Abstract
The phosphatidylinositol 3-kinase subunit PIK3CA is frequently mutated in human cancers. Here we used gene targeting to “knock in” PIK3CA mutations into human breast epithelial cells to identify new therapeutic targets associated with oncogenic PIK3CA. Mutant PIK3CA knockin cells were capable of epidermal growth factor and mTOR-independent cell proliferation that was associated with AKT, ERK, and GSK3β phosphorylation. Paradoxically, the GSK3β inhibitors lithium chloride and SB216763 selectively decreased the proliferation of human breast and colorectal cancer cell lines with oncogenic PIK3CA mutations and led to a decrease in the GSK3β target gene CYCLIN D1. Oral treatment with lithium preferentially inhibited the growth of nude mouse xenografts of HCT-116 colon cancer cells with mutant PIK3CA compared with isogenic HCT-116 knockout cells containing only wild-type PIK3CA. Our findings suggest GSK3β is an important effector of mutant PIK3CA, and that lithium, an FDA-approved therapy for bipolar disorders, has selective antineoplastic properties against cancers that harbor these mutations.
Keywords: GSK3β, lithium, mTOR, phosphatidylinositol 3-kinase, cancer
An attractive target for cancer therapy is the phosphatidylinositol 3-kinase (PI3K) enzyme, as its activity is often dysregulated in many human malignancies. PI3K, along with other key downstream effectors, such as AKT1 (AKT) and mammalian target of rapamycin (mTOR), have been the subject of therapeutic exploitation in many studies. However, cell signaling through the PI3K-AKT-mTOR pathway occurs in many normal tissues as well as other disease states. Thus, the development of inhibitors that are selective for cancer cells with a tolerable side effect profile has remained difficult.
Recently the p110α catalytic subunit of PI3K, PIK3CA, has been shown to be frequently mutated in a number of different human cancers (1, 2). Notably, 3 recurrent oncogenic “hotspot” mutations comprise the majority of somatic PIK3CA mutations. Two of these mutations, E542K and E545K, occur in the helical domain found in exon 9, and the third mutation, H1047R, affects the kinase domain located within exon 20 (3). Although many studies have implicated PIK3CA mutations with features of transformation (4–6), definitive mechanisms describing how these mutations lead to increased cell growth and proliferation have not been fully elucidated. Knowledge of these molecular mediators would greatly assist in the development of inhibitors capable of specifically targeting cancer cells that carry oncogenic PIK3CA mutations.
Although the biological phenotypes of mutant PIK3CA in various cellular systems have been demonstrated, we have recently shown that overexpression of mutant oncogenes via transgene methods may not accurately represent the biologic changes seen when mutant alleles are expressed under the control of a gene's native promoter (7). Thus, similar to knockin mice, we have used gene targeting to knock in pathogenic mutations into human cell lines to further elucidate their physiologic consequences and to validate these mutated genes as bona fide targets for cancer therapy. In the present study, knockin of oncogenic PIK3CA mutations was performed using the nontumorigenic human breast epithelial cell line MCF-10A. Our findings reveal that mutant PIK3CA alters several distinct downstream effectors, and that in addition to AKT/mTOR, other signaling pathways contribute to mutant PIK3CA-mediated cellular proliferation, including increased phosphorylation of GSK3β. Surprisingly, pharmacologic modulation of GSK3β with lithium, an FDA-approved drug for the treatment of bipolar disorder, led to selective growth inhibition of cells harboring mutant PIK3CA. Thus, our study validates the use of human isogenic cell lines for target identification and drug discovery, and suggests the need for targeting several key growth-promoting pathways for optimal antineoplastic therapy against human cancers containing oncogenic PIK3CA.
Results
Gene Targeting of Oncogenic PIK3CA Leads to Growth Factor Independence.
To create models of human epithelial cells harboring PIK3CA mutations expressed in a physiologic manner, we used gene targeting in the nontumorigenic human breast epithelial cell line MCF-10A as previously described (7, 8). Two targeting vectors were created: one designed to introduce the E545K mutation, and the other for knocking in the H1047R mutation (supporting information (SI) Fig. S1). Three E545K and 2 H1047R clones were independently isolated. Further characterization of these clones is described in SI Methods.
Removal of epidermal growth factor (EGF) from the culture medium results in G1 arrest of MCF-10A cells, and the ability of these cells to proliferate in the absence of this growth factor is often associated with an increased malignant potential (9). Cell proliferation assays showed that mutant PIK3CA knockin cells, but not their control counterparts or parental cells, were capable of growth in media without EGF similar to previous studies with forced mutant PIK3CA overexpression (Fig. S2) (5). However, other features of transformation seen with overexpression of mutant PIK3CA, such as abnormal acini formation in Matrigel (5), were not present (Fig. S3). In addition, mutant PIK3CA knockin cells did not form colonies in soft agar or tumors in nude mouse xenograft assays (data not shown). Collectively these studies suggest that mutant PIK3CA imparts some features of transformation but is not sufficient for tumorigenicity.
Oncogenic PIK3CA and mTOR Activation.
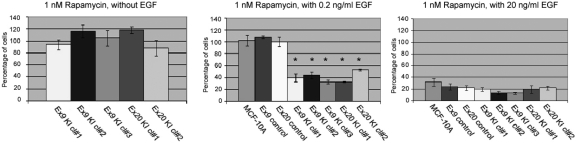
To identify and validate molecular targets of therapy, we used our knockin cell lines to analyze pathways previously implicated in PI3K-AKT-mTOR signal transduction. The dependence of MCF-10A on EGF for cell proliferation allows for a clean analysis of the molecular pathways involved with oncogene-induced cell proliferation, because removal of EGF from the culture medium effectively ablates signaling through the epidermal growth factor receptor (EGFR). We therefore initially evaluated mutant PIK3CA knockin cells for their response to the mTOR pathway inhibitor rapamycin as cell growth mediated by mutant PIK3CA is thought to be regulated partially through activation of the AKT-mTOR pathway (4, 10, 11). Unexpectedly, rapamycin at pharmacological doses of 1 nM had no effect on the proliferation of knockin cells in the absence of EGF, as depicted in Fig. 1. In contrast, the weakly selective PI3K inhibitors LY294002 and wortmannin were capable of inhibiting proliferation of mutant PIK3CA knockin cells in the absence of EGF similar to other studies (Fig. S4) (5, 12). The effect of rapamycin on the proliferation of control cell lines could not be determined because they do not propagate in the absence of EGF, as previously noted. For this reason, we evaluated the effects of rapamycin using EGF at a standard concentration of 20 ng/ml (13). As illustrated in Fig. 1, PIK3CA knockin clones, parental MCF-10A, and control cell lines showed equivalent sensitivity to rapamycin when grown in the presence of 20 ng/ml EGF. We then repeated these assays at 0.2 ng/ml, which represents a more physiologic concentration of EGF (14). These experiments showed a differential sensitivity toward rapamycin between PIK3CA knockin cell lines vs. control and parental cells (Fig. 1). However, the weakly selective PI3K inhibitors showed equal toxicity for both parental MCF-10A and mutant PIK3CA knockin cell lines at this concentration of EGF (Fig. S4).
Fig. 1.
Sensitivity of MCF-10A mutant PIK3CA knockin cell lines to rapamycin. Cell proliferation assays were performed with MCF-10A cells and/or their knockin derivatives. EGF and rapamycin concentrations are shown above each graph. Bars represent the percentage of cell proliferation relative to vehicle only (DMSO) controls. Error bars represent SEM from triplicate samples. *P < 0.0011 for all clones vs. control cell lines.
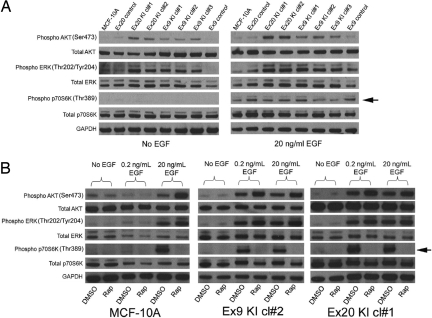
To uncover the mechanism of this effect, Western blotting was performed on PIK3CA mutant knockin cell lines and control cells. As shown in Fig. 2A, mutant PIK3CA knockin cells did not exhibit any appreciable mTOR activation in the absence of EGF as depicted by the lack of p70S6Kinase (S6K) phosphorylation (Thr-389), despite the cells clearly having the capacity to proliferate under these culture conditions. However, in the presence of 20 ng/ml EGF, MCF-10A cells, controls, and PIK3CA mutant knockin cells all exhibited mTOR activation. Western blotting confirmed that mutant PIK3CA knockin cells exhibited a reduced threshold for mTOR activation by EGF compared with parental and control clones, as seen by the increased S6K phosphorylation (Thr-389) only in the knockin cell lines when cultured in 0.2 ng/ml EGF (Fig. 2B and Fig. S5). Importantly, in all of these assays, sensitivity to rapamycin correlated with S6K phosphorylation (Thr-389), which was reversible upon addition of this mTOR inhibitor. Collectively, our findings suggest that oncogenic PIK3CA is neither necessary nor sufficient for mTOR activation. However, cells containing mutant PIK3CA do have a reduced threshold for mTOR activation by EGF as measured by S6K phosphorylation (Thr-389) and sensitivity to rapamycin. Moreover, these cells are capable of proliferation that is independent of EGF and mTOR activation, suggesting that cell growth and proliferation mediated by mutant PIK3CA involves one or more additional pathways that may need to be targeted for effective antineoplastic therapy.
Fig. 2.
Mutant PIK3CA is not sufficient for mTOR activation in MCF-10A cells. (A) Western blot illustrating levels of phosphorylated AKT (Ser-473), total AKT, phosphorylated ERK (Thr-202/Tyr-204), total ERK, phosphorylated p70S6K (Thr-389), and total p70S6K in mutant PIK3CA knockin cell lines and controls in the absence of EGF (Left) or presence of 20 ng/ml EGF (Right). Arrow denotes differences in p70S6Kinase (Thr-389) phosphorylation. GAPDH is shown as a loading control. Note that shorter exposure times were used for AKT phosphorylation (Ser-473) with 20 ng/ml EGF as described in the text. (B) Western blot illustrating levels of phosphorylated AKT (Ser-473), total AKT, phosphorylated ERK (Thr-202/Tyr-204), total ERK, phosphorylated p70S6K (Thr-389), and total p70S6K in MCF-10A parental cells and 2 mutant PIK3CA knockin cell lines in the presence of no EGF, 0.2 ng/ml EGF, and 20 ng/ml EGF in the presence (Rap) and absence (DMSO) of 1 nM rapamycin. Arrow denotes differences in p70S6Kinase (Thr-389) phosphorylation. GAPDH is shown as a loading control. Identical results were obtained for all PIK3CA knockin clones and controls. Note that shorter exposure times were used for AKT phosphorylation (Ser-473) for mutant PIK3CA knockin clones compared with parental and control cells as described in the text.
Oncogenic PIK3CA Leads to Phosphorylated ERK.
Although it has been established that Ras signaling via PIK3CA is necessary for Ras' full oncogenic function (15), mutant PIK3CA leading to MEK/ERK signaling has only recently been reported (5, 16), and the mechanism of this crosstalk has not been fully elucidated. To determine if knockin of oncogenic PIK3CA also exhibited increased ERK signaling, mutant PIK3CA knockin cell lines and control cells were subjected to Western blotting using total and phospho-specific anti-ERK antibodies. As shown in Fig. 2A (Left), in the absence of EGF, ERK was also phosphorylated (Thr-202/Tyr-204) in mutant PIK3CA knockin cell lines relative to control cells. However, upon exposure to 20 ng/ml EGF, control and parental cell lines showed approximately equal amounts of ERK phosphorylation (Thr-202/Tyr-204) relative to total ERK (Fig. 2A Right) when compared with mutant PIK3CA knockin cell lines. In addition, similar to S6K phosphorylation, mutant PIK3CA knockin clones have a relatively lower threshold for MAP kinase pathway activation by EGF, as seen by the increased ERK phosphorylation (Thr-202/Tyr-204) in the presence of 0.2 ng/ml EGF relative to control cell lines (Fig. 2B and Fig. S5). Accordingly, in the absence of EGF, mutant PIK3CA knockin cell lines were sensitive to the MEK inhibitor U0126, but in the presence of 0.2 ng/ml EGF, both parental MCF-10A cells and mutant PIK3CA knockin cells displayed sensitivity to this compound, similar to findings with PI3K inhibitors, although there was slight preferential toxicity for mutant PIK3CA knockin cell lines (Fig. S4).
Oncogenic PIK3CA Leads to Phosphorylated AKT and GSK3β.
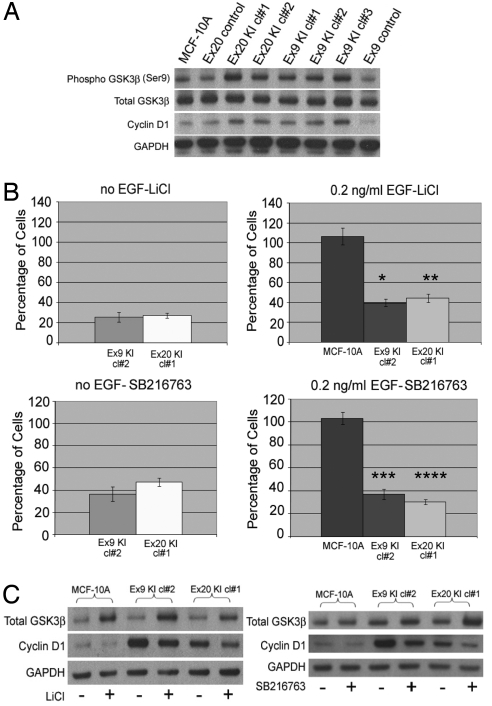
To further characterize mutant PIK3CA-mediated growth, we next analyzed levels of phosphorylated AKT. AKT is a substrate of PI3K, and mediates several distinct signaling pathways involved with cell proliferation and apoptosis (17). Phosphorylated AKT (Ser-473) was consistently elevated in PIK3CA mutant knockin clones relative to parental and control cells regardless of EGF concentration (Fig. 2A, Left vs. Right). Indeed, shorter exposure times were necessary when cells were treated with 20 ng/ml EGF compared with no EGF, to appreciate differences in AKT phosphorylation (Ser-473). In addition, shorter exposure times with mutant PIK3CA knockin clones were also necessary compared with parental and control clones, to fully appreciate the differences in increased AKT phosphorylation (Ser-473) using 0, 0.2, and 20 ng/ml EGF (Fig. 2B and Fig. S5). Thus, although baseline levels of phosphorylated AKT (Ser-473) were elevated in mutant PIK3CA knockin clones relative to parental and control cell lines, further AKT phosphorylation (Ser-473) was seen at relatively lower levels of EGF stimulation compared with parental and control cell lines, akin to the situation with ERK and S6K. Because AKT is itself a protein kinase, we next analyzed downstream targets of AKT to determine if differences in protein phosphorylation of these effectors could account for the increased cellular proliferation observed in mutant PIK3CA cells. For example, phosphorylation of the cyclin-dependent kinase inhibitors (CDKIs) p21 and p27 via AKT results in nuclear exclusion of these CDKIs, rendering them nonfunctional in mediating G1 arrest (18–21). However, Western blotting for phosphorylated p21 and p27 did not reveal any differences between mutant and wild-type PIK3CA-containing cell lines (data not shown). We next examined phosphorylation of GSK3β, a tumor suppressor in the WNT-APC signaling pathway, which has also been shown to be phosphorylated at serine 9 and functionally inhibited by AKT (22). As seen in Fig. 3A and Fig. S6, all mutant PIK3CA knockin clones exhibited increased GSK3β phosphorylation (Ser-9) relative to parental and control cells. This was accompanied by an increase in cyclin D1 levels, a gene known to be regulated by GSK3β (23).
Fig. 3.
Mutant PIK3CA leads to increased GSK3β phosphorylation and sensitizes MCF-10A human breast epithelial cells to lithium and SB216763. (A) Western blot illustrating levels of phosphorylated GSK3β (Ser-9), total GSK3β, and cyclin D1 in mutant PIK3CA knockin cell lines and controls cultured for 24 h in the absence of EGF. GAPDH is shown as a loading control. Results are representative of 3 independent experiments. (B) Cell proliferation assays were performed as described in Methods, with MCF-10A cells and their knockin derivatives grown in the absence of EGF (Left) or presence of 0.2 ng/ml EGF (Right). Bars represent the percentage of cell proliferation in 10 mM lithium chloride (LiCl) (Upper) or 10 μM SB216763 (Lower) relative to cells grown in control medium (water for LiCl, DMSO for SB216763) after 6 days in culture. Error bars represent SEM from triplicate samples. *P < 0.0001, **P < 0.0001, *** P < 0.0002, ****P < 0.0001 compared with parental MCF-10A cells. (C) Western blot illustrating levels of total GSK3β, cyclin D1, and GAPDH in MCF-10A parental cells and 2 mutant PIK3CA knockin cell lines in the presence (+) and absence (−) of 10 mM lithium chloride (LiCl) (Left), and in the presence (+) and absence (−) of 10 μM SB216763 (Right). Cells were cultured in 0.2 ng/ml EGF and harvested for cell lysates after 24 h.
Mutant PIK3CA Sensitizes Cells to Lithium in Vitro and in Vivo.
Our findings presented the intriguing possibility that mutant PIK3CA cells may be more sensitive to the effects of GSK3β inhibitors such as lithium chloride (24) and SB216763 (25). Although inhibition of GSK3β would theoretically lead to an increase in mitotic activity, previous studies have shown that lithium and other GSK3β inhibitors can in fact inhibit cell proliferation (26–28). Though this effect is paradoxical, it has been postulated that inhibition of GSK3β initially leads to cellular proliferation, but further GSK3β inhibition by lithium and SB216763 beyond a critical threshold results in an antiproliferative effect, as it has been demonstrated that complete inhibition of GSK3β is deleterious for cells because GSK3β knockout mice are embryonic lethal (29). Moreover, depending on the genetic context of the cell, recent reports suggest that GSK3β may have a pro-oncogenic effect in mixed lineage leukemias (MLL) leukemias (28). Thus, our rationale for testing lithium and SB216763 on our cell lines was based on these prior studies. Treatment with lithium chloride led to a decrease in cell proliferation in both EGF-free and EGF-containing medium (0.2 ng/ml) with high selectivity for mutant PIK3CA knockin cells (Fig. 3B). Control and parental cell lines grown in 0.2 ng/ml EGF proliferate at approximately the same rate as mutant PIK3CA knockin cells in the absence of EGF (Figs. S2 and S7). Thus, the antiproliferative effects of lithium are not simply the result of increased cell proliferation seen in mutant PIK3CA knockin clones. Although lithium is known to have other cellular effects, the small-molecule competitive ATP inhibitor SB216763 has been shown to be a highly specific GSK3 kinase inhibitor (25). Using a relatively lower concentration of SB216763 than previously reported in the literature, identical effects of decreased cell proliferation were found in mutant PIK3CA knockin cell lines but not control cell lines (Fig. 3B). Western blotting revealed that lithium and SB216763 led to an increase in total GSK3β, with a concomitant decrease in cyclin D1 (Fig. 3C), suggesting that in epithelial cells these compounds decrease cell proliferation via upregulating GSK3β rather than inhibiting its activity, an effect more consistent with the known role of GSK3β as a tumor suppressor. In accord with these findings, GSK3β phosphorylation (Ser-9) was decreased in mutant PIK3CA knockin clones treated with lithium and SB216763, although the kinetics of this decrease varied between both of these agents (data not shown). These findings suggest that lithium and SB216763 can selectively modulate GSK3β levels, resulting in decreased cell proliferation preferentially in cells with mutant PIK3CA.
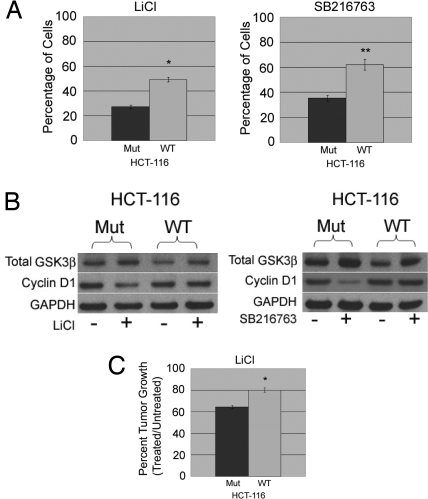
The generality of our findings was then rigorously tested. We first analyzed the effects of lithium and SB216763 using the breast cancer cell lines MCF-7 and ZR-75–1, which are mutant (E545K) and wild type for PIK3CA, respectively. As illustrated in Fig. S8A, MCF-7 cells also exhibited sensitivity toward the toxic effects of lithium and SB216763, whereas ZR-75–1 cells were relatively resistant against both reagents. Western blotting confirmed that lithium and SB216763 led to an increase in total GSK3β and a decrease in cyclin D1 in MCF-7 cells, similar to mutant PIK3CA knockin clones (Fig. S8B). We then verified our findings using another paired isogenic system: HCT-116 human colorectal cancer somatic cell knockouts, containing either a single wild-type or mutant PIK3CA allele (12). Similar to our breast epithelial knockin clones, cell proliferation assays showed that lithium and SB216763 had selective toxicity for HCT-116 knockout cells harboring a mutant PIK3CA gene, compared with knockout derivates carrying a single PIK3CA wild-type allele (Fig. 4A). Western blotting of treated vs. untreated cells showed identical results seen with MCF-10A mutant PIK3CA knockin clones (Fig. 4B). However, the magnitude of the difference in cell proliferation was not as pronounced as in our breast epithelial knockin cell lines, nor the breast cancer cell lines, because wild-type PIK3CA HCT-116 cells also displayed partial sensitivity toward GSK3β inhibitors. This may reflect the fact that HCT-116 cells, unlike breast cancer cells, have an activating KRAS mutation, which may alter the response to lithium and SB216763 through additional activation of PI3K.
Fig. 4.
Mutant PIK3CA sensitizes HCT-116 human colon cancer cells to lithium and GSK3β inhibitors in vitro and in vivo. (A) Cell proliferation assays were performed as described in Methods using the HCT-116 somatic cell knockout derivatives containing either a single mutant (Mut) or wild-type (WT) PIK3CA allele in the presence of either 10 mM lithium chloride (LiCl) (Left) or 1 μM SB216763 (Right). Bars represent the percentage of cell proliferation in LiCl or SB216763 relative to cells grown without these inhibitors. Error bars represent SEM from triplicate samples. *P < 0.0003, **P < 0.0005. (B) Western blot illustrating levels of total GSK3β and cyclin D1 in the HCT-116 somatic cell knockout derivatives containing either a single mutant (Mut) or wild-type (WT) PIK3CA allele in the presence of either 10 mM lithium chloride (LiCl) (Left) or 1 μM SB216763 (Right) cultured for 24 h. GAPDH is shown as a loading control. (C) LiCl treatment and xenograft assays were conducted, with percentage of average tumor volumes (n = 10 for each group) shown for lithium- and sham-treated mice at day 18 postinoculation. *P < 0.04.
To confirm our in vitro data, we performed nude-mouse xenograft assays using the isogenic HCT-116 PIK3CA knockout cell lines to assess the effects of lithium in vivo. Mice were given lithium orally for 10 days before tumor inoculation, to achieve serum levels comparable to therapeutic doses in humans being treated for bipolar disorder (30, 31). As shown in Fig. 4C, xenografts of HCT-116 cells containing a single mutant PIK3CA allele had significantly less tumor growth when administered oral lithium compared with sham-treated controls 18 days postinoculation, and this effect was statistically significant when compared with nude mice bearing xenografts of HCT-116 cells harboring a single wild-type PIK3CA allele (P < 0.04). Thus, the use of 2 separate isogenic gene-targeted somatic cell lines, derived from different tissue types (breast and colon), along with our additional experiments in estrogen receptor (ER)-positive breast cancer cell lines and in vivo xenograft experiments, strongly suggest that mutant PIK3CA sensitizes human cells to the GSK3β inhibitors lithium chloride and SB216763.
Discussion
In this study, we attempted to identify downstream therapeutic targets of mutant PIK3CA by knocking in oncogenic mutations into nontumorigenic human breast epithelial cells. Somewhat surprisingly, knockin of mutant PIK3CA was neither necessary nor sufficient for activating the downstream effector mTOR, but did lower the threshold for mTOR activation by the EGFR pathway. Because receptor tyrosine kinase signaling is known to be mediated in part through the p85 subunit of PI3K, this suggests that signaling through EGFR is augmented by mutant PIK3CA, but that oncogenic mutations of this gene do not directly lead to mTOR activation. Currently there are a number of clinical trials addressing the effectiveness of mTOR inhibitors in various human malignancies, and many of these trials include correlative studies examining whether oncogenic PIK3CA mutations can serve as predictive biomarkers of response. Our findings suggest that PIK3CA mutations could be a positive predictor of response depending on serum EGF levels. However, the absence of these mutations may not be informative, because increased activation of EGFR signaling by other means, such as amplification, could also lead to mTOR activation and sensitivity to mTOR inhibitors.
Our study confirmed previous reports that oncogenic PIK3CA can also lead to activation of the MAP kinase pathway as demonstrated by increased (Thr-202/Tyr-204) phosphorylation of ERK (5, 16). The precise molecular mechanism of how this occurs requires further elucidation, although recent studies have shown the necessity of Ras and PIK3CA interaction for tumorigenesis induced by mutant Kras (15). It is tempting to speculate that Ras/PIK3CA binding is also required to realize the full oncogenic effects of mutant PIK3CA, and that disruption of this interaction may effectively ablate MAP kinase signaling seen with mutant PIK3CA. Further study of this testable hypothesis may yield new insight into the nature of this complex signaling pathway.
Knockin mutant PIK3CA clones displayed EGF- and mTOR-independent cell proliferation that was mediated at least in part by GSK3β, as illustrated by increased GSK3β phosphorylation (Ser-9) in mutant PIK3CA knockin clones, and the selective growth inhibition seen with GSK3β inhibitors. Serine 9 phosphorylation of GSK3β has been shown to be associated with AKT activation in chicken cells expressing exogenous oncogenic PIK3CA mutations (6), although isogenic PIK3CA knockout HCT-116 and DLD-1 colon cancer cells were reported by Samuels et al. (12) as having no consistent differences in GSK3β phosphorylation between mutant and wild-type PIK3CA clones. However, 3 of 4 wild-type PIK3CA clones in their study appear to have decreased GSK3β phosphorylation relative to parental and mutant-only PIK3CA clones (12). In our hands, there was a slight decrease in baseline GSK3β phosphorylation in the wild-type PIK3CA HCT-116 clone when compared with mutant PIK3CA HCT-116 cells (data not shown). These findings could be due to the microsatellite instability phenotype of these colon cancer cell lines, leading to increased clonal variability.
Treatment with the GSK3β inhibitors lithium chloride and SB216763 in vitro was selectively toxic for mutant PIK3CA knockin clones, as well as breast and colon cancer cell lines known to harbor mutant PIK3CA. Oral administration of lithium chloride using doses comparable in humans treated for bipolar disorder (30, 31) resulted in a statistically significant growth inhibition of human colorectal cancer xenografts containing a single mutant PIK3CA allele compared with isogenic xenografts with a single wild-type PIK3CA allele. Interestingly, increased levels of phosphorylated GSK3β (Ser-9) and augmented sensitivity to GSK3β inhibitors have been described in the SKOV-3 ovarian cancer cell line (26), and this cell line has also been shown to contain an oncogenic PIK3CA mutation (32). More recently, others have demonstrated that lithium and SB216763 treatment of thyroid cancer cells can lead to a decrease in cell proliferation (27). These investigators also identified that activation of ERK through overexpression of Raf-1 results in increased GSK3β phosphorylation (Ser-9), and similarly Ding et al. reported that activated ERK can also lead to GSK3β phosphorylation (Ser-9) (33). These studies and ours suggest that the increased GSK3β phosphorylation (Ser-9) seen in PIK3CA mutant knockin clones may be a consequence of both increased AKT and ERK activation.
Despite decades of clinical use, the precise mechanisms of lithium's actions have not been fully elucidated. However, in most studies examining the molecular basis of lithium for the treatment of bipolar disorder, lithium has been shown to inhibit GSK3β (33), which in principle should lead to a cell proliferative response, thereby mimicking the baseline status of mutant PIK3CA knockin cell lines. In this study, we observed an increase in GSK3β upon treatment with lithium chloride and SB216763, supporting that the antiproliferative effects of these compounds are due to an increase in total GSK3β, along with a decrease in the known downstream target gene CYCLIN D1. However, lithium is known to have a number of other cellular targets, including inhibition of inositol monophosphatase, which could theoretically lead to a decrease in phosphatidylinositol bisphosphate (PIP2), the substrate of PI3K (34). It is possible that cells with mutant PIK3CA may be adversely affected by this depletion of PIP2, relative to cells with normal PI3K activity. Arguing against this, SB216763 has been described as a GSK3-specific inhibitor and has shown little or no activity against a number of protein kinases (25), yet treatment with this compound also resulted in growth inhibition, with a similar increase in total GSK3β. More recently, Wang et al. (28) reported that in human leukemia cells defined by mutations in the MLL oncogene, treatment with lithium and SB216763 led to an antineoplastic effect both in vitro and in vivo. In their system, GSK3β appears to have a paradoxical oncogenic effect involving a downstream link with p27 destabilization; however, we did not see any effects on p27 stability in our studies (data not shown). How these molecules increase GSK3β in human breast and colon cancer cells is presently unknown, but based on the current study and extensive literature, their effects are dependent on the tissue type and genetic context of the cell.
In summary, our findings suggest that oncogenic PIK3CA mutations reduce the threshold for mTOR activation, and that cell proliferation mediated by mutant PIK3CA involves additional critical pathways. Specifically, we found that modulation of GSK3β also contributes significantly to EGF- and mTOR-independent cellular proliferation induced by mutant PIK3CA, as evidenced by our findings with lithium chloride and SB216763. Our study has important consequences for the design of clinical trials, as combinations of drugs targeting multiple pathways may be necessary to achieve optimal effective antineoplastic therapy against cancers that contain PIK3CA mutations. The identification of GSK3β as a potential therapeutic target may allow for the emergence of more efficacious drugs to treat the significant number of human malignancies that harbor mutant PIK3CA.
Methods
Targeted Knockin of the PIK3CA Oncogene.
Targeting vectors were designed to introduce a single oncogenic mutation within PIK3CA to separately create E545K and H1047R knockin clones. Vector transduction, colony selection, clone screening, and Cre recombinase removal of the neomycin resistance gene were done as previously described (7, 35).
Cell Proliferation Assays.
Cell proliferation assays were performed as previously described (7, 13) with and without pathway inhibitors.
Xenograft Assays.
Lithium chloride (1,200 mg/l) in 5% sucrose (wt/vol) and water was administered orally 10 days before xenograft inoculation as previously described (30). Sham-treated mice received 5% sucrose in their drinking water. Drinking water was monitored to ensure no differences in consumption between treatment and control groups. Xenograft assays were performed as previously described (7).
Additional details and methods are described in SI Methods.
Supplementary Material
Acknowledgments.
We thank Ramon Parsons for helpful suggestions and insights, and Giovanni Parmigiani for helpful discussions with statistics. This work was supported by the Avon Foundation, Susan G. Komen for the Cure, the Mary Kay Ash Charitable Foundation, the Stewart Trust Fund, National Institutes of Health Grants CA109274 and CA88843, the Flight Attendant Medical Research Institute (FAMRI), and the Breast Cancer Research Foundation. J. P. Gustin is a recipient of Department of Defense Breast Cancer Research Program Predoctoral Fellowship Award W81XWH-06–1-0325. M.B.W. is supported by NIH T32DK067872. J. P. Garay is a recipient of a Research Supplement to Promote Diversity in Health-Related Research. H.K. is a recipient of a Young Clinical Scientist Award from FAMRI and was also supported by the Yasuda Medical Research Foundation and the Kanzawa Medical Research Foundation. D.C. receives support from National Institutes of Health Grant T32 CA09071–27 and the American Society of Clinical Oncology's Young Investigator Award. J.L. is a recipient of a Young Clinical Scientist Award from FAMRI; A.M.A. and M.I.V. are recipients of a Susan G. Komen Foundation Postdoctoral Fellowship Award; and G.W. is a recipient of a Medical Scientist Training Program Fellowship.
Footnotes
Conflict of interest statement: Under an agreement between The Johns Hopkins University and Genzyme Corporation, Dr. Vogelstein is entitled to a share of royalty received by the University on sales of products related to PIK3CA mutations. Johns Hopkins University has also licensed HCT-116 cell lines with particular mutations of PIK3CA to Horizon Discovery Corporation and other pharmaceutical companies. The terms of these arrangements are being managed by The Johns Hopkins University in accordance with its conflict of interest policies. B.H.P. receives research support from GlaxoSmithKline, though the studies reported here were not supported by this contract. K.E.B. is currently an employee of GlaxoSmithKline, though all of the work reported here was during his faculty appointment at the University of Maryland.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813351106/DCSupplemental.
References
- 1.Samuels Y, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 2.Bachman KE, et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004;3:772–775. doi: 10.4161/cbt.3.8.994. [DOI] [PubMed] [Google Scholar]
- 3.Karakas B, Bachman KE, Park BH. Mutation of the PIK3CA oncogene in human cancers. Br J Cancer. 2006;94:455–459. doi: 10.1038/sj.bjc.6602970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci USA. 2005;102:802–807. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isakoff SJ, et al. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 2005;65:10992–11000. doi: 10.1158/0008-5472.CAN-05-2612. [DOI] [PubMed] [Google Scholar]
- 6.Bader AG, Kang S, Vogt PK. Cancer-specific mutations in PIK3CA are oncogenic in vivo. Proc Natl Acad Sci USA. 2006;103:1475–1479. doi: 10.1073/pnas.0510857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konishi H, et al. Knock-in of mutant K-ras in nontumorigenic human epithelial cells as a new model for studying K-ras mediated transformation. Cancer Res. 2007;67:8460–8467. doi: 10.1158/0008-5472.CAN-07-0108. [DOI] [PubMed] [Google Scholar]
- 8.Lauring J, et al. The multiple myeloma associated MMSET gene contributes to cellular adhesion, clonogenic growth, and tumorigenicity. Blood. 2008;111:856–864. doi: 10.1182/blood-2007-05-088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ignatoski KM, Lapointe AJ, Radany EH, Ethier SP. erbB-2 overexpression in human mammary epithelial cells confers growth factor independence. Endocrinology. 1999;140:3615–3622. doi: 10.1210/endo.140.8.6939. [DOI] [PubMed] [Google Scholar]
- 10.Sekulic A, et al. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–3513. [PubMed] [Google Scholar]
- 11.Ikenoue T, et al. Functional analysis of PIK3CA gene mutations in human colorectal cancer. Cancer Res. 2005;65:4562–4567. doi: 10.1158/0008-5472.CAN-04-4114. [DOI] [PubMed] [Google Scholar]
- 12.Samuels Y, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–573. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Bachman KE, et al. p21(WAF1/CIP1) mediates the growth response to TGF-beta in human epithelial cells. Cancer Biol Ther. 2004;3:221–225. doi: 10.4161/cbt.3.2.666. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka Y, et al. Growth factor contents of autologous human sera prepared by different production methods and their biological effects on chondrocytes. Cell Biol Int. 2008;5:505–514. doi: 10.1016/j.cellbi.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, et al. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell. 2007;129:957–968. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 16.Zhao L, Vogt PK. Helical domain and kinase domain mutations in p110alpha of phosphatidylinositol 3-kinase induce gain of function by different mechanisms. Proc Natl Acad Sci USA. 2008;105:2652–2657. doi: 10.1073/pnas.0712169105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang J, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8:1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- 19.Shin I, et al. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8:1145–1152. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- 20.Viglietto G, et al. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med. 2002;8:1136–1144. doi: 10.1038/nm762. [DOI] [PubMed] [Google Scholar]
- 21.Zhou BP, et al. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001;3:245–252. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- 22.Cross DA, et al. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 23.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coghlan MP, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7:793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 26.Cao Q, Lu X, Feng YJ. Glycogen synthase kinase-3beta positively regulates the proliferation of human ovarian cancer cells. Cell Res. 2006;16:671–677. doi: 10.1038/sj.cr.7310078. [DOI] [PubMed] [Google Scholar]
- 27.Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, Chen H. Inactivation of glycogen synthase kinase-3beta, a downstream target of the raf-1 pathway, is associated with growth suppression in medullary thyroid cancer cells. Mol Cancer Ther. 2007;6:1151–1158. doi: 10.1158/1535-7163.MCT-06-0665. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, et al. Glycogen synthase kinase 3 in MLL leukaemia maintenance and targeted therapy. Nature. 2008;455:1205–1209. doi: 10.1038/nature07284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoeflich KP, et al. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 30.Dehpour AR, Farsam H, Azizabadi-Farahani M. Inhibition of the morphine withdrawal syndrome and the development of physical dependence by lithium in mice. Neuropharmacology. 1995;34:115–121. doi: 10.1016/0028-3908(94)00121-8. [DOI] [PubMed] [Google Scholar]
- 31.Severus WE, et al. What is the optimal serum lithium level in the long-term treatment of bipolar disorder—a review? Bipolar Disord. 2008;10:231–237. doi: 10.1111/j.1399-5618.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- 32.Whyte DB, Holbeck SL. Correlation of PIK3Ca mutations with gene expression and drug sensitivity in NCI-60 cell lines. Biochem Biophys Res Commun. 2006;340:469–475. doi: 10.1016/j.bbrc.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 33.Ding Q, et al. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell. 2005;19:159–170. doi: 10.1016/j.molcel.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Phiel CJ, Klein PS. Molecular targets of lithium action. Annu Rev Pharmacol Toxicol. 2001;41:789–813. doi: 10.1146/annurev.pharmtox.41.1.789. [DOI] [PubMed] [Google Scholar]
- 35.Konishi H, et al. A PCR-based high-throughput screen with multiround sample pooling: Application to somatic cell gene targeting. Nat Protoc. 2007;2:2865–2874. doi: 10.1038/nprot.2007.409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.