, TX, and an unrestricted grant from the Research to Prevent Blindness, New York, NY
The authors acknowledge Ms. Jenny Song, MD, MS, CNWC, from HealthCruiser, Fort Worth, for her statistical analyses.
References
- 1.McCulley JP, Aronowicz JD, Uchiyama E, et al. Correlations in a change in aqueous tear evaporation with a change in relative humidity and the impact. Am J Ophthalmol. 2006;141:758–760. doi: 10.1016/j.ajo.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 2.Uchiyama E, Aronowicz JD, Butovich IA, et al. Increased evaporative rates in laboratory testing conditions simulating airplane cabin relative humidity: an important factor for dry eye syndrome. Eye Contact Lens. 2007;33:174–176. doi: 10.1097/01.icl.0000252881.04636.5e. [DOI] [PubMed] [Google Scholar]
- 3.Nilforoushan MR, Latkany RA, Speaker MG. Effect of artificial tears on visual acuity. Am J Ophthalmol. 2005;140:830–835. doi: 10.1016/j.ajo.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Korb DR, Scaffidi RC, Greiner JV, et al. The effect of two novel lubricant eye drops on tear film lipid layer thickness in subjects with dry eye symptoms. Optom Vis Sci. 2005;82:594–601. doi: 10.1097/01.opx.0000171818.01353.8c. [DOI] [PubMed] [Google Scholar]
- 5.Christensen MT, Cohen S, Rinehart J, et al. Clinical evaluation of an HP-guar gellable lubricant eye drop for the relief of dryness of the eye. Curr Eye Res. 2004;28:55–62. doi: 10.1076/ceyr.28.1.55.23495. [DOI] [PubMed] [Google Scholar]
- 6.Gifford P, Evans BJ, Morris J. A clinical evaluation of Systane. Cont Lens Anterior Eye. 2006;29:31–40. doi: 10.1016/j.clae.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Khanal S, Tomlinson A, Pearce EI, et al. Effect of an oil-in-water emulsion on the tear physiology of patients with mild to moderate dry eye. Cornea. 2007;26:175–181. doi: 10.1097/ICO.0b013e31802b492d. [DOI] [PubMed] [Google Scholar]
- 8.Ubels JL, Clousing DP, Van Haitsma TA, et al. Pre-clinical investigation of the efficacy of an artificial tear solution containing hydroxypropyl-guar as a gelling agent. Curr Eye Res. 2004;28:437–44. doi: 10.1080/02713680490503787. [DOI] [PubMed] [Google Scholar]
- 9.Ousler GW, Michaelson C, Christensen MT. An evaluation of tear film breakup time extension and ocular protection index scores among three marketed lubricant eye drops. Cornea. 2007 Sep;26:949–52. doi: 10.1097/ICO.0b013e3180de1c38. [DOI] [PubMed] [Google Scholar]
- 10.Mathers WD, Binarao G, Petroll M. Ocular water evaporation and the dry eye. A new measuring device. Cornea. 1993;12:335–340. doi: 10.1097/00003226-199307000-00010. [DOI] [PubMed] [Google Scholar]
- 11.McCulley JP, Shine WE, Aronowicz J, et al. Presumed hyposecretory/hyperevaporative KCS: tear characteristics. Trans Am Ophthalmol Soc. 2003;101:141–152. [PMC free article] [PubMed] [Google Scholar]
- 12.Rolando M, Refojo MF. Tear evaporimeter for measuring water evaporation rate from tear film under controlled conditions in humans. Exp Eye Res. 1983;36:25–33. doi: 10.1016/0014-4835(83)90086-6. [DOI] [PubMed] [Google Scholar]
- 13.McCulley JP, Uchiyama E, Aronowicz JD, et al. Impact of evaporation on aqueous tear loss. Trans Am Ophthalmol Soc. 2006;104:121–128. [PMC free article] [PubMed] [Google Scholar]