Abstract
During the first 7 weeks of postnatal life, short day lengths inhibit the onset of puberty in many photoperiodic rodents, but not in Syrian hamsters. In this species, timing of puberty and fecundity are independent of the early postnatal photoperiod. Gestational day length affects postnatal reproductive development in several rodents; its role in Syrian hamsters has not been assessed. We tested the hypothesis that cumulative effects of pre- and postnatal short day lengths would restrain gonadal development in male Syrian hamsters. Males with prenatal short day exposure were generated by dams transferred to short day lengths 6 weeks, 3 weeks, and 0 weeks prior to mating. Additional groups were gestated in long day lengths and transferred to short days at birth, at 4 weeks of age, or not transferred (control hamsters). In pups of dams exposed to short day treatment throughout gestation, decreased testis growth was apparent by 3 weeks and persisted through 9 weeks of age, at which time maximum testis size was attained. A subset of males (14%), whose dams had been in short days for 3 to 6 weeks prior to mating displayed pronounced delays in testicular development, similar to those of other photoperiodic rodents. This treatment also increased the percentage of male offspring that underwent little or no gonadal regression postnatally (39%). By 19 weeks of age, males housed in short days completed spontaneous gonadal development. After prolonged long day treatment to break refractoriness, hamsters that initially were classified as nonregressors underwent testicular regression in response to a 2nd sequence of short day lengths. The combined action of prenatal and early postnatal short day lengths diminishes testicular growth of prepubertal Syrian hamsters no later than the 3rd week of postnatal life, albeit to a lesser extent than in other photoperiodic rodents.
Keywords: seasonality, day length, gestation, puberty, testis, rodent
Northern temperate zone muroid rodents typically breed from late March through September, during which time females usually produce more than 1 litter (Bronson, 1989; Paul et al., 2008). Young born in the field at the beginning of the breeding season reach sexual maturity at ∼6 weeks of age and reproduce in the season of birth (Schwarz et al., 1964; Sadleir, 1969). Those born near the end of the breeding season typically overwinter as juveniles, first reaching puberty at 5 to 6 months of age (Sadleir, 1969). Seasonal arrest of reproductive development in late-born cohorts presumably evolved because the energetic challenge posed by winter conditions reduced fitness in individuals that bred at this time (Prendergast et al., 2002).
Seasonal changes in day length are sufficient to control pubertal development; the decrease in day length after the summer solstice inhibits reproductive development in both males and females (Butler et al., 2007a, 2007b). In many rodent species, short day (SD) lengths imposed at birth arrest reproductive development for the 1st several months of life (white-footed mice, Johnston and Zucker, 1980; deer mice, Whitsett et al., 1984; Siberian hamsters, Hoffmann, 1978; meadow voles, Dark et al., 1990; montane voles, Horton, 1984; field voles, Craven and Clarke, 1982; grasshopper mice, Frost and Zucker, 1983; marsh rice rats, Edmonds and Stetson, 1995; Turkish hamsters, Hong and Stetson, 1986). The Syrian hamster—Mesocricetus auratus—is unusual in that no effect of postnatal short day lengths on reproductive development has been detected prior to 7 weeks of age. Females are fecund at this age and males produce sperm regardless of postnatal day length conditions (Cherry, 1987). Darrow et al. (1980, p 447), echoing conclusions of several others, stated that “the onset of puberty is independent of early lighting conditions, since during the 1st 7 weeks of age, the male reproductive organs developed at similar rates regardless of photoperiod or blinding.”
The neonatal rodent's gestational photoperiodic history can influence postnatal reproductive and somatic development. Day length information is transduced by the duration of nocturnal melatonin secretion. Longer duration melatonin signals produced in SDs act on the neuroendocrine axis to restrain reproductive development (Carter and Goldman, 1983; Bartness et al., 1993). Prenatal melatonin exposure affects postnatal reproductive development in montane and meadow voles (Horton, 1984; Lee and Zucker, 1988; Lee, 1993) and Siberian hamsters (Horton et al., 1990; Weaver et al., 1987; Elliott and Goldman, 1989), but whether or not Syrian hamsters respond similarly is unknown.
In earlier studies of Syrian hamsters, females were transferred from long to short day lengths at parturition (Reiter et al., 1970) or at 3 to 5 days (Darrow et al., 1980), 4 days (Cherry, 1987), or 6 to 10 days prior to birth of the young (Sisk and Turek, 1987). Because several weeks elapse after the transition from long to short day lengths before the circadian system entrains to the shorter day length (Larkin et al., 2002) and the duration of nocturnal melatonin secretion increases (Elliott and Tamarkin, 1994, for Syrian hamsters; Illnerová et al., 1984, for Siberian hamsters), fetuses in prior studies of Syrian hamsters were not exposed to melatonin signals that impart a SD photoperiodic history. Such signals may be required to induce early postnatal responsiveness to SDs in this species. We evaluated this hypothesis by maintaining female hamsters in SDs for 0 to 6 weeks prior to mating, or at parturition. Two additional groups of offspring were gestated and weaned in long days; 1 was transferred to SDs at 4 weeks of age, and the other was maintained as a long day (LD) control. The combination of prenatal and early postnatal day lengths is here shown to exert inhibitory effects on prepubertal reproductive development of males.
MATERIALS AND METHODS
Animals and Housing
Syrian hamsters, 2 to 3 generations descended from stock (Mesocricetus auratus, HsdHan:AURA) obtained from Harlan Sprague-Dawley (Haslett, MI), were housed in polypropylene cages (48 × 25 × 15 cm) on Tek-Fresh bedding (Harlan Teklad, Madison, WI). Hamsters were maintained in LD lengths (14 h light/day) and SD lengths (10 h light/day) for different durations described below. Dark onset was 1600 h PST in both photoperiods. Food (Prolab RMH 3000, Lab Diet, Richmond, IN) and tap water were available ad libitum. Ambient temperature was 22 ± 2 °C. All procedures were approved by the Animal Care and Use Committee of the University of California, Berkeley.
Mating Procedure
Females were monitored for regular estrous cycles beginning at 8 weeks of age by recording post-estrous vaginal discharge (Orsini, 1961) for 2 months. At 16 weeks of age they were mated to males that shared no common ancestors within our colony. On the day before expected post-estrous discharge, a LD-housed male was placed in the female's home cage 1.5 h before onset of darkness and mated to satiety or until the female became aggressive. If the female did not display lordosis within 5 min, or the male did not mate within 20 min of pairing, he was replaced with a different male. The test was terminated if females still failed to display lordosis; these females were retested for up to 3 more consecutive days. This occurred most frequently in females that had been in SDs for 6 weeks prior to mating. Additional females were mated to ensure that experimental males in each group would be derived from at least 5 dams. Postweaning estrous cycles of SD dams were monitored to assess responsiveness to SD.
Estimated Testicular Volume Measurements
Beginning at weaning (3 weeks of age) and at 2-week intervals thereafter, estimated testis volume (ETV) and body mass of male offspring were recorded. The length and width of the right testis were measured externally (±0.1 mm) under anesthesia induced with isoflurane vapors. The product of testis width squared × testis length provides a measure of estimated testis volume in Syrian hamsters that is highly correlated with testis weight (Watson-Whitmyre and Stetson, 1985), which in turn is highly correlated with spermatogenesis and a male's ability to sire offspring (Beery et al., 2007).
Classification of Regression and Recrudescence
Because of within-group variation in profiles of testicular development, all males were assigned to 1 of 4 categories. “Complete regressors” underwent rapid testicular development followed by a reduction in ETV of >1000 mm3 in ≤4 weeks; “intermediate regressors” did not reach the criteria of “complete regressors” but displayed rapid gonadal growth followed by regression exceeding 500 mm3, typically over a longer time period; “nonregressors and weak regressors” displayed rapid testicular development without substantial regression (ETV decrease < 500 mm3); “delayed developers” exhibited late onset testicular development (after 7 weeks) without subsequent regression. Assignment to categories was done independently by 2 investigators without knowledge of each hamster's treatment group.
Onset of recrudescence was defined as the 1st week after week 11 in which ETV exceeded 1000; a previous study in our laboratory demonstrated that SD-housed adult Syrian hamsters with ETVs below this threshold were infertile (Beery et al., 2007). In animals displaying weak regression, onset of recrudescence was defined as the 1st week in which 20% growth was sustained over 2 successive measures. Nonregressors that did not sustain such growth were excluded from analysis of recrudescence.
Procedure
Part 1: Effects of Photoperiodic History on Offspring Reproductive Development
Six groups of males (n = 18 per group) were generated by dams with differing durations of SD treatment (Fig. 1). Two groups of dams were transferred to SDs 6 and 3 weeks before mating (6-MSD and 3-MSD, respectively; MSD denotes maternal SD exposure before mating). Two groups of LD-housed females were transferred to SDs on the day after mating (SDgest) or the day of parturition (SD-birth). One group of LD gestated offspring was transferred to SDs 4 weeks after birth (SD-4wk), and another remained in LDs (LD control; Fig. 1). Seven or more females were mated within each group (6-MSD = 16 females; 3-MSD = 9 females; SD-gest, SD-birth, SD-4wk, and LD = 7 females) and 2 to 4 males from each litter were used to generate the final sample size of 18.
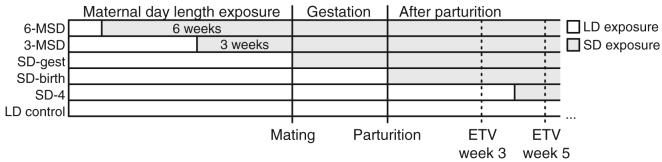
Figure 1.
Time line of day length treatments in each group. White and gray horizontal bars denote maintenance in LDs and SDs, respectively, for dams and/or their offspring. Mating and parturition occurred within the same 4-day date range for all groups. LD = long day length; SD = short day length; 6-MSD and 3-MSD = dams transferred to SDs 6 and 3 weeks before mating; SD-gest = LD-housed females transferred to SDs on the day after mating; SD-birth = LD-housed females transferred to SDs on the day of parturition; SD-4wk = LD-gestated offspring transferred to SDs 4 weeks after birth.
Male pups were weaned at 3 weeks of age and housed 2 per cage from the time of weaning. ETV was assessed every 2 weeks from 3 to 31 weeks of age. Males were separated to single housing in cases where any antagonism was detected (20 of 54 pairs).
Part 2: Effects of Early Photoperiod on Adult Photoresponsiveness
Assessment of photoresponsiveness was performed on a subset of males from part 1 that included 3 hamsters per group that had undergone complete gonadal regression, and all males classified in part 1 as weak or nonregressors or delayed developers. Twelve LD control hamsters were also tested. Hamsters were transferred to or maintained in LDs for 25 weeks, which breaks photorefractoriness and restores responsiveness to antigonadal effects of SDs (Reiter 1980; Stetson et al., 1983; Bittman and Zucker, 1981). ETV was assessed at the end of this LD treatment; hamsters were then transferred to SDs for 18 weeks, during which time ETVs were determined at weeks 4, 6, 9, 12, 15, and 18.
Data Analysis
Repeated measures analysis of the ETV time series indicated significant differences between the treatment groups (p < 0.001), so differences between treatment groups were analyzed at specific time points. Significant analyses of variance (ANOVAs) were followed by pairwise comparisons using Tukey's HSD. Effects of gestation and maintenance in LDs versus SDs on ETV were analyzed by t tests assuming unequal variances. LD and SD-4wk groups were treated as a single LD-gestated and maintained cohort for comparisons involving the week 3 ETV (prior to SD transfer of the SD-4wk group). Logistic regression was used to analyze developmental trajectory by cumulative SD exposure. Differences between groups were considered significant at p < 0.05; all p values lower than 0.001 are reported as p < 0.001. All statistical analyses were performed using JMP 7.0 (SAS Institute Inc., Cary, NC). Mean ± SEM values are reported throughout.
RESULTS
Gestational Day Length and Pup Gonadal Development
Initial Testis Growth
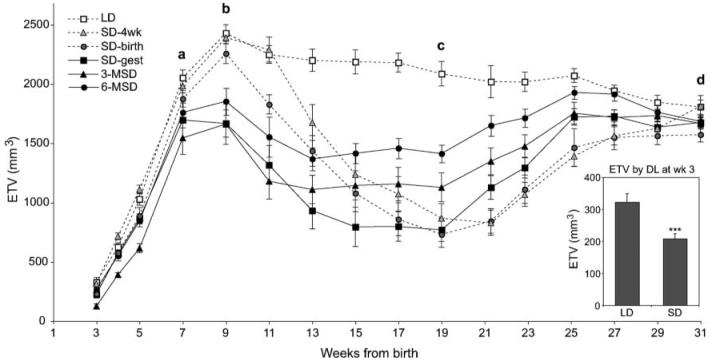
Testis growth was reduced in males gestated in SD photoperiods (Fig. 2). Differences were apparent at the time of the 1st ETV measurement at 3 weeks of age (Fig. 2, inset; SD-gestated vs LD-gestated and maintained pups; p < 0.001, t test), representing combined data from 6-MSD, 3-MSD, and SD-gest groups for the SD-gestated cohort (n = 54), and LD and SD-4wk groups for the LD-gestated and maintained cohort (n = 36). This difference remained statistically significant at weeks 5, 7, and 9 despite comparison of the SD-gestated groups to only the LD control group after transfer of SD-4wk males to SDs (p < 0.001, for each week). Hamsters transferred between day lengths after birth (SD-birth and SD-4wk) were statistically indistinguishable from LD controls during this interval.
Figure 2.
Maintenance of dams in short day lengths (SDs) before or during gestation retards reproductive development of offspring. Estimated testicular volume values are shown for the 1st 8 months of life. Groups are ordered in the key according to increasing pup and maternal exposure to SDs. Points connected with dotted and solid lines indicate groups gestated in long day lengths (LDs) and SDs, respectively. Pairwise comparisons of all groups were made at the 4 lettered time points (a-d). At week 7 (a), testes of LD hamsters were significantly larger than those of SD-gest and 3-MSD hamsters; the SD-4wk group also differed significantly from the 3-MSD group. By week 9 (b), estimated testis volumes (ETVs) of LD and SD-4wk hamsters were significantly greater than those of the 3 SD-gestated groups; ETVs of SD-birth hamsters were significantly higher than those of SD-gest and 3-MSD, but not 6-MSD hamsters. At week 19 (c), LD hamsters had larger testes than all the other groups; mean ETV of 6-MSD hamsters was significantly higher than those of the other SD groups, and the 3-MSD value was significantly greater than that of the SD-gest, SD-birth, and SD-4wk hamsters. By week 31 (d) the analysis of variance was not significant and groups were indistinguishable from one another. (Inset) ETV differs between SD-gestated groups (6-MSD, 3-MSD, SD-gest) and LD-gestated and maintained groups (LD, SD-4wk) by week 3. *** = p < 0.001. 3-MSD and 6-MSD = dams transferred to SDs 3 and 6 weeks before mating; SD-gest = LD-housed females transferred to SDs on the day after mating; SD-birth = LD-housed females transferred to SDs on the day of parturition; SD-4wk = LD-gestated offspring transferred to SDs 4 weeks after birth.
Peak Testis Dimensions
Maximum testicular size was attained in all groups by 9 weeks of age (Fig. 2, comparison b). Hamsters gestated in SDs had lower maximum ETVs than did LD-gestated males (1727 ± 78 and 2360 ± 41, respectively; p < 0.001) before initiating gonadal regression. Week 9 ETVs were significantly higher in LD and SD-4wk groups than in each of the SD-gestated groups (SD-gest, 3-MSD, 6-MSD) in pairwise comparisons; ETVs of SD-birth hamsters did not differ from the other LD-gestated groups or 6-MSD hamsters, but were significantly greater than those of SD-gest and 3-MSD (ANOVA followed by Tukey's HSD).
Testicular Regression
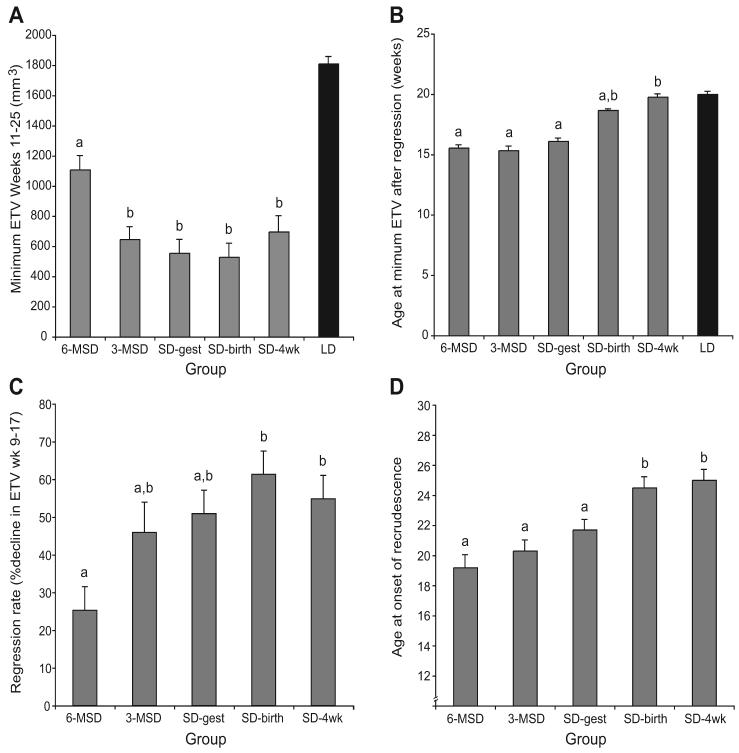
After week 9, each SD-treated group experienced declining ETVs. The rate of gonadal regression during maintenance in SDs was calculated as the percent decrease in ETV between 9 and 17 weeks of age (excluding the 5 delayed developers who underwent substantial growth during this period). The rate of regression was lower in the 6-MSD group than in each of the groups gestated in LDs (Fig. 3C; SD-birth, SD-4wk; Tukey's HSD, pairwise comparisons). The other SD-gestated groups did not have significantly lower rates of regression during this interval.
Figure 3.
(A) Mean ± SEM of individual minimum estimated testis volumes (ETVs) in each group. All SD-exposed groups underwent regression to equivalent minima except 6-MSD. (B) Mean ± SEM age of individuals in each group at the time of minimum ETV. The timing of minima varied between groups, with earlier minima in SD-gestated groups than in the SD-4wk group. (C) Mean ± SEM rate of regression, defined as the percent decrease in ETV between week 9 and 17. The 6-MSD group exhibited a decreased rate of regression relative to groups gestated in LDs. (D) Mean ± SEM age at which individuals in each group demonstrated onset of recrudescence. Groups labeled “a” versus “b” differ significantly from each other. Values for LD hamsters (black bars, panels A and B) are included for reference only, and were not included in the statistical analysis as these hamsters did not undergo photoperiod-mediated regression. LD = long day length; SD = short day length; 6-MSD and 3-MSD = dams transferred to SDs 6 and 3 weeks before mating; SD-gest = LD-housed females transferred to SDs on the day after mating; SD-birth = LD-housed females transferred to SDs on the day of parturition; SD-4wk = LD-gestated offspring transferred to SDs 4 weeks after birth.
By week 19 (Fig. 2, comparison c), ETVs of LD hamsters exceeded those of all other groups. Testicular regression was significantly attenuated in 6-MSD hamsters at week 19 compared with all other groups housed in SDs (Tukey's HSD); attenuated regression was also exhibited by 3-MSD hamsters compared with SD-gest, SD-birth, and SD-4wk groups (Tukey's HSD).
The grouped data (Fig. 2) may obscure differences in peak and minimal ETV resulting from differences in timing of these events. This was evaluated by determining the mean of individual maximum and minimum ETVs for each treatment group. For maximum ETV, this analysis yielded results nearly identical to the week 9 group data in Figure 2; individual analysis of minimum ETV, however, uncovered several differences. Although 3-MSD hamsters in Figure 2 appear to undergo less gonadal regression than do SD-gest hamsters, this reflects lack of synchronization in the timing of the minimum ETV, not the extent of regression. The mean minimum ETV from each male's time series (Fig. 3A) indicates that testes of all SD-challenged groups reached statistically equivalent minima except for 6-MSD hamsters, which underwent significantly less gonadal regression (Tukey's HSD, Fig. 3A). Minimum ETV before recrudescence was also reached earlier in the SD-gestated groups (Fig. 3B), with a significant difference in timing between each of the SD-gestated groups and the SD-4wk group (Tukey's HSD).
Recrudescence
Recrudescence occurred significantly earlier in groups gestated in SDs than in each group gestated in LDs (Fig. 3D), but the interval between minimum ETV and onset of recrudescence did not differ between groups (cf. Loudon et al., 1998); differences in timing of recrudescence reflect differences in timing of regression (minimum ETV; Fig. 3B).
By week 31 (Fig. 2, comparison d) all SD-housed groups had completed gonadal recrudescence; no differences between groups were detectable by ANOVA, nor did pooled LD- and SD-gestated groups differ from each other (p = 0.28, t test).
Body Mass
During initial reproductive development, LD-gestated and maintained groups weighed slightly more than did SD-gestated groups (p < 0.001, t tests at 3 and 5 weeks). By week 7, no influence of gestational day length was detectable at a single time point. Repeated measures ANOVA of the full time series indicated a significant effect of day length on body mass (p < 0.05); analysis by treatment group reveals that this difference is attributable to the significantly higher body mass of the SD-4wk group throughout. All other treatment groups are indistinguishable.
Individual Differences in Testicular Development
Because hamsters within each group differed in their developmental profiles, individual males were classified as manifesting complete, intermediate, or little to no regression, as described in the Materials and Methods section. Figure 4A-D illustrates sample individual time series for each of these categories. Group ETVs reflect the average of males with testes that undergo full regression and others with modest or no involution. Within litters, pups followed a mix of developmental trajectories. For example the 5 delayed developers originated from 4 different litters that included siblings from all developmental profiles (complete, intermediate, weak/nonregressor, and delayed developer).
Figure 4.
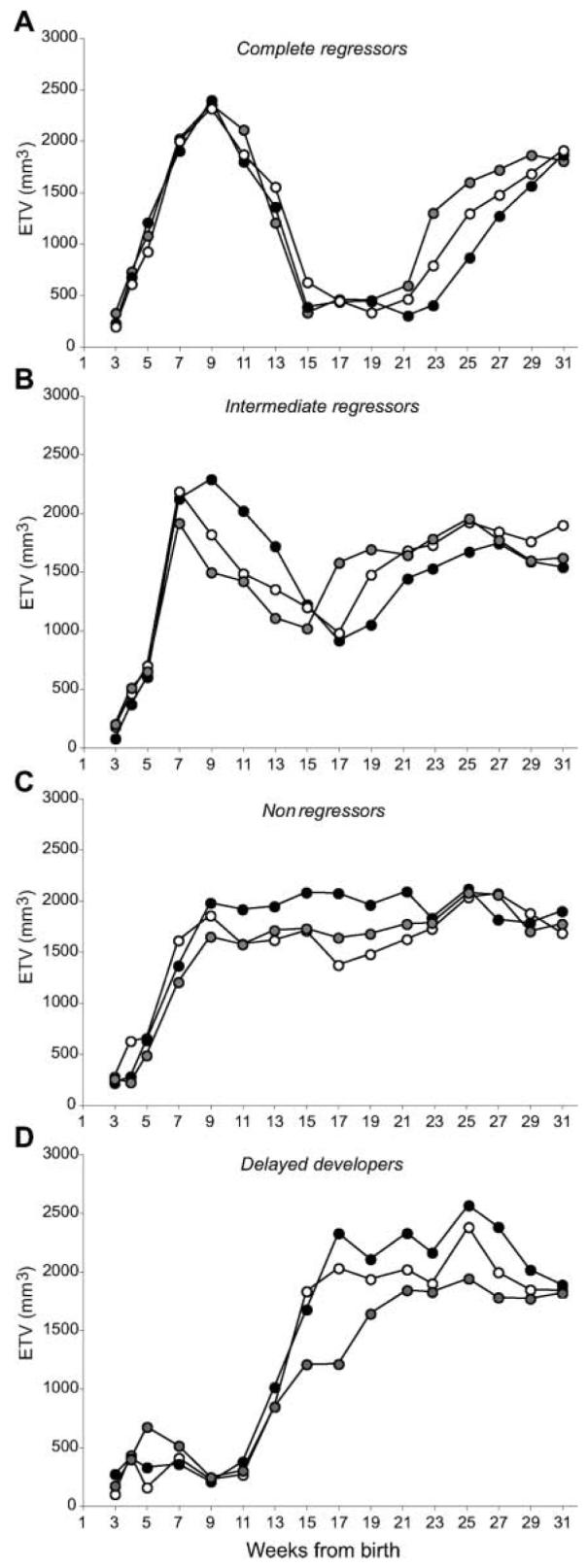
Sample profiles from individual hamsters with different developmental trajectories in each of 4 categories: complete regressors (A), intermediate regressors (B), nonregressors (C), and delayed developers (D). Regression profiles of some animals were intermediate between those illustrated in panels B and C and were considered weak regressors. These animals were grouped with nonregressors for purposes of statistical analysis.
The number of representatives in each category varied between groups (Table 1). Chi-square analysis of the full table is not indicated based on the high frequency of low and zero counts, but a 2 × 2 analysis of a regression category (complete vs incomplete or no regression) and treatment (pre-gestation vs later exposure to SDs) indicates that rows and columns are not independent (Pearson's χ2 = 8.13, df = 1, p < 0.05). Analysis of developmental category ordered by weeks of SD exposure reveals that longer maintenance of dams in SDs prior to mating was positively correlated with a reduction in the number of complete regressors (p < 0.005, logistic regression), no change in intermediate regressors (p = .34), an increase in weak and nonregressors (p < 0.005), and an increase in delayed developers (p < 0.05). Maternal gestational photoperiodic history substantially influenced the probability of different postnatal testicular developmental trajectories.
Table 1.
Regression categorization by SD treatment group (18 males/group).
| Group |
Complete Regression |
Intermediate Regression |
Weak Regression and Nonregression |
Delayed Development |
|---|---|---|---|---|
| 6-MSD | 4 | 3 | 9 | 2 |
| 3-MSD | 9 | 1 | 5 | 3 |
| SD-gest | 12 | 3 | 3 | 0 |
| SD-birth | 12 | 3 | 3 | 0 |
| SD-4wk | 12 | 3 | 3 | 0 |
NOTE: Number of males in each group that exhibited complete regression, intermediate regression, weak or nonregression, or were classified as delayed developers. Rows sum across to 18 males per treatment group. The number of intermediate and weak regressors did not vary with treatment group, but the number of complete regressors increased with decreasing exposure to SDs (p < 0.005, logistic regression) and the number of delayed developers decreased with decreasing exposure to SDs (p < 0.05, logistic regression). LD = long day length; SD = short day length; 6-MSD and 3-MSD = dams transferred to SDs 6 and 3 weeks before mating; SD-gest = LD-housed females transferred to SDs on the day after mating; SD-birth = LD-housed females transferred to SDs on the day of parturition; SD-4wk = LD-gestated offspring transferred to SDs 4 weeks after birth.
Long-Term Effects of Gestational Day Length on Photorefractoriness
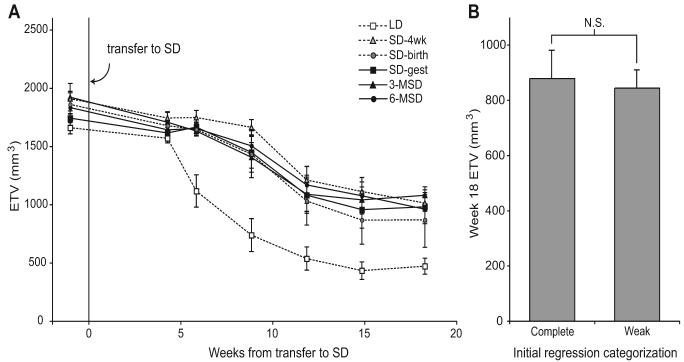
After all SD groups had undergone gonadal development, a subset of hamsters including all weak or nonregressors was treated with 25 weeks of LDs followed by 18 weeks of SDs. The LD control group (n = 12) underwent earlier and more marked testicular regression than did hamsters with a prior SD photoperiodic history (Fig. 5A). After 6 weeks in SDs, mean ETV in the LD control group was significantly lower than that of any of the other groups (Tukey's HSD); the week 18 value for the LD group was still significantly lower than that for all but the SD-birth group (Tukey's HSD). Despite earlier differences in testicular development, all SD groups underwent marked gonadal regression with indistinguishable ETV values at week 18 (Tukey's HSD). There were no detectable differences between mean final ETV values of hamsters initially assigned to the category “weak/nonregressor” versus “complete regressor” (Fig. 5B; p = 0.85, ANOVA). Hamsters unresponsive to SDs early in life were responsive to such day lengths later in life, and were equivalent to all other SD-reared hamsters.
Figure 5.
(A) Testicular regression in SDs after 25 weeks of long-day exposure. Regression occurred significantly earlier and more completely in hamsters that were experiencing SDs for the 1st time, than in those with a prior SD photoperiodic history. Timing of earlier SD treatment did not affect rates of regression during this life phase. (B) Estimated testis volume (ETV) at week 18 did not differ by initial regression categorization within the groups. Three complete regressors were followed from each group, as well as all weak and nonregressors. Sample sizes for each group were: n = 10 (3-MSD), n = 12 (6-MSD), n = 12 (LD), and n = 6 (each in SD-4wk, SD-birth, and SD-gest). LD = long day length; SD = short day length; 6-MSD and 3-MSD = dams transferred to SDs 6 and 3 weeks before mating; SD-gest = LD-housed females transferred to SDs on the day after mating; SD-birth = LD-housed females transferred to SDs on the day of parturition; SD-4wk = LD-gestated offspring transferred to SDs 4 weeks after birth.
Cessation of Estrous Cycles in Postpartum Dams
We assessed whether maintenance of dams in SD lengths prior to insemination would affect postpartum display of estrous cycles. In nonpregnant females, estrous cycles typically cease after approximately 6 weeks of SD treatment (Seegal and Goldman, 1975). The majority of 6-MSD and 3-MSD dams that littered in SD did not resume their estrous cycles after weaning their young, although 2 dams generated 1 and 3 cycles, respectively, before onset of acyclicity. In contrast, SD-birth and SD-gest dams generated a number of 4-day cycles (9 ± 1.5 and 10 ± 2.4, respectively) before the onset of acyclicity.
DISCUSSION
The apparent unresponsiveness of the male Syrian hamster reproductive axis to SD lengths during the 1st 7 weeks of postnatal life—reported in several earlier studies (Reiter et al., 1970; Darrow et al., 1980; Cherry, 1987; Sisk and Turek, 1987)—was confirmed; the present analysis, however, establishes that early postnatal testicular development is reduced in males gestated by dams maintained in short photoperiod. Testes of these males were substantially smaller by 3 weeks of age than those of males gestated by LD dams and the difference persisted for the next 6 weeks. The testes of nearly all SD-treated males eventually achieved adult LD-like dimensions and most subsequently underwent regression, but males gestated in SDs exhibited decreased peak testis size, and those whose dams had been exposed to 6 weeks of SDs before mating exhibited attenuated testicular regression. The lack of full regression probably results in the maintenance of fertility in at least some individuals gestated in SDs. In a prior study, 94% of female Syrian hamsters reared in SDs postnatally produced litters when mated at 6 to 7 weeks of age and SD- and LD-housed males exhibited equivalent presence of sperm in penile smears (Cherry, 1987).
All SD-gestated groups in the present study reached minimum ETV values sooner than the SD-4wk group and initiated recrudescence earlier than SD-4wk and SD-birth groups (Fig. 3B, D). This suggests that the interval timer that controls onset of recrudescence (Park et al., 2006) may be triggered earlier in SD-gestated groups. The demonstration that prenatal day length affects Syrian hamster testicular development is consistent with results obtained in other photoperiodic rodents, yet the extent of the photoperiodic effect is not as great in Syrian hamsters. These observations are congruent with the finding that melatonin injections initiated at 3 weeks of age (that extend the duration of the nocturnal melatonin signal) induce decreases in testicular size of Syrian hamsters by 7 weeks of age (Rissman, 1980).
The failure of previous studies to detect influences of day length on testicular development of Syrian hamsters reflects the absence of sufficient gestational SD exposure; the use of, at most, 6 to 10 days of gestational SD treatment in earlier research was insufficient to entrain the dams' circadian rhythms to generate the expanded SD melatonin secretion pattern essential for induction of the SD phenotype. Syrian hamsters transferred from 14 L to constant darkness do not expand the duration of the locomotor activity rhythm (alpha, Elliott and Tamarkin, 1994), an excellent proxy for the duration of nocturnal melatonin secretion (Hastings et al., 1987), during the 1st week in constant darkness. However, pineal melatonin peak width and activity duration were both 4 to 5 h longer after 12 or 27 weeks than after 5 or 6 days in continuous darkness. In a separate study, Syrian hamsters transferred from LDs to SDs at 22 °C did not expand alpha during the 1st week, but showed a significant lengthening in alpha that achieved a steady state by the end of the 2nd week in SDs (Larkin et al., 2002). In the present study, estrous cycles of females treated with SDs beginning 6 and 3 weeks prior to mating ceased substantially sooner after weaning than those of females in which SDs were imposed at the time of mating or at parturition. This confirms that the transition to the SD phenotype was initiated prior to insemination. At least some females exposed to SDs beginning at conception communicated a SD melatonin signal to their progeny, as SD-gest offspring had reduced testis development compared with males from dams treated with SDs beginning postnatally.
Maternal exposure to SDs prior to gestation (groups 6-MSD and 3-MSD) affected the proportion of offspring with alternative developmental trajectories; increased duration of maternal exposure to SD was associated with fewer hamsters undergoing complete testicular regression, more weak and nonregressors, and more males with substantially delayed testicular development (Table 1). The probability of each developmental strategy was positively correlated with duration of premating exposure of dams to SDs; the proportion of dams that communicate an intermediate- or long-duration melatonin signal to their fetuses apparently increases with increased maternal exposure to SDs. Based on analysis in Siberian hamsters (Weaver et al., 1987), we surmise that maternally generated melatonin signals communicated to fetuses in the last few days of gestation contribute to postnatal differences in gonadal development. The several differences among males whose dams were treated with short days for 6 versus 3 weeks prior to mating suggest that there may be differences unrelated to melatonin signaling that dams from these 2 groups communicate to their off-spring.
The diversity of developmental phenotypes in offspring born to SD-treated dams may represent different seasonal strategies on the part of the offspring. The testicular development profile of the delayed developers resembles that of Siberian hamsters, montane voles, meadow voles, white-footed mice, Turkish hamsters, and grasshopper mice born into declining or SD lengths typical of the early autumn (see introductory section). Siberian hamsters in this circumstance display a complete absence of testicular growth prior to 18 weeks of age (Hoffmann, 1978), followed by spontaneous gonadal development (Park et al., 2006). Syrian hamster dams whose offspring displayed delayed gonadal growth experienced a decline in day lengths prior to gestation; their offspring delayed development until 11 weeks, followed by spontaneous growth in SDs. Arrested testicular development may be advantageous for hamsters born into decreasing day lengths, as the increased costs of reproduction in autumn and winter can be postponed until the next breeding season (Prendergast et al., 2002, Butler et al., 2007a). The pattern of Syrian hamster development before regression in laboratory SDs is unusual and does not serve an obvious adaptive purpose. It is possible that nonphotic cues in natural environments (such as cold or lack of food), combined with exposure of dams to decreasing day lengths over several weeks prior to mating, are sufficient to prevent this development or alter its profile.
Hamsters that developed large testes but failed to undergo regression in SDs (nonregressors) potentially represent males unresponsive to short photoperiods or “nonresponders” (Nelson, 1987; Prendergast et al., 2001). This is unlikely in the present study, as males that presented as weak and nonregressors during initial development subsequently underwent as complete testicular regression as males originally classified as complete regressors when treated for 25 weeks with LDs prior to transfer to SDs. The failure of these males to manifest regression early in life may potentially be an adaptive response produced by gestation in SDs.
The unusual gonadal profile of Syrian hamsters first exposed to SDs at birth should not obscure the clear demonstration that Syrian hamster offspring respond to day length signals imparted during gestation. The unnatural transition in a single day from LD to SD lengths employed in most studies of Syrian hamsters (see Gorman and Zucker, 1998, for hazards associated with this approach) may contribute to the failure of SD lengths imposed at birth to affect development. Syrian hamsters respond to gestational SD signals by altering developmental profiles and strategies, although this response is attenuated relative to that of many other photoperiodic rodents.
ACKNOWLEDGMENTS
This research was supported by grant MH-61171 from the National Institute of Mental Health, a NDSEG fellowship to A. Beery, and a NINDS NRSA (F32-NS0581352) fellowship to M. Paul. We are grateful to Chris Tuthill for laboratory management and to the Office of Laboratory Animal Care.
REFERENCES
- Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: What has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Beery AK, Trumbull JJ, Tsao JM, Costantini RM, Zucker I. Sex differences in the onset of seasonal reproductive quiescence in hamsters. Proc R Soc Lond B Biol Sci. 2007;274:281–286. doi: 10.1098/rspb.2006.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittman EL, Zucker I. Photoperiodic termination of hamster refractoriness: Participation of the pineal gland. Biol Reprod. 1981;24:568–572. doi: 10.1095/biolreprod24.3.568. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Mammalian Reproductive Biology. University of Chicago Press; Chicago: 1989. [Google Scholar]
- Butler MP, Trumbull JJ, Turner KW, Zucker I. Timing of puberty and synchronization of seasonal rhythms by simulated natural photoperiods in female Siberian hamsters. Am J Physiol. 2007a;293:R413–R420. doi: 10.1152/ajpregu.00216.2007. [DOI] [PubMed] [Google Scholar]
- Butler MP, Turner KW, Park JH, Butler JP, Trumbull JJ, Dunn SP, Villa P, Zucker I. Simulated natural day lengths synchronize seasonal rhythms of asynchronously born male Siberian hamsters. Am J Physiol. 2007b;293:R402–R412. doi: 10.1152/ajpregu.00146.2007. [DOI] [PubMed] [Google Scholar]
- Carter DS, Goldman BD. Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters (Phodopus sungorus sungorus): Duration is the critical parameter. Endocrinology. 1983;113:1261–1267. doi: 10.1210/endo-113-4-1261. [DOI] [PubMed] [Google Scholar]
- Cherry JA. The effect of photoperiod on development of sexual behavior and fertility in golden hamsters. Physiol Behav. 1987;39:521–526. doi: 10.1016/0031-9384(87)90383-0. [DOI] [PubMed] [Google Scholar]
- Craven RP, Clarke JR. Gonadotrophin levels in male voles (Microtus agrestis) reared in long and short photoperiods. J Reprod Fertil. 1982;66:709–714. doi: 10.1530/jrf.0.0660709. [DOI] [PubMed] [Google Scholar]
- Dark J, Spears N, Whaling CS, Wade GN, Meyer JS, Zucker I. Long day lengths promote brain growth in meadow voles. Dev Brain Res. 1990;53:264–269. doi: 10.1016/0165-3806(90)90016-r. [DOI] [PubMed] [Google Scholar]
- Darrow JM, Davis FC, Elliott JA, Stetson MH, Turek FW, Menaker M. Influence of photoperiod on reproductive development in the golden hamster. Biol Reprod. 1980;22:443–450. doi: 10.1095/biolreprod22.3.443. [DOI] [PubMed] [Google Scholar]
- Edmonds KE, Stetson MH. Effects of prenatal and postnatal photoperiods and of the pineal gland on early testicular development in the marsh rice rat (Oryzomys palustris) Biol Reprod. 1995;52:989–996. doi: 10.1095/biolreprod52.5.989. [DOI] [PubMed] [Google Scholar]
- Elliott JA, Goldman BD. Reception of photoperiodic information by fetal Siberian hamsters: Role of the mother's pineal gland. J Exp Zool. 1989;252:237–244. doi: 10.1002/jez.1402520305. [DOI] [PubMed] [Google Scholar]
- Elliott JA, Tamarkin L. Complex circadian regulation of pineal melatonin and wheel-running in Syrian hamsters. J Comp Physiol A. 1994;174:469–484. doi: 10.1007/BF00191713. [DOI] [PubMed] [Google Scholar]
- Frost D, Zucker I. Photoperiod and melatonin influence seasonal gonadal cycles in the grasshopper mouse (Onychomys leucogaster) J Reprod Fertil. 1983;69:237–244. doi: 10.1530/jrf.0.0690237. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Zucker I. Mammalian photoperiodism: New perspectives from the use of simulated natural photoperiods. In: Touitou Y, editor. Biological Clocks: Mechanisms and Applications. Elsevier Science; Amsterdam: 1998. pp. 195–204. [Google Scholar]
- Hastings MH, Walker AP, Herbert J. Effect of asymmetrical reductions of photoperiod on pineal melatonin, locomotor activity and gonadal condition of male Syrian hamsters. J Endocrinol. 1987;114:221–229. doi: 10.1677/joe.0.1140221. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. Effects of short photoperiods on puberty, growth and moult in the Djungarian hamster (Phodopus sungorus) J Reprod Fertil. 1978;54:29–35. doi: 10.1530/jrf.0.0540029. [DOI] [PubMed] [Google Scholar]
- Hong SM, Stetson MH. Functional maturation of the gonads of Turkish hamsters under various photoperiods. Biol Reprod. 1986;35:858–862. doi: 10.1095/biolreprod35.4.858. [DOI] [PubMed] [Google Scholar]
- Horton TH. Growth and reproductive development of male Microtus montanus is affected by the prenatal photoperiod. Biol Reprod. 1984;31:499–504. doi: 10.1095/biolreprod31.3.499. [DOI] [PubMed] [Google Scholar]
- Horton TH, Stachecki SA, Stetson MH. Maternal transfer of photoperiodic information in Siberian hamsters. IV. Peripubertal reproductive development in the absence of maternal photoperiodic signals during gestation. Biol Reprod. 1990;42:441–449. doi: 10.1095/biolreprod42.3.441. [DOI] [PubMed] [Google Scholar]
- Illnerová H, Hoffmann K, Vaněcek J. Adjustment of pineal melatonin and N-acetyltransferase rhythms to change from long to short photoperiod in the Djungarian hamster Phodopus sungorus. Neuroendocrinology. 1984;38:226–231. doi: 10.1159/000123895. [DOI] [PubMed] [Google Scholar]
- Johnston PG, Zucker I. Photoperiodic regulation of reproductive development in white-footed mice (Peromyscus leucopus) Biol Reprod. 1980;22:983–989. doi: 10.1095/biolreprod22.4.983. [DOI] [PubMed] [Google Scholar]
- Larkin JE, Jones J, Zucker I. Temperature dependence of gonadal regression in Syrian hamsters exposed to short day lengths. Am J Physiol. 2002;282:R744–R752. doi: 10.1152/ajpregu.00299.2001. [DOI] [PubMed] [Google Scholar]
- Lee TM. Development of meadow voles is influenced postnatally by maternal photoperiodic history. Am J Physiol. 1993;265:R749–R755. doi: 10.1152/ajpregu.1993.265.4.R749. [DOI] [PubMed] [Google Scholar]
- Lee TM, Zucker I. Vole infant development is influenced perinatally by maternal photoperiodic history. Am J Physiol. 1988;255:R831–R838. doi: 10.1152/ajpregu.1988.255.5.R831. [DOI] [PubMed] [Google Scholar]
- Loudon AS, Ihara N, Menaker M. Effects of a circadian mutation on seasonality in Syrian hamsters (Mesocricetus auratus) Proc R Soc Lond B Biol Sci. 1998;265:517–521. doi: 10.1098/rspb.1998.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ. Photoperiod-nonresponsive morphs: A possible variable in microtine population-density fluctuations. Am Nat. 1987;130:350–369. [Google Scholar]
- Orsini MW. The external vaginal phenomena characterizing the stages of the estrous cycle, pregnancy, pseudopregnancy, lactation, and the anestrous hamster Mesocricetus auratus Waterhouse. Proc Anim Care Panel. 1961;11:193–206. [Google Scholar]
- Park JH, Kauffman AS, Paul MJ, Butler MP, Beery AK, Costantini RM, Zucker I. Interval timer control of puberty in photoinhibited Siberian hamsters. J Biol Rhythms. 2006;21:373–383. doi: 10.1177/0748730406292315. [DOI] [PubMed] [Google Scholar]
- Paul MJ, Zucker I, Schwartz WJ. Tracking the seasons: The internal calendars of vertebrates. Philos Trans R Soc Lond B Biol Sci. 2008;363:341–361. doi: 10.1098/rstb.2007.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Kriegsfeld LJ, Nelson RJ. Photoperiodic polyphenisms in rodents: Neuroendocrine mechanisms, costs, and functions. Q Rev Biol. 2001;76:293–325. doi: 10.1086/393989. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Nelson RJ, Zucker I. Mammalian seasonal rhythms: Behavior and neuroendocrine substrates. In: Pfaff DW, Arnold A, Etgen A, Fahrbach S, Rubin R, editors. Hormones, Brain and Behavior. Academic Press; San Diego: 2002. pp. 93–156. [Google Scholar]
- Reiter RJ. The pineal and its hormones in the control of reproduction in mammals. Endocr Rev. 1980;1:109–131. doi: 10.1210/edrv-1-2-109. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Sorrentino S, Hoffman RA. Early photoperiodic conditions and pineal antigonadal function in male hamsters. Int J Fertil. 1970;15:163–170. [PubMed] [Google Scholar]
- Rissman EF. Prepubertal sensitivity to melatonin in male hamsters. Biol Reprod. 1980;22:277–280. doi: 10.1095/biolreprod22.2.277. [DOI] [PubMed] [Google Scholar]
- Sadleir RMFS. The Ecology of Reproduction in Wild and Domestic Mammals. Methuen; London: 1969. [Google Scholar]
- Schwarz SS, Pokrovski AV, Istchenko VG, Olenjev VG, Ovtsshinnikova NA, Pjastolova OA. Biological peculiarities of seasonal generations of rodents, with special reference to the problem of senescence in mammals. Acta Theriol (Warsz) 1964;8:11–43. [Google Scholar]
- Seegal RF, Goldman BD. Effects of photoperiod on cyclicity and serum gonadotropins in the Syrian hamster. Biol Reprod. 1975;12:223–231. doi: 10.1095/biolreprod12.2.223. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Turek FW. Reproductive responsiveness to short photoperiod develops postnatally in male golden hamsters. J Androl. 1987;8:91–96. doi: 10.1002/j.1939-4640.1987.tb00956.x. [DOI] [PubMed] [Google Scholar]
- Stetson MH, Watson-Whitmyre M, Tate-Ostroff B. Role of the pineal and its hormone melatonin in the termination of photorefractoriness in golden hamsters. Biol Reprod. 1983;29:689–696. doi: 10.1095/biolreprod29.3.689. [DOI] [PubMed] [Google Scholar]
- Watson-Whitmyre M, Stetson MH. A mathematical method for estimating paired testes weight from in situ testicular measurements in three species of hamster. Anat Rec. 1985;213:473–476. doi: 10.1002/ar.1092130313. [DOI] [PubMed] [Google Scholar]
- Weaver DR, Keohan JT, Reppert SM. Definition of a prenatal sensitive period for maternal-fetal communication of day length. Am J Physiol. 1987;253:E701–E704. doi: 10.1152/ajpendo.1987.253.6.E701. [DOI] [PubMed] [Google Scholar]
- Whitsett JM, Lawton AD, Miller LL. Photosensitive stages in pubertal development of male deer mice (Peromyscus maniculatus) J Reprod Fertil. 1984;72:269–276. doi: 10.1530/jrf.0.0720269. [DOI] [PubMed] [Google Scholar]