Abstract
G protein-coupled receptors (GPCRs) play pivotal roles in regulating the function and plasticity of neuronal circuits in the nervous system. Among the myriad of GPCRs expressed in neural cells, class II GPCRs which couples predominantly to the Gs–adenylate cyclase–cAMP signaling pathway, have recently received considerable attention for their involvement in regulating neuronal survival. Neuropeptides that activate class II GPCRs include secretin, glucagon-like peptides (GLP-1 and GLP-2), growth hormone-releasing hormone (GHRH), pituitary adenylate cyclase activating peptide (PACAP), corticotropin-releasing hormone (CRH), vasoactive intestinal peptide (VIP), parathyroid hormone (PTH), and calcitonin-related peptides. Studies of patients and animal and cell culture models, have revealed possible roles for class II GPCRs signaling in the pathogenesis of several prominent neurodegenerative conditions including stroke, Alzheimer's, Parkinson's, and Huntington's diseases. Many of the peptides that activate class II GPCRs promote neuron survival by increasing the resistance of the cells to oxidative, metabolic, and excitotoxic injury. A better understanding of the cellular and molecular mechanisms by which class II GPCRs signaling modulates neuronal survival and plasticity will likely lead to novel therapeutic interventions for neurodegenerative disorders.
Keywords: Secretin-like, G protein-coupled receptor (GPCR), neurodegeneration, promiscuity, Alzheimer's disease (AD), Parkinson's disease (PD), neuroprotection, neuropeptide
G Protein-Coupled Receptors and Their Classification
G protein-coupled receptors (GPCRs) share a common molecular architecture and a common signaling mechanism involving interaction with G proteins (heterotrimeric GTPases) to regulate the synthesis of intracellular second messengers such as cyclic adenosine monophosphate (cAMP), inositol phosphates, diacylglycerol, and calcium ions (Ca2+). All GPCRs have seven-transmembrane domains (7TM), three extracellular loops (EC1, EC2, EC3), three intracellular loops (IC1, IC2, and IC3), an amino-terminal extracellular domain and an intra-cellular carboxyl terminus (Lefkowitz, 2004). This topology is predicted from the analysis of hydropathy profiles and from a limited amount of experimental evidence, most importantly from the crystal structure of the visual pigment rhodopsin (Palczewski et al., 2000), the prototypical class I GPCR for which the activating stimulus is light.
GPCRs are one of the most important classes of signalling proteins as they allow organisms to sense their environment and respond to endogenous hormones and exogenous agents. The superfamily of GPCRs can be divided into six primary classes (denoted below). All of the receptors in each class have several common characteristics, e.g., 7TM repeats, extracellular N-terminus, an intracellular carboxy terminus, and the ability to functionally couple to heterotrimeric G proteins.
Class I
Class Ia receptors share a high degree of sequence similarity and tend to bind to small molecular ligands such as catecholamines, small peptides such as opiates, and also small lipids such as the cannabanoid anandamide. The receptor-activating hormones in this family of receptors bind deep in the heptahelical transmembrane bundle. This family is often referred to as “rhodopsin-like” receptors because they are all typified by the light-sensing rhodopsin receptor.
Class Ib receptors are very similar to class Ia but tend to interact with larger ligands, e.g., cytokines, thrombin, and fMLP-peptide. In contrast to Ia receptors ligands for these receptors interact largely with the superficial areas of the heptahelical bundle, extracellular loops, and the N-terminus.
Class Ic receptors differ significantly from the Ia and Ib families owing to their larger N-terminus, often being more than 200 residues in size. This family of receptors also interacts with larger glycoprotein hormones such as luteinizing hormone and thyroid-stimulating hormone. Similar to the Ib family, the receptor–ligand interaction tends to occur mainly with the N-terminus, superficial regions of the helical bundle, and the extra-cellular loops.
Class II
Although these receptors share the heptahelical transmembrane structure with the class I receptors, the amino acid sequences of class II and class I receptors are dissimilar. These receptors interact with large, glycoprotein hormones such as secretin, glucagons, and parathyroid hormone via their extremely large (up to 400 residues) extracellular N-terminus. In contrast to the Ic class, the N-terminus appears to be the primary area of receptor–ligand interaction. We discuss the molecular mechanism of the functioning of these receptors and their implications in neuronal physiology and therapeutic neuroprotection in this review.
Class III
Similar to class II, these receptors also possess extremely large N-termini that interact with the activating hormone. However in contrast to class II, the ligands are all relatively small in mass, e.g., glutamate, Ca2+, and γ-aminobutyric acid. This GPCR family shares a global structural analogy with classes I and II, but shares very little amino acid sequence identity.
Class IV
As with classes II and III, this family shares little sequence identity with class I but does share the classical GPCR properties described in the opening paragraph. These vomeronasal (VN) receptors interact with putative pheromone molecules.
Class V
These receptors consist of the “frizzled” and “smoothened” receptors involved in embryonic development and in particular in cell polarity and segmentation.
Class VI
This class consists only of the Dictyostelium discoideum cAMP receptors. These have only been found in D. discoideum and are involved in chemo-taxis of this slime mold.
General Receptor Function
GPCRs have evolved to interact with a chemically diverse array of native ligands, e.g., endogenous compounds like amines, peptides, pheromones, and Wnt proteins (i.e., secreted proteins activating frizzled receptors); endogenous cell surface adhesion molecules; photons and even exogenous compounds like odorants. This review will concentrate on a particular subset of these tremendously important trans-membrane proteins, i.e., the class II family of GPCRs. Class II, often referred to as the secretin-receptor family of GPCRs, is a small but structurally and functionally diverse group of proteins that includes receptors for large polypeptide hormones (Laburthe et al., 1996). Class II GPCRs have been found in all animal species investigated, including mammals, Caenorhabditis elegans, and Drosophila melanogaster, but not in plants, fungi, or prokaryotes. In this article, the structures and functions of class II GPCRs will be described as well as an in-depth review of the roles of these signaling molecules in neuronal degeneration and survival mechanisms. Ligands acting in these systems may, in the near future, prove to be useful therapeutics for neurodegenerative disorders.
The combined use of site-directed mutagenesis and molecular-modeling approaches have provided detailed insights into the molecular mechanisms of ligand binding, receptor activation, G protein-coupling, and regulation of GPCRs. The vast majority of class I, II, III, and VN receptors are able to transduce signals into cells through heterotrimeric G protein-coupling. However, G protein-independent signaling mechanisms have also been reported for many GPCRs. Site-directed mutagenesis and molecular dynamics simulations have revealed that the inactive state conformations are stabilized by specific interhelical and intrahelical salt bridge interactions and hydrophobic-type interactions. Constitutively activating mutations of receptors or agonist binding disrupts such constraining interactions leading to receptor conformations that associate with and activate G proteins.
Characteristics of Class II GPCRs
The first cloned receptor of the class II family was that for secretin (Ishihara et al., 1991). This receptor was predicted to have the classical heptahelical transmembrane topology reminiscent of class I receptors. The receptor was capable of productively interacting with the Gas heterotrimeric G protein, thus elevating intracellular cAMP. In addition, coupling to Gαq and Gαi-type G proteins has been demonstrated. Interestingly, the primary amino acid sequence was found to be only distantly homologous to the other known existing class I GPCRs and therefore the secretin receptor became the archetypical member of the class II GPCR family.
Since the discovery of the secretin receptor, at least 33 human genes encoding receptor members of class II have been identified. Within class II, three distinct subfamilies of GPCRs can be identified. A simplified nomenclature has been proposed by Harmar (2001), i.e., B1, B2, and B3, for these sub-families. Subclass B1 consists of receptors for polypeptide hormones from 27 to 141 amino-acid residues in length. In this subclass, nine of the mammalian receptors respond to ligands that are structurally related to one another: glucagon, glucagon-like peptides (GLP-1, GLP-2); glucose-dependent insulinotropic polypeptide (GIP); secretin; vasoactive intestinal peptide (VIP), pituitary adenylate cyclase-activating polypeptide (PACAP) and growth hormone-releasing hormone (GHRH). All members of this subfamily have been shown to be capable of regulating intracellular concentrations of cAMP by coupling to adenylate cyclase (AC) through Gαs. Some members of the subfamily are capable of signalling through additional G protein-coupled signalling pathways, for example through Gαi/Gαq-mediated activation of phospholipase C (PLC). This subclass is by far the most studied of the class II receptors and thus we shall concentrate more intensively upon this subfamily. The subclasses B2 and B3 consist of receptors that can interact with epidermal growth factor (EGF), i.e., EGF-7TM (McKnight and Gordon, 1996: B2) and the D. melanogaster Methuselah gene product (Lin et al., 1998; for review see Harmar, 2001).
The greatest extent of knowledge of GPCR structure and function so far has been gathered upon the class I receptors (primarily the rhodopsin-like class). We will therefore concentrate on the comparison between classes I and II in this review. The heptahelical bundle of class II receptors shows only a small degree of amino acid sequence identity with the class I receptors. The class II receptors share approx 20–45% sequence identity among themselves and less than 10% identity with other families.
Class II GPCRs are structurally characterized by a relatively long N-terminus (∼120–140 amino acids) containing a set of six cysteine (Cys) residues connected by three disulphide bonds, forming a globular domain. These cysteines are completely conserved across class II receptors. The disulphide bond pattern seems to be conserved in all receptors, suggesting a very similar three dimensional structure (PTH-1R: Grauschopf et al., 2000; CRF-1R: Qi et al., 1997; GLP-1R: Bazarsuren et al., 2002). The N-terminal domain represents the receptor fragment where most of the hormone-binding activity resides. This putative hormone-binding domain, which contains three or four conserved cysteine residues and two conserved tryptophan residues, also contains an aspartate, which may be critical for ligand binding, e.g., splice variation in this region of the PACAP receptor has been shown to influence ligand-binding specificity and affinity (Dautzenberg et al., 1999).
Archetypical class I GPCR motifs, such as the E/DRY and NPX2-3Y motifs, are missing, as is the palmitoylated cysteine in the C-terminus of the protein. Conserved cysteine residues within extracellular loops EC1 and EC2 probably form a disulphide bridge similar to those in class I GPCRs. In contrast to class I GPCRs, which rely on internal hydrophobic sequences for targeting to the plasma membrane, most class II GPCRs appear to have an amino-terminal signal peptide for plasma membrane insertion. Site-directed mutagenesis and the construction of chimeras between hormone receptors in class II have shown that the amino-terminal extracellular domain is essential for ligand binding but that transmembrane domains and extracellular loops can assist with encoding for specific interaction with ligands.
One of the most important structural regions of class I receptors is the IC3, which allows productive interaction with heterotrimeric G proteins. The IC3 of class II GPCRs also contains the major determinants required for specific G protein-coupling as splice variation in this region can give rise to PACAP receptors that differ in their ability to couple to different G proteins (Pisegna et al., 1996). Alternative splicing in IC1 of the CRH1 receptor (Nabhan et al., 1995) and calcitonin (Nussenzveig et al., 1995) receptors has also been reported to influence G protein coupling.
An interesting difference between class I and class II GPCRs is that many of the receptors in class II can naturally exhibit a high degree of agonist-independent (constitutive) activity in the wild-type form, e.g., naturally occurring point mutations of the human PTH receptor have been reported to be associated with constitutive activation of the PTH receptor in Jansen-type metaphyseal chondrodysplasia causing severe hypocalcaemia and hyper-calciuria (Schipani et al., 1999). Mutagenesis of the equivalent amino acid residues in the GIP receptor (Tseng and Lin, 1997) and the VPAC1 receptor (Gaudin et al., 1998) also leads to their constitutive activity.
Class II Receptor Ligands
The primary ligands for the class II (subgroup B1) are listed in Table 1. Most endogenous functional hormones that bind class II receptors are peptides 30–40 amino acids long. Many of the hormones are created as much larger pro-hormones that are then processed into the active hormones. In addition, there seem to be multiple biologically active truncated forms of the processed hormones present.
Table 1.
Class II Peptide Hormones and Receptors
| Receptor: Gene name | Functional ligand | Primary original function |
|---|---|---|
| Secretin receptor: SCTR | Secretin | Pancreatic secretion (HCO3−: K+) (Drucker et al., 2000) |
| Glucagon receptor: GCGR | Glucagon | Glycogenolysis and gluconeogenesis, pancreatic insulin secretion (Drucker et al., 2000) |
| Glucagon-like peptide 1 receptor: GLP1R | Glucagon-like peptide 1 (GLP-1) | Pancreatic secretion of insulin and glucagons (Drucker et al., 2000). |
| Glucagon-like peptide 2 receptor: GLP2R | Glucagon-like peptide 2 (GLP-2) | Proliferation of intestinal epithelium (Drucker et al., 2000). |
| Growth hormone-releasing hormone receptor: GHRHR | Growth hormone releasing hormone (GHRH) | Pituitary relase of growth hormone (Drukcer et al., 2000). |
| PAC1 receptor: ADCYAP1R1 | Pituitary adenylate cyclase activating peptide (PACAP) | Glucose homeostasis, nociception, learning/memory, circadian rhythm (Otto et al., 2001; Hannibal et al., 2001; Jongsma et al., 2001; Jamen et al., 2000). |
| VPAC1 receptor: VIPR1 | Vasoactive intestinal peptide (VIP)/PACAP | Neuromodulation, T-cell differentiation |
| VPAC2 receptor: VIPR2 | VIP/PACAP | Circadian rhythms (Shen et al., 2000) |
| Corticotropin-releasing hormone 1 receptor: CRHR1 | Corticotropin-releasing hormone (CRH)/ urocortin | Secretion of adrenocorticotropin hormone (ACTH) (Lewis et al., 2001) |
| Corticotropin-releasing hormone 2 receptor: CRHR2 | Urocortin, urocortin II, urocortin II (CRH) | Stress-related behavioural and neuroendocrine function (Lewis et al., 2001). |
| Parathyroid hormone receptor 1: PTHR1 | Parathyroid hormone (PTH), PTH-related peptide (PTHrP) | Ca2+ homeostasis (bone and kidney), tissue differentiation (Dunbar et al., 1999; Wysolmerski et al., 1998). |
| Parathyroid hormone receptor 2: PTHR2 | Tuberoinfundibular peptide of 39 residues (TIP-39) | Renal vessel dilation (Eichinger et al., 2002) |
| Calcitonin receptor: CALCR | Calcitonin (+RAMP2/3) Amylin (AMY) (+RAMP1/3) Calcitonin gene-related peptide (CGRP) (+RAMP1) | Calcitonin, Ca2+ homeostasis: Amylin postprandial glucagon secretion |
| Calcitonin receptor-like receptor: CALCRL | CGRP (+RAMP1) Adrenomedullin (AM) (+RAMP2/3) | Microvascular tone |
| Gastric inhibitory polypeptide receptor: GIPR | Gastric inhibitory peptide (GIP) | Pancreatic secretion of insulin (Drucker et al., 2000) |
The genes encoding the structurally related class II peptides secretin, glucagon, GLP-1, GLP-2, GHRH, and GIP are primarily expressed in the gastrointestinal tract and/or brain but can also be found in many other tissues in the body. Asingle proglucagon gene in mammals (Irwin, 2001) encodes three distinct structurally related peptides, glucagon, GLP-1 and GLP-2, which exhibit unique biological actions mediated by separate receptors (Drucker, 2000). Several members of the secretin peptide family, including secretin, GLP-1 (7-36 amide), and GHRH are amidated at the carboxyl terminus, however amidation is not always an invariant requirement for biological activity, as the nonamidated GLP-1 (7-37) is equipotent with GLP-1 (7-36 amide) (Orskov et al., 1993). Similarly, GHRH, GLP-1, GIP and GLP-2 are excellent substrates for the enzyme dipeptidyl peptidase IV, which inactivates these peptides following cleavage at the position 2 alanine or proline (Lambeir et al., 2002).
Interaction of Class II Peptides With Their Receptors
Structure-activity studies have indicated that receptor activation determinants are grouped in the extreme N-terminal end of the class II peptides, which can be referred to as their “activation domain.” For example, in the GLP-1/glucagon subgroup, amino acids Histidine1, Aspartate/Glutamate3, Phenylalanine6 and Threonine/Serine7 in this region are very highly conserved and define their signature. In GLP-1, the first histidine, in particular, is essential for efficacy. Its removal renders a peptide devoid of agonistic properties (Adelhorst et al., 1994). The same effect is seen in other peptides, e.g., glucagon (Unson et al., 1993). This general scheme even applies to the distantly related PTH, which does not share a high primary sequence with GLP-1 at all, but nonetheless deletion of the first two amino acids also converts it into a potent competitive antagonist (Nutt et al., 1990). Interestingly, in this case, it has been postulated that the determinant responsible for selective signalling may not be equally located in the hormone. For example, although the nine N-terminal residues of PTH are critical for receptor-mediated AC activation, residues 29–32 have been proposed to be determinants for PLC activation (Jouishomme et al., 1994). This postulate, however, has not been explored in other class II ligands. Amino acids contributing to binding affinity are seemingly more widely dispersed along the hormone molecule. In general terms, the structural features most important for receptor binding appear to be clustered in the C-terminal half of the peptides, which can be designated the “principal binding domain.” Residues located in this region provide the high affinity required for potent and specific interaction with the various class II receptors. For instance, the last four residues of GLP-1 are thought to determine much of the selectivity for GLP-1R (Hjorth et al., 1994; Xiao et al., 2001). However some binding determinants located in the N-terminal region of the hormone are also required for high-affinity binding, e.g., GLP-1 is very sensitive to N-terminal modification as alanine-substitution of a single residue, His1, reduces its affinity >100-fold (Adelhorst et al., 1994). In summary, the highly conserved first residues appear to be involved in receptor activation, carrying a common message for all ligands of class II GPCRs. On the other hand, the more heterogeneous C-terminus of the hormone would determine binding selectivity of each hormone for its cognate receptor.
As a general working rule, the C-terminus of the peptides interact with the N-terminus of their receptors while the N-terminus of the hormones contact the receptor juxtamembrane region (Bergwitz et al., 1996; Gardella and Juppner, 2001). Using chimeric PTH-1/secretin receptors, the proximal N-terminus in PTH-1R has been singled out as a high-affinity interaction site for PTH, but seems not to be required for full activation of the receptor. In contrast, the portion near transmembrane (TM) domain 1 (TM1) is proposed as a low-affinity binding site but with a more pronounced role in signal transduction (Vilardaga et al., 2001). Extensive studies with chimeric and truncated receptors have established that the N-terminal domain is the major high-affinity determinant. Deletion or exchange of this domain leads to a great loss of specific binding (Buggy et al., 1995; Bergwitz et al., 1996; Vilardaga et al., 2001). The most definite proof of the outstanding significance of the N-terminus is that this isolated domain can be expressed (in E. coli and mammalian cells) as an independent unit able to bind the ligands, albeit with a much reduced affinity (SecR: Chow, 1997; PTH-1R: Grauschpof et al., 2000; CRFR: Perrin et al., 2001; GLP-1R: Bazarsuren et al., 2002; Lòpez de Maturana et al., 2003). Therefore, in general, the ligand-binding pocket is primarily located in the extracellular domains of these receptors.
Promiscuity of Class II Receptor–Ligand Interactions
As mentioned previously, many of the class II ligands are structurally related (for sequences see Table 2) and at least three of the receptors can respond selectively to different ligands that are all synthesized by posttranslational processing of proglucagon. The VPAC1 and VPAC2 receptors can respond to physiological concentrations of two different ligands (PACAP and VIP), whereas the PAC1 receptor responds selectively only to PACAP. Two receptors have been identified that bind corticotropin-releasing hormone (CRH). CRH is the primary ligand for the CRHR1 receptor while the most potent agonists for the CRHR2 receptor are the urocortins. Urocortins comprise a family of peptides structurally related to CRH but are encoded by different genes with different expression patterns (Lewis et al., 2001). In a similar manner the PTH1 and PTH2 receptors appear to be specific for different physiological ligands. Hence, PTH1 receptors respond to parathyroid hormone and parathyroid hormone-related protein (PTHrP), whereas PTH2 receptors respond to the “tuberoinfundibular peptide of 39 residues” (TIP39), a novel peptide isolated from bovine hypothalamus (Christopolous et al., 1999).
Table 2.
Class II Peptide Hormone Sequences
| 1 | 10 | 20 | 30 | 40 | ||
|---|---|---|---|---|---|---|
| PACAP | ||||||
| human/rat/mouse | HSDGIFTDSYSRYRKQMAVKKYLAAVLGKRYKQRVKNK | |||||
| GHRH | ||||||
| human | YADAIFTNSYRKVLGQLSARKLLQDIMSRQQGESNQERGARARL | |||||
| rat | HADAIFTSSYRRILGQLYARKLLHEIMNRQQGERNQEQRSRFN | |||||
| mouse | HVDAIFTTNYRKLLSQLYARKVIQDIMNKQ-GERIQEQRARLS | |||||
| VIP | ||||||
| human/rat/mouse | HSDAVFTDNYTRLRKQMAVKKYLNSILN | |||||
| PHM/PHI | ||||||
| human PHM | HADGVFTSDFSKLLGQLSAKKYLESLM | |||||
| rat/mouse PHI | HADGVFTSDYSRLLGQISAKKYLESLI | |||||
| Glucagon | ||||||
| human/rat | HSQGTFTSDYSKYLDSRRAQDFVQWLMNT | |||||
| GLP-1 | ||||||
| human/rat | HDEFERHAEGTFTSDVSSYLEGQAAKEFIAWLVKGR | |||||
| GLP-2 | ||||||
| human | HADGSFSDEMNTILDNLAARDFINWLIQTKITD | |||||
| rat | HADGSFSDEMNTILDNLATRDFINWLIQTKITD | |||||
| Secretin | ||||||
| human | HSDGTFTSELSRLREGARLQRLLQGLV | |||||
| rat | HSDGTFTSELSRLQDSARLQRLLQGLV | |||||
| GIP | ||||||
| human | YAEGFTISDYSIAMDKIHQQDFVNWLLAQKGKKNDWKHNITQ | |||||
| rat | YAEGFTISDYSIAMDKIRQQDFVNWLLAQKGKKNDWKHNLTQ | |||||
| mouse | YAEGFTISDYSIAMDKIRQQDFVNWLLAQRGKKSDWKHNLTQ | |||||
| PTH | ||||||
| human | SVSEIQLMHNLGKHLNSMERVEWLRKKLQDVHNF | |||||
| PTHrP | ||||||
| human | AVSEHQLLHNKGKSIQDLRRRFFLRHHLIAEITA | |||||
| TIP-39 | ||||||
| human | SLALADDAAFRERARLLAALERRHWLNSYMHKLLVLDAP | |||||
| Calcitonin family | ||||||
| human αCGRP | ACDTATCVTHRLAGLLSRSGGVVKNNFVPTNVGSKAF | |||||
| rat αCGRP | SCNTATCVTHRLAGLLSRSGGVVKDNFVPTNVGSEAF | |||||
| human βCGRP | ACNTATCVTHRLAGLLSRSGGMVKSNFVPTNVGSKAF | |||||
| rat βCGRP | SCNTATCVTHRLAGLLSRSGGVVKDNFVPTNVGSKAF | |||||
| human AMY | KCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTY | |||||
| rat AMY | KCNTATCATQRLANFLVRSSNNLGPVLPSTNVGSNTY | |||||
| human AM | GCRFGTCTVQKLAHQIYQFTDKDKDNVAPRNKISPQGY | |||||
| rat AM | GCRFGTCTMQKLAHQIYQFTDKDKDGMAPRNKISPQGY | |||||
| human CT | CGNLSTCMLGTYTQDFNKFHTFPQTAIGVGAP | |||||
A family of three small accessory proteins called receptor activity-modifying proteins (RAMPs) play a significant role in determining the ligand-binding specificity of two hormone receptors in subfamily B1 in a manner that has not been described for any other class of GPCRs. These auxiliary proteins, RAMPs 1, 2, and 3, consist of a large extracellular N-terminal domain and a single transmembrane spanning domain and a relatively small intracellular carboxyl terminus. Co-expression of the human calcitonin-receptor-like (CALCRL) gene with RAMP1 results in a receptor selective for calcitonin-gene-related peptide (CGRP), whereas RAMP2 and RAMP3 promote the expression of receptors selective for adrenomedullin (Hilairet et al., 2001). Expression of the calcitonin receptor (CALCR) gene with RAMP2 results in a receptor responding most potently to calcitonin, whereas expression of CALCR with RAMP3 results in a receptor responding to both calcitonin and the pancreatic hormone amylin. Finally, the expression of CALCR with RAMP1 promotes the formation of a receptor responding most potently to amylin and CGRP(McLatchie et al., 1998; Foord et al., 1999; Sexton et al., 2001). Thus, the interaction of the products of two receptor genes with the three RAMPs is potentially capable of generating six pharmacologically distinct receptors.
How RAMPs contribute to the specific binding event is still a subject of much investigation. In addition to the calcitonin-family receptors, RAMPs have recently been shown to interact with other class II GPCRs, namely the VPAC1, PTH-1, PTH-2, and glucagon receptors (Christopoulos et al., 2003). The association between VPAC1 and RAMP2 did not modify the affinity and specificity of the receptor–ligand interaction, however, the VPAC1 R–RAMP2 heterodimer displayed a higher efficacy for PLC activation with no change in AC activation compared with the VPAC1R alone. This suggests that RAMPs may modulate receptor function in a way we are now only beginning to appreciate. If this phenomenon proves to be more widespread than is known at present, it will change the way molecular pharmacologists view the function of GPCRs and how they associate with their cognate ligands.
Class II Receptor Activation Mechanisms
A “two-site” model for the interaction of class II GPCRs with their ligands has been proposed. The helical sequences of the peptide hormones appear to recognise high-affinity epitopes located in the N-terminal receptor domain and also other low-affinity binding determinants in outer regions of TM helices and extracellular loops. The initial seven residues of the hormone sequence seems to interact with an activation site within the juxtamembrane region, triggering a series of conformational changes in the receptor that culminate in the activation of intracellular signalling cascades. It is unclear whether the ligand interacts with its receptor sequentially (at the two sites) or not. It has been suggested that high-affinity binding to the proximal region of the N-terminal receptor domain facilitates the low-affinity association of the peptide with other epitopes closer to the juxtamembrane region, i.e., distal N-terminal domain, extracellular loops and outer portions of TM helices, thus allowing initiation of the signal transduction process. Although most of the detailed experimental evidence used to delineate this mechanism comes from studies with PTH-1R, this generic model seems to apply to all class II GPCRs (e.g., GLP-1R; Al-Sabah and Donnelly, 2003).
Class I receptor activation typically occurs with the early protonation of a highly conserved aspartate residue in IC2 (below TM3) that forms part of a three residue motif (aspartate[D]-arginine[R]-tyrosine[Y]) often referred to as the DRY motif. Two hydrophobic conserved residues in class II GPCRs, tyrosine and leucine, are predicted to be aligned with the highly conserved aspartate and arginine in the DRY motif at the bottom of TM3 of the GLP-1 receptor (Frimurer and Bywater, 1999). Single mutations of these residues to alanine did not markedly affect VIP affinity but impaired cAMP accumulation mediated by the VPAC1R (Knudsen et al., 2001). G protein coupling was shown to be defective in these mutant receptors. Thus, the highly divergent chemical properties of the hydrophobic YL motif and charged DRY motif could implicate a key functional difference between the two families. The study of chimeric receptors has identified other receptor segments that could play a role in signal transduction, such as TM3 and TM6 (Vilardaga et al., 2001), the end of ECL1 (Holtmann et al., 1996), ECL2 (Holtmann et al., 1996), and ECL3 (Vilardaga et al., 2001).
Deletion or substitution of the N-terminal domain of the receptors results in no or very little activation, which is attributed to defective binding. Indeed, a truncated PTH-1 receptor in which the N-terminus had been replaced by the first nine residues of the PTH peptide was able to autoactivate (Shimizu et al., 2000). A parallel report exists for the CRH receptor (Nielsen et al., 2000). Removal of this segment in constitutively active glucagon receptors however does not seem to affect their activation (Hjorth et al., 1998). Chimeric GLP-1 receptors incorporating the N-terminus of the GIP receptor still bind GLP-1 but are, however, not activated (Xiao et al., 2001). Furthermore, substitution of only a short sequence at the beginning of this domain in the GLP-1 receptor with the corresponding segment of the GIP receptor completely abrogates activation of the chimera (Gelling et al., 1997). This suggests a more direct role of the N-terminal domain in receptor activation, at least in the GLP-1R.
Class II GPCR Peptides in the Central Nervous System and Neuroprotection
The signal transduction pathways activated by class II GPCRs are known to be involved in the regulation of a variety of neuronal functions including synaptic transmission and plasticity, and the behaviors they control including learning and memory, emotions, and sensory and motor functions. Although one or more class II GPCR and their ligands are expressed in most (if not all) brain regions, very little is known of their normal functions in those neuronal circuits. In the remainder of this review article we will address the possible functions of class II GPCRs in the brain, and will also consider their roles in neurodegenerative disorders.
Several of the most common neurological disorders involve degeneration and death of specific populations of neurons in particular regions of the brain or spinal cord (Table 3). In Alzheimer's disease (AD), pyramidal neurons in the entorhinal cortex, hippocampus and frontal and temporal lobes degenerate (Mattson, 2004). Neurons in the substantia nigra that employ the neurotransmitter dopamine degenerate in Parkinson's disease (Barzilai and Melamed, 2003), whereas medium spiny neurons in the caudate and putamen die in Huntington's disease (Sieradzan and Mann, 2001). Amyotrophic lateral sclerosis (ALS) is characterized by selective degeneration of motor neurons in the spinal cord (Bruijn et al., 2004). In stroke, neurons in the brain region(s) that receive blood from the affected artery degenerate (Mergenthaler et al., 2004), whereas in traumatic brain or spinal cord injury, tissues at and adjacent to the site of injury are affected (Dias, 2004). Although the causes of each of these neurodegenerative conditions are different, they do share mechanisms that include oxidative stress, metabolic compromise, and disruption of cellular calcium homeostasis (Rao and Balachandran, 2002; Hashimoto et al., 2003; Mattson, 2003). GPCRs for class II peptides are expressed in neurons and/or glial cells in regions of the central nevous system (CNS) affected in neurodegenerative conditions, and increasing evidence suggests important roles for these signaling pathways in modifying the neurodegenerative process. The remainder of this review article considers the roles of class II GPCRs in neuronal cell survival in the context of neurodegenerative disorders.
Table 3.
Characteristics of Neurodegenerative Disorders
| Disorder | Neurons affected | Major symptoms |
|---|---|---|
| Alzheimer's disease | Entorhinal cortex, hippocampal pyramidal, basal forebrain cholinergic, amygdala, temporal and frontal lobes. brainstem monoaminergic | Cognitive impairment, emortional disturbances (depression, agitation), disturbed circadian rythyms |
| Parkinson's disease | Substantia nigra dopaminergic, cerebral cortical | Motor dysfunction, emotional disturbances, cognitive impairment |
| Huntington's disease | Striatal medium spiny neurons (GABAergic/ motor dysfunction, emotional enkephalinergic), cerebral cortical |
Disturbances, cognitive impairment |
| Stroke | Depends on vessel affected: may involve cortical, basal ganglia, brainstem or cerebellum | Sensory and/or motor deficits, cognitive impairment, motor dysfuntion |
| Amyotrophic lateral sclerosis | Spinal cord and brainstem lower motor neurons, cerebral cortical upper motor neurons | Progressive paralysis |
| Spinocerebellar ataxia | Cerebellar Purkinje cells | Impaired motor control |
Secretin
Secretin, a 27- residue peptide, was the first functional “hormone” discovered in human history (1902). Nevertheless, its function as a neuropeptide has been overlooked in the past. Secretin was first identified and sequenced from secretory granule-containing endocrine S-cells from which it is secreted into the duodenum and proximal jejunum (Mutt, 1980). Secretin acts on epithelial cells lining the pancreatic and biliary ducts, leading to the secretion of alkaline bicarbonate-rich fluid. This, in turn, helps to neutralize the acidic chyme emptied from the stomach. Secretin also slows gastric emptying to further protect the duodenum from excessively acidic chyme. Secretin is produced in both the periphery and brain (Fremeau et al., 1983; Itoh et al., 1991; Konturek et al., 2003). It is considered part of the brain–gut axis having a role in digestive activities and feeding behavior. The classical sites for secretin receptor localization are on epithelial cells within the pancreatic and biliary ducts (Ulrich et al., 1998). Additionally, secretin receptors are present on pancreatic acinar cells, gastric epithelial cells, intestinal epithelial cells, Brunner's glands, gastric and intestinal smooth muscle cells, and certain areas of the brain. Like many of the class II receptors, the secretin receptor has been shown to couple to Gαs however the secretin receptor also couples to Gαq. Receptor activation leads to increases in both cAMP and intracellular calcium (Trimble et al., 1987). Promiscuous coupling is typical of this receptor family and, like other members, the Gs coupling and cAMP signaling occur at the lowest concentrations of hormone and represents the physiological signaling pathway. The Gαq coupling and intracellular calcium response occur in response to concentrations of secretin more than 100-fold higher than those stimulating the other pathway, suggesting that this is an auxiliary signalling function.
The early literature about the occurrence of secretin in the CNS was relatively scant (Mutt et al., 1979; O'Donohue et al., 1981). However, receptors for secretin in several brain regions have been demonstrated. The concentration of secretin receptors has been observed to be highest in the cerebellum, intermediate in the cortex, thalamus, striatum, hippocampus, and hypothalamus, and lowest in the brainstem and medulla (Fremeau et al., 1983; Yung et al., 2001). Expression of secretin and of the secretin receptor has also subsequently been detected in the central amygdala, hippocampus, area postrema, nucleus of the tractus solitarus, and cerebellum (Tay et al., 2004). Interestingly, secretin can pass from the systemic circulation to the brain as secretin administered intravenously can be recovered intact in biologically significant quantities from various brain regions including the hypothalamus and hippocampus (Banks et al., 2002). Secretin administered intravenously induces c-fos protein expression in several brain regions as well as the area postrema, a circumventricular organ (Goulet et al., 2003). A direct neuronal effect could be initiated from peripheral secretin crossing the blood–brain barrier, however the possibility exists for systemic secretin to have CNS activity through vagal actions and/or effects on neurons located within circumventricular organs. Intracerebroventricular administration of secretin can stimulate pancreatic function and also induces c-fos protein expression in similar brain regions as those targeted by peripheral secretin administration (Welch et al., 2003) demonstrating a tight connection between central and peripheral functions of this hormone.
With respect to the neurophysiological and/or therapeutic actions of secretin Horvath et al. (1998) described improved behavioral and language skills in autistic children who received intravenous porcine secretin during endoscopic procedures. Subsequent controlled studies in autism (Dunn-Geier et al., 2000; Lightdale et al., 2001; Owley et al., 2001), have either failed to confirm the Horvath study, or have demonstrated a modest trend toward behavioral improvements (Coniglio et al., 2001). Secretin has also been described as being potentially therapeutic for disorders distantly related to autism such as schizophrenia (Sheitman et al., 2004). As far as a clinical mechanism for these potential therapeutic effects of secretin it has been demonstrated that secretin released from the somatodendritic regions of cerebellar cortex purkinje cells can serve as a retrograde messenger modulating GABAergic afferent activity by facilitating evoked, spontaneous and miniature inhibitory postsynaptic currents (Yung et al., 2001).
In addition to endogenous central secretin recently two peptides known as “hypocretins” (also called orexins) with substantial sequence homology to secretin, have recently been described in the hypothalamus (DeLecea et al., 1998). Animal studies suggest that the hypocretins contribute to the regulation of sleep, arousal and motivation (Siegel, 1999). Levels of hypocretins were reported to be decreased in the cerebrospinal fluid of PD patients (Drouot et al., 2003). Whether therapeutic modulation of secretin and these novel neuropeptides will prove to be beneficial for neurodegenerative disorders largely remains to be investigated.
Glucagon and Glucagon-Like Peptides (GLPs)
Glucagon is a 29-amino acid peptide originally isolated from a side fraction of purified insulin (Kenny, 1955) as a hyperglycemic factor originating from the pancreas. Its primary structure is identical in most mammals including man, although some amino acid sequence changes are noted in glucagons from guinea pig and non-mammalian vertebrates (Irwin, 2001). Glucagon is synthesized mainly in the α-cells at the periphery of the islets of Langerhan's (Baum et al., 1962). Glucagon can be detected in specific cells in the stomach and intestine in some species (Baetens et al., 1976), as well as in specialized neurons of the CNS. Isolation of cDNAs encoding glucagon (Lund et al., 1982) showed that the peptide is produced from a 160-amino acid precursor, proglucagon, which also contains two additional glucagon-like sequences at its carboxyl terminus, GLP-1 and GLP-2, which were subsequently shown to display specific biological activities (Drucker, 1998). Rodbell and coworkers (Rodbell et al., 1971) established that the glucagon receptor is involved in the Gαs-dependent activation of AC. At very low doses glucagons may also activate the PLC/inositol phosphate pathway leading to Ca2+ mobilization. In at least some cell types, such as BHK fibroblasts, both receptor-mediated Gαs and Gαi activation contributes to the Ca2+ response to glucagon (Wakelam et al., 1986; Hansen et al., 1998). In addition, GLP-1-dependent stimulation of intracellular calcium can occur via a ryanodine-sensitive pathway (Holz et al., 1999) in a cAMP-dependent, protein kinase A (PKA)-independent manner through heterotrimeric or small monomeric G proteins distinct from Gαs, e.g., Rab3, Gαi, Gαq (Kang et al., 2001; Kashima et al., 2001; Hallbrink et al., 2001). GLP-1 stimulation can also induce closure of ATP-sensitive potassium (KATP) channels providing a cellular mechanism for the glucose-sensitivity of GLP-1 action in β-cells (Light et al., 2002).
The glucagon receptor was found to be expressed mainly in liver and kidney and, to a lesser extent, in heart, adipose tissue, spleen, thymus, adrenal glands, pancreas, cerebral cortex, and throughout the gastrointestinal tract of rats (Svoboda et al., 1994; Dunphy et al., 1998). Specifically, in the rat brain, radioligand-binding studies detected glucagon binding sites in the olfactory tubercle, hippocampus, anterior pituitary, amygdala, septum, medulla, thalamus, olfactory bulb, and hypothalamus (Hoosein and Gurd, 1984). In rat brain, GLP-1 receptors have been found in the lateral septum, subfornicalorgan, thalamus, hypothalamus, interpeduncular nucleus, posterodorsal tegmental nucleus, area postrema, inferior olive, and nucleus of the solitary tract (Goke et al., 1995; Shughrue et al., 1996).
GLP-1 has CNS effects resulting in delayed gastric emptying (Schirra et al., 1997) and appetite regulation (Turton et al., 1996; Gutzwiller et al., 1999). Systemic administration of GLP-1 in rodents activates the sympathetic nervous system leading to increased tyrosine hydroxylase gene transcription, enhanced sympathetic outflow, and increased heart rate and blood pressure (Barragan et al., 1999). As with secretin the circulating peptide may gain access to the brain from the periphery by simple diffusion (Kastin et al., 2002).
Activation of brain GLP-1 receptors likely occurs via GLP-1 produced in the brainstem, which then is transported to distant regions of the CNS (Drucker, 1988; Jin et al., 1988; Larsen et al., 1997; Merchenthaler et al., 1999) such as the area postrema (Kastin et al., 2002; Yamamoto et al., 2002). GLP-1 receptor activation has been proposed as a therapeutic strategy for treatment of peripheral diabetic neuropathy and other neurodegenerative processes. GLP-1, and a longer-acting analogue exendin-4, protects cultured rat hippocampal neurons against glutamate-induced apoptosis and protected basal forebrain cholinergic neurons against excitotoxic damage in rats (Perry et al., 2002a). In addition, GLP-1 protected neurons against the toxicity of amyloid β-peptide in a model relevant to AD (Perry et al., 2003). The mechanism whereby GLP-1 protects neurons involves cAMP production and stabilization of cellular calcium homeostasis (Perry et al., 2002a; Gilman et al., 2003). GLP-1 can also promote nerve growth factor-mediated differentiation in rat phaeochromocytoma (PC12) cells (Perry et al., 2002b). Activation of GLP-1 receptor signalling can also promote proliferative and anti-apoptotic actions in the endocrine pancreas, providing a potential opportunity for interventions directed at expanding β-cell mass in subjects with diabetes (Li et al., 2003; Drucker, 2003).
GLP-2, like GLP-1, possesses central actions and has been shown to activate AC in rat hypothalamic and pituitary membranes (Hoosein and Gurd, 1984). AcDNA encoding a specific GLP-2 receptor was isolated from hypothalamic and intestinal cDNA libraries (Munroe et al., 1999). In experimental scenarios GLP-2 can activate cAMP production in rodent and human cells transfected with the rat or human GLP-2 receptors (Munroe et al., 1999; Yusta et al., 1999). With respect to its effect on cellular metabolism, GLP-2 promotes enlargement of the gut mucosa in rodents in vivo, while only promoting a weak direct proliferative response in fibroblasts transfected with the rat GLP-2 receptor (Yusta et al., 1999). As with GLP-1, GLP-2 can prevent apoptosis; for example, it inhibited cycloheximide-induced apoptosis in BHK-GLP-2R cells, with reduced DNA fragmentation and improved cell survival in association with a reduced activation of caspase-3 and decreased poly(ADP-ribose) polymerase cleavage (Yusta et al., 2000). GLP-2 can also reduce mitochondrial cytochrome c release and decrease the cycloheximide-induced cleavage of caspase-3 independently of PKA (Yusta et al., 2000). Similarly, GLP-2 increased cell survival following cycloheximide in the presence of the MAP kinase inhibitor PD98054 and the phosphatidylinositol 3-kinase inhibitor LY294002 (Yusta et al., 2000). The anti-apoptotic actions of the rat GLP-2 receptor in transfected cells are not strictly dependent on phosphatidylinositol 3-kinase (PI3-K) or Akt, as GLP-2 directly promotes cell survival, enhances glycogen synthase kinase-3 and BAD phosphorylation, and reduces mitochondrial-associated BAD and Bax following LY294002-induced apoptosis in a PKA-dependent manner (Yusta et al., 2002). More importantly, along with its effects upon cell survival it has recently been shown that GLP-2-stimulated PKA activity can significantly reduce glutamate-induced excitotoxic injury in hippocampal cells. These findings demonstrate that GLP-2 in a similar manner to GLP-1 exerts cytoprotective actions in cells derived from the CNS (Lovshin et al., 2004)
Growth Hormone-Releasing Hormone
GHRH is a peptide hormone of 42–44 amino acids, depending on the species, which is proteolytically processed from a larger precursor protein of 103–108 amino acids (Mayo et al., 1983, 1985; Frohman et al., 1989). GHRH is released from neurosecretory cells in the arcuate nuclei of the hypothalamus (Merchenthaler et al., 1984; Sawchenko et al., 1985). The predominant site of GHRH receptor expression is the pituitary gland (Lin et al., 1992; Mayo, 1992), within the pituitary gland, its expression is confined to the anterior lobe (Lin et al., 1992; Mayo, 1992). It remains uncertain whether pituitary cells other than growth hormone-secreting somatotrophs express the GHRH receptor mRNA.
The regulation of somatic growth in vertebrate species is under complex hormonal control. Pituitary growth hormone (GH) synthesis and secretion is regulated by direct neuroendocrine signals from the brain, as well as numerous peripheral feedback cues. GHRH directly stimulates growth hormone synthesis and secretion while somatostatin directly opposes the action of GHRH and functionally suppresses growth hormone secretion.
GHRH receptor stimulation results in activation of AC and PKA(Lin et al., 1992; Mayo, 1992; Gaylinn et al., 1993). In somatotroph cells, GHRH can also stimulate an influx of calcium (Holl et al., 1988), most likely through activation of voltage-sensitive Ca2+ channels (VSCCs). Although GHRH is reported to stimulate the phospholipase C-inositol phosphate-calcium mobilization pathway in pituitary cells (Canonico et al., 1983; Ohlsson and Lindstrom, 1990), other studies have reported no activation of this pathway (Escobar et al., 1986; French et al., 1990). Recent studies demonstrate that GHRH receptor signaling can also lead to the activation of the MAP kinase pathway and ERK phosphorylation, at least in some cell types (Pombo et al., 2000; Zeitler and Siriwardana, 2000). It is likely that this function relates to the ability of GHRH to stimulate somatotroph cell proliferation.
GH, secreted from the pituitary gland in response to GHRH, acts on many peripheral target tissues to alter cellular metabolism, proliferation, and differentiation. Many of the effects of GH are also mediated by the generation and action of insulin-like growth factor (IGF)-I, a strong proliferative growth factor and a potent neuroprotective compound (for review, see Mattson et al., 2004). In turn, there is considerable evidence that IGF-I can participate in the physiological regulation of GH. The IGF-I receptor is expressed both in the hypothalamus, and the pituitary (Goodyer et al., 1984: Rosenfeld et al., 1984). In the hypothalamus, IGF-I can regulate GH secretion as it stimulates somatostatin release (Berelowitz et al., 1981) and concomitantly can inhibit GHRH release (Shibaski et al., 1986). Thus, in the pituitary IGF-I has been shown to reduce the secretion and synthesis of GH (Yamashita and Melmed, 1986). Indeed IGF-I has also been shown to downregulate the expression of GHRH receptor itself (Sugihara et al., 1999), thus demonstrating a close and complex relationship between GH, IGF-1 functionality and the effective capacity of GHRH.
Concerning matters of neurodegeneration, GH secretion has been shown to decline with aging. Parallels between normal aging and the signs and symptoms of adult premature GH deficiency have led to interest in the potential utility of replacing or stimulating GH release to improve physical and psychological function thus prolonging the capacity for independent living in older adults. Aging and its associated neurodegeneration is often correlated with the declining activity of the GH–IGF-I axis resulting in changes in body composition, function, and metabolism that show strict similarities with those of younger adults with pathological GH deficiency (Lanfranco et al., 2003). The age-related changes of the GH–IGF-I axis activity are mainly dependent on variations in the hypothalamic control of somatotroph function. This can also be affected by changes in peripheral hormones and metabolic input. Thus the term “somatopause” indicates the potential link between the possible dys-function of GHRH and the age-related decline in GH and IGF-I levels that cause the changes in body composition, structural functions, and metabolism characteristic of aging.
The involvement of the GHRH–GH–IGF-I system in neurodegenerative disorders has been suggested from several studies. Some studies have reported blunted GH responses to GHRH in patients with AD (Nemeroff et al., 1989; Lesch et al., 1990), whereas other studies have been inconclusive (Heuser et al., 1992). Interestingly, in PD, there appears to be an impairment in the stimulation of GH release by serotonin, although GH responses to GHRH may remain intact (Volpi et al., 1997). GH-releasing molecules such as GHRH could be used therapeutically to restore the activity of the GH–IGF-I axis with beneficial anabolic, anti-aging results. However, the beneficial action of such treatment in elderly subjects or patients with neurodegenerative disorders has not yet been established (Lanfranco et al., 2003).
Another potential therapeutic mechanism used to interdict and ameliorate the deleterious effects of aging is dietary caloric restriction. It has been shown that in response to moderate caloric restriction (a treatment that increases mean and maximal lifespan by 30–40%), age-related decreases in GH secretion are ameliorated (despite a decline in plasma levels of IGF-I) suggesting that some of the effects of caloric restriction are mediated by modifying the regulation of the GH–IGF-I axis (Sonntag et al., 2000).
The aging pituitary remains responsive to GHRH and to GHrelin-mimetic GH secretagogues (GHS), and these agents have both theoretical and practical potential advantages as alternatives to the use of GH itself in this setting. It has been reported that the age-related changes of the GH system seem to be the result of the reduction in GHRH-positive neurons, but not by somatostatin positive neurons. (Kuwahara et al., 2004). In aging patients, administration of GHRH, stimulating GH secretion, when given repeatedly can elevate IGF-I levels to those seen within younger adult normal ranges. Compared to placebo, GHRH treatment improved scores in certain tests of cognitive performance. Administration of GHRH (to increase endogenous release of GH) or direct administration of IGF-I was shown to reverse the age-related decline in spatial working and reference memory (Thornton et al., 2000). Interestingly, antagonism of IGF-I action in the brains of young animals impaired both learning and reference memory. IGF-I has been shown to regulate age-related alterations in N-methyl d-aspartate receptor subtypes (e.g., NMDAR2A and R2B), to increase local cerebral glucose utilization in hippocampal CA1 cells (Lynch et al., 2001) and to ameliorate the age-related decline in hippocampal neurogenesis (Lichtenwalner et al., 2001). The beneficial role of growth hormone and IGF-1 in ameliorating vascular and brain aging are however counterbalanced by their well-recognized roles in age-related pathogenesis, e.g., cancer (Yakar et al., 2004). With respect to the neuroprotective functions of IGF-1, age-related decreases in endogenous GHRH function may contribute to the defective GH secretion during ageing and therefore functionality of IGF-1, an important neurotrophic factor. Indeed, IGF-1 has been shown to protect neurons in cell culture and animal models of AD and stroke (Cheng and Mattson, 1992; Guan et al., 2001; Gasparini and Xu, 2003).
Pituitary Adenylate Cyclase Activating Peptide (PACAP)
PACAP was first identified as a 38 amino acid peptide (PACAP-38) from ovine hypothalamus that stimulated adenylate cyclase in rat anterior pituitary cells (Miyata et al., 1989). A carboxyl-terminally truncated form, PACAP-27, was subsequently identified from the same source (Miyata et al., 1990). In the CNS PACAP is found in highest levels in the hypothalamus and it is also present in many other areas of the brain e.g., olfactory bulb, frontal cortex, basal ganglia, hippocampal CA1-3 pyramidal cells, nucleus accumbens, dentate gyrus, superior colliculus, substantia nigra, pituitary gland, locus coeruleus and pontine and raphe nuclei (Masuo et al., 1992).
As stated previously there are multiple types of Class II receptors that respond to PACAP, however PACAP itself exerts the majority of its effects through the PAC1 receptor. The PAC1 receptors exhibit a high affinity for PACAP and a much lower affinity for VIP, whereas the related VPAC1/2 receptors have similar affinity for both PACAP and VIP. PAC1 and VPAC1/2 receptor stimulation activates AC and stimulates inositol phosphate accumulation/Ca2+ mobilization. PAC1 receptors are particularly abundant in the brain and pituitary and adrenal glands whereas the two VPAC receptors are expressed primarily in the lung, liver, and testis. The highest density of PACAP in the brain is typically found in magnocellular region of the hypothalamic PVN and SON (Köves et al., 1990; Masuo et al., 1992). Here PACAP increases the firing rate activity causing membrane depolarization of magnocellular neurons. Intracerebroventricular and intracisternal injection of PACAP can cause dose-dependent elevations in plasma vasopressin concentration (Murase et al., 1993; Seki et al., 1995). Similarly, in the neural lobe of the pituitary, PACAP can stimulate the release of both oxytocin and vasopressin (Lutz-Bucher et al., 1996). PACAP has also been shown to modulate the activity of various other hypothalamic neuronal populations, for example PACAP can elevate GnRH, somatostatin and corticotrophin-releasing hormone (CRH) gene expression (Li et al., 1996; Grinevich et al., 1997).
PACAP plays an important role during the development of the nervous system and in regeneration following nervous injuries. It has strong anti-apoptotic effects in several types of cultured neurons and in vivo. PACAP also protects neurons against various toxic insults in vitro, has anti-inflammatory actions and stimulates the release of neuroprotective substances from astrocytes. In vivo, the protective effects of PACAP have been shown in various models of brain injuries, including cerebral ischemia, Parkinson's disease, physical trauma and nerve transections. The upregulation of PACAP following several types of nerve injuries indicates that endogenous PACAP plays a role in the post-traumatic recovery of the nervous system (for review, see Somogyvari-Vigh and Reglodi, 2004). For example, ischemic death of hippocampal and cortical neurons can be largely prevented by infusing PACAP (Uchida et al., 1996; Tamas et al., 2002). PACAP is still effective in protecting against cell death when treatment is started 24 hours after the ischemia, which suggests that PACAP may have a tremendous therapeutic potential in the treatment of cerebral injuries, considering the often extended time delay between the onset of stroke and the commencement of therapy.
At the cellular level, PACAP can inhibit cerebellar granule neuron apoptosis (Cavallaro et al., 1996; Gonzalez et al., 1997; Villalba et al., 1997; Vaudry et al., 2000) and also stimulates neurite outgrowth (Gonzalez et al., 1997). PACAP-dependent cell survival is in part mediated through activation of AC, leading to phosphorylation of the extracellular signal-regulated kinase (ERK) (Villalba et al., 1997) associated with an increase in c-fos gene expression (Vaudry et al., 1998a,b). PACAPalso stimulates calcium mobilization (Gonzalez et al., 1996; Mei, 1999) and blocks transient potassium currents (Zerr and Feltz, 1994), two processes closely involved in the regulation of programmed cell death. PACAP can also directly protect cultured cortical neurons from the cytotoxic effects of high concentrations of glutamate (Morio et al., 1996). A neuroprotective effect of PACAP on glutamate-induced neurotoxicity also has been reported in cultured retinal neurons (Shoge et al., 1999). Most of the actions of PACAP on cortical neurons are mediated through the cAMP pathway (Martin et al., 1995; Morio et al., 1996) although it has been reported that PACAP can directly modulate N-methyl-d-aspartate receptors independently of intracellular second messengers (Liu and Madsen, 1997). In cortical neurons, PACAP can also prevent the neurotoxic effect of other insults such as lipopolysaccharide (Kong et al., 1999), and in mesencephalic dopaminergic neurons PACAP can counteract the neurotoxic effect of 6-hydroxydopamine (Takei et al., 1998). In a study relevant to AD, it was shown that PACAP can protect PC12 cells against death induced by amyloid β-peptide (Onoue et al., 2002). These results again confirm the potential therapeutic benefits of pharmacological modulation of PACAP receptors for neurodegenerative disorders. In addition to its direct actions on neurons, PACAP may affect neuronal vulnerability to injury and disease by acting on glial cells. For example, Delgado et al. (1999) reported that PACAP can inhibit macrophage activation, suggesting a role for PACAP in suppressing inflammatory processes that are believed to contribute to neuronal damage in stroke and neurodegenerative disorders.
Corticotropin-Releasing Hormone
Corticotropin-releasing hormone (CRH) is a 41-amino acid peptide that functions as a potent mediator of endocrine, autonomic, and immune responses to stress (Holsboer and Barden, 1996; Owens and Nemeroff, 1991). It has also been implicated in the modulation of a wide range of different types of behavior, including motor function, arousal, food intake, reproduction and anxiety-related behavior (Dunn and Berridge, 1990). CRH is released from parvocellular neurons of the paraventricular nucleus (PVN) of the hypothalamus, the major site of CRH-containing cell bodies (Merchenthaler, 1984; Swanson and Simmons, 1989), into portal vessels where it activates the hypothalamic–pituitary–adrenal (HPA) axis by triggering the immediate release of adrenocorticotropic hormone (ACTH) from the anterior pituitary (Holsboer and Barden, 1996; Owens and Nemeroff, 1991; Vale et al., 1981). ACTH in turn stimulates the secretion of glucocorticoids from the adrenal gland. Outside of the PVN, regions with high densities of CRH-containing neurons include the bed nucleus of the stria terminalis, with projections to brainstem areas involved in autonomic functioning, and interneurons of prefrontal, cingulate, and insular cortical areas, which are likely to be involved in regulating the behavioral effects of CRH (Owens and Nemeroff, 1991). Several studies have implicated CRH in the etiology of anxiety, depression, substance abuse, stress-related gastrointestinal disorders, and preterm labor.
The effects of CRH are mediated by two biochemically and pharmacologically distinct receptors. Two different human genes for CRH receptors have been isolated encoding CRHR1 and CRHR2 (Chen et al., 1993; Liaw et al., 1996). CRHR1 and CRHR2 are 71% homologous in their amino acid sequences (Perrin and Vale, 1999). Expression of CRHR1 mRNA is distinct from that of CRHR2 mRNA and is most abundant in cerebellar, neocortical, hippocampal, and sensory relay structures of the rat brain (Behan et al., 1996). Both CRHR1 and CRHR2 typically stimulate AC activity. Transgenic mice deficient in CRHR1 have been shown to exhibit impaired stress responses and reduced anxiety due to an improperly developed HPA axis (Smith et al., 1998; Timpl et al., 1998). On the other hand, evidence from mice deficient in CRHR2 suggests that this receptor also can modulate stress responses following activation of the HPA axis (Coste et al., 2000; Kishimoto et al., 2000).
Brain areas affected in the progressive neuro-degenerative disorder AD, the most common cause of senile dementia, show morphological abnormalities in the CRH neurons and also dramatic reductions in CRH content (Bissette et al., 1985). Moreover, cognitive impairment in AD patients is accompanied by decreased concentrations of CRH in cerebrospinal fluid (Pomara et al., 1989). With respect to the involvement of CRH in neuronal protection mechanisms an interesting dichotomy appears to exist. In some experimental paradigms CRH appears to be involved in neuronal damage and death but in other scenarios it can exert a potent neuroprotective action. Hence, it has been shown that in rat traumatic injury models CRH is rapidly upregulated in the PVN and the amygdala. Using a CRH receptor antagonist a marked neuroprotective effect against a percussion injury was observed suggesting that CRH is directly involved in the pathogenesis of the brain trauma (Roe et al., 1998). In marked contrast though, CRH has been shown to exert a PKA-dependent protective action in CRH R1-receptor-expressing neurons against oxidative cell death (Lezoualc'h et al., 2000). This protective action coincided with a reduction in the transcriptional activity of NF-κB. In another study, CRH protected cultured rat hippocampal neurons from being degraded by glutamate, lipid peroxidation, and amyloid β-peptide, insults relevant to the pathogenesis of AD (Pedersen et al., 2001). CRH was also shown to potently prevent glutamate-induced neurotoxicity, before or after the actual administration of glutamate to organotypic hippocampal cultures (Elliot-Hunt et al., 2002). In this model it was shown that the downstream signaling factors induced by glutamate, c-Jun N-terminal kinase (JNK)/stress-activated protein kinase (SAPKs), were functionally inhibited by the CRH treatment.
Urocortin and urocortin II are members of the CRH family of neuropeptides that are expressed in multiple regions of the brain, with particularly high levels of expression in certain populations of brainstem and midbrain neurons (Kozicz et al., 1998). Urocortin has recently been shown to modify neuronal survival under stressful conditions. For example, urocortin (but not urocortin II) was more potent than CRH in protecting cultured hippocampal neurons against death induced by glutamate and amyloid β-peptide (Pedersen et al., 2002). Urocortin's neuroprotective action was apparently mediated by activation of CRHR1, protein kinase C and mitogen-activated protein kinase. Consistent with the latter findings, Facci et al. (2003) showed that urocortin can protect cerebellar granule cells from the toxicity of a PI3 kinase inhibitor and can protect cortical neurons from being degraded by amyloid β-peptide. The neuroprotective mechanism in the latter study appeared to involve cAMP generation, activation of Akt and phosphorylation of glycogen synthase kinase-3. All together, these findings suggest possible roles for CRH and urocortin in neurodegenerative processes in disorders such as ischemic stroke and AD.
Vasoactive Intestinal Peptide
Vasoactive intestinal peptide (VIP) was first isolated from porcine intestine as a 28 amino acid peptide capable of inducing vasodilation in the canine femoral artery (Said and Mutt, 1970). Subsequently, VIP has been shown to have many other actions such as a neuroendocrine hormone and a putative neurotransmitter. The presence of VIP and specific VIP-binding sites in defined pathways in the brain indicates that it may play an important role in CNS function (Besson et al., 1986; Martin et al., 1987). The hypothalamic suprachiasmatic nucleus (SCN), a master circadian oscillator in mammals, contains VIP neurons. Intracranial injection of VIP elicited hyperglycemia by enhancing neural activities of the sympathetic nerves and by the suppression of the insulin secretion and enhanced secretion of adrenaline and glucagon. These findings suggested that VIP neurons in the SCN could regulate the blood glucose level through the enhancement of the sympathetic activity (Nagai, 2004). Among its other central and peripheral actions, VIP can induce prolactin secretion from the pituitary (Reichlin, 1988) and catecholamine release from the adrenal medulla (Malhotra et al., 1988). Other actions of VIP include stimulation of electrolyte secretion, smooth muscle relaxation, and protection against oxidant injury (Gozes and Brenneman, 1989; Salomon et al., 1993; Said, 1991). In common with the precursors of several other neuroendocrine peptides, the VIP precursor polypeptide (prepro-VIP) contains sequences encoding additional biologically active peptides, including peptide histidine isoleucine (PHI; Jensen et al., 1981), peptide histidine methionine (PHM, the human equivalent of PHI; Itoh et al., 1983), and peptide histidine valine (PHV), a C-terminally extended form of PHI and PHM (Yiangou et al., 1987). PHI, PHM, and PHV probably exert their actions through the same receptors as VIP because there is little evidence for the existence of distinct receptors selective for these peptides.
Early on, it was demonstrated that VIP can potently promote neuronal survival (Brenneman and Eiden, 1986) and regulate glycogen metabolism in the cerebral cortex (Sorg and Magistretti, 1992). It was subsequently shown that, to exert neuro-protective activity, VIP requires glial cell secretion of auxiliary neuroprotective proteins (Brenneman and Eiden, 1986). Activity-dependent neurotrophic factor (ADNF) is a recently isolated factor secreted by glial cells under the action of VIP (Blondel et al., 2000; for review, see Gozes and Brenneman, 2000). This protein protected neurons from death associated with a functional blockade of electrical activity. ADNF is produced by glial cells and acts directly on neurons to promote glutamate responsiveness and morphological development. ADNF may mediate these effects in part by causing the secretion of the neurotrophic factor neurotrophin 3 (NT-3). The VIP, ADNF, and NT-3 neuronal-glial pathway has been shown to regulate glutamate responses from an early stage in the synaptic development of excitatory neurons and may also contribute to the known effects of VIP on learning and behavior in the adult nervous system (Blondel et al., 2000). VIP and its control of ADNF has also been shown to exert a neuroprotective action against toxicity from familial amyotrophic lateral sclerosis-linked mutant SOD1 both in vitro and in vivo (Chiba et al., 2004). ADNF additionally has been shown to ameliorate the neurodegenerative toxicity induced by mutations in presenilin-1 (Guo et al., 1999), amyloid β-peptide, glutamate excitoxicity, and the neurotoxic HIV envelope coat protein gp120 (Glazner et al., 1999). The neuroprotective mechanism of ADNF could in part be mediated by the increased expression of molecular chaperone heat shock protein 60 (hsp60) observed in rat cerebral cortical cultures. In this scenario, ADNF was able to significantly attenuate the deleterious amyloid β-peptide-induced suppression of hsp60 (Zamostiano et al., 1999). Thus, ADNF and related truncated peptides (ADNF-14/9) exhibit protective activity in experimental models of AD. In addition, there appears to be another mediator of VIP-controlled neuroprotection, i.e., ADNP is another glial mediator of VIP-associated neuroprotection (Bassan et al., 1999). NAP, an eight-amino-acid peptide derived from ADNP (sharing structural and functional similarities with ADNF-9), has been identified as the most potent neuroprotectant described to date in an animal model of apolipoprotein E-deficiency (knockout mice) (Pinhasov et al., 2003; Gozes et al., 2003).
As well as being a useful agent that could protect against AD-related neuropathological events, it has also been shown that VIP could exert a prophylactic action against Parkinson's disease. VIP can ameliorate the toxicity of dopamine, 6-hydroxydopamine (6-OHDA) and 1-methyl-4-phenylpyridinium ion (MPP+) in rat PC12 cells, human neuroblastoma (SH-SY5Y), and rat cerebellar granular cells (Offen et al., 2000). Cerebellar granule neurons are also protected against 6-OHDA toxicity by VIP. Dopamine toxicity is linked to the redox state of intracellular glutathione. The mechanism of VIP/NAP neuroprotection may be mediated through raising cellular resistance against oxidative stress as VIP has been shown to protect neurons against buthionine sulfoximine, a selective inhibitor of glutathione synthesis, which causes a marked attenuation in reducing glutathione in neuroblastoma cells (Offen et al., 2000). These data suggest that VIP and NAP peptides have potential as protective therapies against Parkinson's disease.
In addition to ADNF and related peptides, IGF-1 may play an important role in the neuroprotective actions of VIP. Both VIP- and ADNF-stimulated neural growth can be blocked by anti-IGF-I antibodies, whereas anti-ADNF-14 sera had no detectable effect on IGF-I-induced growth (Servoss et al., 2001). In addition, IGF-I itself seemed to be directly controlled by VIP, i.e., treatment with VIP resulted in a twofold increase in embryonic neuronal IGF-I mRNA. Thus, there appears to be a prominent role for IGF-1 as a downstream mediator of VIPand ADNF in a regulatory pathway coordinating embryonic growth (Servoss et al., 2001). Because IGF-1 exerts a strong neuroprotective action, through Akt-1 activation, VIP/ADNF regulation can also entrain this additional level of neuroprotective activity.
In contrast to VIP and ADNF, very little work has been done to determine if and how PHM and PHV may be involved in neurodegenerative disorders. It was reported that levels of PHM and PHV are significantly decreased in the cerebrospinal fluid of AD patients compared with levels in samples from neurologically normal age-matched subjects (Yasuda et al., 1993; Yasuda et al., 1995). In regards to biological effects on neurons, it has been reported that PHM can stimulate neurite outgrowth (Heraud et al., 2004). Experiments to determine if and how PHM and/or PHV affect neuronal survival remain to be performed.
Parathyroid Hormone
Parathyroid hormone (PTH) is a large glycoprotein hormone, 84 residues in length, whose primary function was originally thought to be the control of serum calcium levels. Despite its large size, it has been shown that the biological activity of PTH resides primarily in its first 35 residues. PTH secretion from the parathyroid glands is controlled by feedback via calcium levels in the blood. In addition to PTH, two other related peptides have been identified, i.e., parathyroid hormone-related peptide (PTHrP) and tuberoinfundibular peptide of 39 residues (TIP39). PTHrP was isolated from tumors associated with hypercalcaemia (Mangin et al., 1990) whereas TIP39 was isolated from bovine hypothalamus (Usdin et al., 1999). Perhaps concomitant with the presence of three hormones there are three distinct types of PTH receptors, PTH1, 2, and 3. PTH and PTHrP primarily interact with PTH1 receptors, while it seems that the cognate ligand for PTH2 receptors is TIP39, however, a third form of PTH-receptor has as yet only been found in zebrafish.
PTH appears to mainly regulate calcium home-ostasis in bone and kidney via the PTH1 receptor. PTHrP, probably also through PTH1 receptors, regulates skeletal, pancreatic, epidermal, and mammary gland differentiation and bladder and vascular smooth muscle relaxation. The PTH1 receptors couple to both Gαs and Gαq (Guo et al., 1995; Segre, 1993). As with the other class II receptors the PTH1 receptor has an affinity for Gαs that seems to be an order of magnitude or more greater than for Gαq (Guo et al., 1995).
PTH1 and PTH2 receptors, as well as their cognate hormones, are widely expressed in the CNS (Clemens et al., 2001). PTH itself is expressed in hypothalamic nuclei with projections to the portal system, and there is evidence that there it can regulate prolactin secretion (Clemens et al., 2001; Harvey and Hayer, 1993). Usdin et al. (1999) discovered the second receptor, termed the PTH-2 receptor, in a cerebral cortical cDNA library (Usdin et al., 1995) along with its cognate ligand TIP39. This ligand only possesses nine amino acids in common with bovine PTH. Of the three endogenous ligands, two (PTH and TIP 39) seem to be expressed in highly discrete locations, whereas PTHrPis widely expressed in neuronal populations throughout the brain.
PTHrP possesses important roles in the CNS based on identification of selective PTH2 receptor activation events. Of all the PTH receptors PTH2 receptor expression is greatest in the CNS, where it is concentrated in limbic, hypothalamic, and sensory areas, especially hypothalamic peraventricular neurons, nerve terminals in the median eminence, superficial layers of the spinal cord dorsal horn, and the caudal part of the sensory trigeminal nucleus (Weaver et al., 1995). It is also present at lower levels in a number of endocrine cells. Thus, TIP39 and PTH2 receptor-influenced functions may range from pituitary and pancreatic hormone release to pain perception. On the basis of the localization of PTH2Rs and TIP39 in the CNS and recent neurobehavioral studies, it appears likely that one of the important actions of TIP39 could be to facilitate the response to painful stimuli (LaBuda and Usdin, 2004).
The CNS was one of the first sites to be examined carefully for PTHrP gene expression, and the gene was found to be widely expressed in neurons of the cerebral cortex, hippocampus, and cerebellum (Weir et al., 1990). The PTH1 receptor was also found co-localized with PTHrP gene expression in a number of sites (Weaver et al., 1995). It was noted at the time that several neuronal populations in which the PTHrP gene is highly expressed share the features of abundant L-type voltage-sensitive calcium channels (L-VSCCs) as well as excitatory amino acid content and a known sensitivity to excitotoxicity (Weir et al., 1990). In agreement with a role for PTH in excitotoxicity, it has also been reported that in hippocampal organotypic cultures, sustained high levels of PTH cause a toxic increase in intracellular calcium. Therefore, in the brain, PTH may induce degeneration because of Ca2+ overload via activation of dihydropyridine-sensitive L-VSCCs (Hirasawa et al., 2000). One neuronal population that both produces PTHrP and bears the PTH type 1 receptor is the cerebellar granule cell. PTHrP gene expression in granule cells has been shown to be controlled by depolarization-dependent L-VSCCCa2+ influx, which signals to the nucleus via the calmodulin–CaM kinase cascade (Holt et al., 1996). As in smooth muscle cells, PTHrP appears to negatively regulate L-VSCC Ca2+ influx in granule cells and in so doing is able to protect these cells against the long-latency form of excitotoxicity produced by excitotoxins such as kainic acid (Brines et al., 1999; Weiss et al., 1990). PTHrP-knockout mice display systemic chondrodysplasia that is lethal at birth (Karapalis et al., 1994). This transgenic mouse however, was “rescued” by a transgenic replacement strategy, generating a mouse that is PTHrP-sufficient in chondrocytes but PTHrP-null in all other locations (Philbrick et al., 1996: Wysolmerski et al., 1998). The rescued transgenic mouse demonstrated a sixfold increase in neuronal kainic acid sensitivity. In accordance with this observation, PTHrP can counteract kainic acid toxicity in mixed cerebral cortical cultures prepared from both rescued and control littermates. In this paradigm, PTHrP-mediated negative regulation of L-VSCC-induced Ca2+ influx in the neuroblastoma cells potentially underlies its neuroprotective effect.
Calcitonin Family Ligands
The calcitonin family of peptides comprises five known members: calcitonin (CT), amylin, α and β calcitonin gene-related peptide (CGRP), and adrenomedullin (AM). These hormones differ in their primary sequences but they have analogous secondary structures, i.e., amidated carboxyl ter-mini and a six/seven amino acid ring structure formed by an intramolecular disulphide bond. The peptides are widely distributed in various peripheral tissues as well as in the peripheral nervous system and the CNS. They can induce multiple biological effects including potent vasodilatation (CGRP and AM), reduction in nutrient intake (amylin), and decreased bone resorption (calcitonin). α-CGRP was cloned from the gene-encoding calcitonin (Amara et al., 1982). Alternate splicing of the calcitonin gene leads to the production, especially in nervous tissues, of α-CGRP, a 37-amino acid peptide. The second CGRP homolog, β-CGRP, differs from human α-CGRP by three amino acids and in the rat by one amino acid. β-CGRP is encoded by its own unique gene, with high homology to the calcitonin gene (Steenbergh et al., 1985). Adrenomedullin (AM), 52 residues in length, was isolated from human adrenal pheochromocytomas (Kitamura et al., 1993). AM, while possessing potent vasoactive properties, has been reported to be present in neurons in the CNS and in tumors of neural and glial origin. Amylin is a 37-amino acid peptide originally isolated from amyloid deposits of human insulinoma and the pancreas of type-2 diabetic patients (Cooper et al., 1987). Circulating levels of amylin are raised in response to meal ingestion where it can oppose the metabolic actions of insulin in skeletal muscle (Hoppener et al., 2000). The peptide also can potently inhibit gastric emptying and gastric acid secretion. Knock-out of the amylin gene leads to weight gain in mice, suggesting an important physiological role for the peptide in weight control, interestingly these transgenic mice also show reduced pain perception (Gebre-Medhin et al., 1998).
The receptors for these peptides allow multiple calcitonin-family members to functionally interact with them. Initially, the calcitonin-like (CL) receptor was considered an orphan receptor. Evidence for CGRP receptor function of the CL receptor was first obtained by Aiyar et al. (1996), who observed CGRP binding and CGRP-dependent cAMP accumulation in a single subclone of HEK293 cells stably expressing the CL receptor. The important breakthrough came when an expression cloning approach demonstrated that the CL receptor required a single transmembrane domain protein, RAMP1, to function as a CGRP receptor (McLatchie et al., 1998). The RAMP family of proteins comprises three members, RAMP1, -2, and -3. Detailed studies of the pharmacology displayed by CT and CL calcitonin receptors, expressed with or without all three RAMPs, have now been reported by several laboratories (Bühlmann et al., 1999; Christopoulos et al., 1999; Fraser et al., 1999; Muff et al., 1999; Aldecoa et al., 2000; Husmann et al., 2000; Leuthäuser et al., 2000; Tilakaratne et al., 2000; Zumpe et al., 2000; Aiyar et al., 2001; Oliver et al., 2001). They have all revealed receptors that bind and respond to CT, CGRP, AM, and amylin can be generated by differential RAMP association with the two calcitonin (CL and CT) receptors and the multiple RAMPS. The CL receptor and RAMP2 or -3 reconstitute AM receptors that show the pharmacological characteristics of the native receptors. RAMP2 and RAMP3 enable the CL receptor to generate AM receptors that are pharmacologically similar. Amylin receptors can be reconstituted in cellular systems by co-expressing the CT receptor with RAMPs. These amylin receptors have similar and high affinities for CT and amylin and lower affinities for CGRP. In this respect, the CT receptor/RAMP combination represents a similar amylin receptor to that characterized in native tissues (Chen et al., 1997).
The signalling of all these receptors is relatively similar in that all the related peptides activate AC. In addition CGRP activates calcium and potassium channels via pertussis toxin-sensitive G proteins, however, comparable studies have not been performed with other family peptides (Main et al., 1998). The calcitonin receptor is linked to the stimulation of adenylate cyclase and phospholipase C, but alternate coupling to phospholipase D and inhibition of adenylate cyclase (at high agonist concentration) have also been observed (Purdue et al., 2002). Amylin stimulates the formation of cAMP as the principal mediator in skeletal muscle (Pittner et al., 1994; Beaumont et al., 1995). In view of the structural similarity between the calcitonin family of peptides, it is not surprising that cross-reactivity between them at their cognate receptors has been demonstrated. Therefore, two GPCR proteins and three RAMPs can reconstitute receptors for the whole calcitonin family of peptide ligands creating a novel level of signal transduction complexity to this class of receptors. In addition, it seems that some cells appear to express more than one RAMP and there is evidence to support the hormonal regulation of RAMP expression at the mRNA level.
With respect to neuroprotection mechanisms, perhaps the most interesting of the calcitonin peptide family is amylin. Amylin is also known as islet amyloid peptide (IAP). Human amylin possesses amyloidogenic properties and has a profile of neurotoxicity similar to that of amyloid β-peptide (Mattson and Goodman, 1995). Despite a low primary sequence homology, amylin shares many biophysical properties with amyloid β-peptide, i.e., an ability to aggregate into β-pleated sheets in aqueous solution and the ability to modulate ion channel function. (Kawahara et al., 2000; Kourie and Henry, 2002). Reinforcing their similarity, it has been reported that amylin induces apoptotic cell death, similar to aggregated amyloid β-peptide, in cultured neurons as well as pancreatic β-islet cells (May et al., 1993). Most importantly, amylin and the amylin receptor may be directly implicated in AD, as demonstrated by Jhamanadas and MacTavish (2004). These researchers demonstrated that antagonists of the human amylin receptor can effectively block the deleterious neuronal effects of amyloid β-peptide. In addition, studies in transgenic rodents have pointed to the role of amylin in the development of type 2 diabetes, an important risk factor for neurodegenerative processes. Therefore, it will be interesting to see in clinical settings whether pharmacological interdiction of the amylin peptide and receptor system could have prophylactic or therapeutic potential for amyloidogenic neurodegenerative disorders such as AD.
With respect to the other members of the calcitonin family, significant neuroprotective mechanisms have been identified. For example, CGRP and its N-terminal fragments have been shown to exert multiple inhibitory and facilitatory actions on neuronal nicotinic acetylcholine (ACh) receptors (nAChRs). Small CGRP fragments can reversibly enhance agonist sensitivity of nAChRs (without directly activating the receptor), whereas longer fragments and full-length CGRPcan inhibit nAChR-mediated responses via an apparently competitive type of receptor antagonism (for review, see Di Angelantonio et al., 2003). Therefore, therapeutic manipulation of the CGRP system may be of specific benefit in conditions of ACh functional deficit such as neurodegenerative conditions like AD and Parkinson's disease.
Focal brain ischemia is the most common event leading to stroke in humans, and a role for AM in this condition has been suggested. Using the rat focal stroke model of middle cerebral artery occlusion (MCAO), Wang et al. (1995) demonstrated significant increases in AM mRNA expression in the ischemic cortex. AM was also localized to the ischemic neuronal processes. However, this response could be part of a neuroprotective response as Dogan et al. (1997), using spontaneously hypertensive rats, showed that pre- or post-infusion of AM attenuated the reduction in regional blood flow after MCAO and decreased the degree and extent of eventual ischemic brain injury. Thus, it may be that AM has an indirect role in preventing ischemic brain injury by increasing collateral circulation. Post-ischemia and reperfusion it has been shown that the number of AM-positive neurons increased and that neurons immunoreactive for AM possessed a normal morphology, whereas nonimmunoreactive neurons were clearly damaged, demonstrating a potential cell-specific neuroprotective role for AM (Serrano et al., 2002, 2002a). Thus, it seems that AM is part of a CNS response mechanism to hypoxic injury. AM appears also to be regulated by other forms of neuronal injury, e.g., following intraperitoneal injection of the organometal trimethyltin (TMT: causing neurodegeneration of the hippocampal CA3-4 pyramidal cell layer) there was increased AM, TNF-α and IL-α immunoreactivity in CA4 cells suggesting an interaction between the proinflammatory cytokines and glia response in the regulation of AM in response to injury (Jahnke et al., 2001).
Outlook
The class II receptors and the peptides that activate them seem to play a pivotal role in controlling the balance of both peripheral and central energy metabolism as they are all principally involved in digestive and central neuronal processes. The multiplicity of molecular neuroprotective mechanisms mediated by class II GPCR activation is demonstrated in Fig. 1. Current themes of research in neurodegenerative disorders have highlighted crucial links between the control of peripheral and central energy metabolism. The expanding knowledge of both the structural biology of both the peptide hormones and the complex yet elegant receptors they interact with will undoubtedly yield the potential to generate several new classes of important pharmacotherapeutic compounds. Already there is great promise in the manipulation of several of the class II receptor systems with specific respect to AD (GLP-1 and amylin) and Parkinson's disease (CGRPand VIP). Even the first generation of pharmacological compounds which are peptidergic in nature possess a great advantage compared to hormones acting on class I receptors as they seem to have an excellent CNS bioavailability. The passage of VIP/PACAP/secretin family members to the CNS will also potentially open up new insights for understanding the CNS effects of peripherally administrated peptides. If an adequate delivery is possible then such receptor-interacting (agonistic or antagonistic) peptides may prove to be useful in the treatment of serious neurological diseases including AD, amyotropic lateral sclerosis, Parkinson's disease, AIDS-related neuropathy, diabetic neuropathy, autism, stroke, and nerve injury.
Fig. 1.
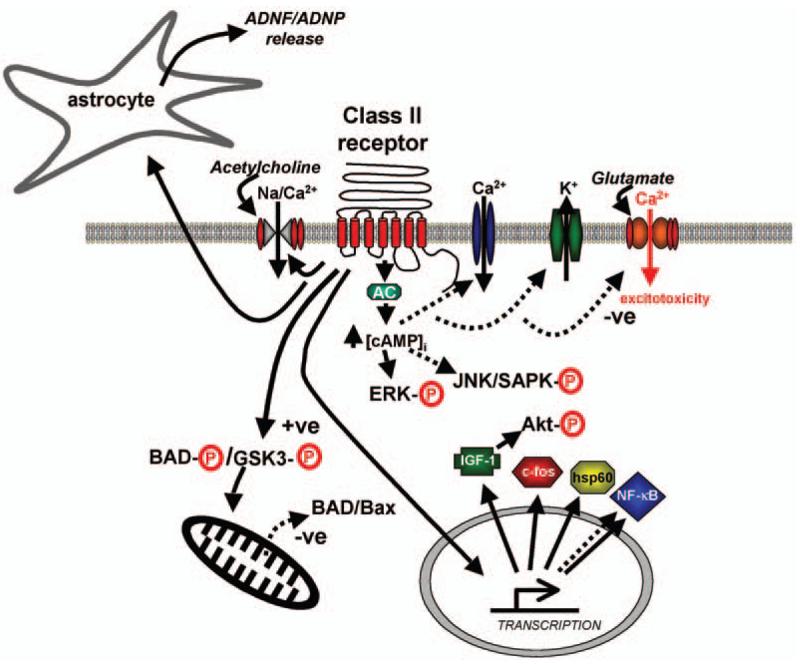
Multiple neuromodulatory pathways emanating from the class II G protein-coupled receptors (GPCRs). Solid lines display positive regulation of the pathways, whereas dashed lines represent an inhibitory action of class II GPCR stimulation. Acetylcholine action is shown upon the nicotinic acetylcholine receptor, whereas gultamate is shown acting at the the N-methyl d-aspartate (NMDA) ionotropic receptor. JNK, c-Jun N-terminal kinase; SAPK, stress-activated protein kinase.
References
- Adelhorst K, Hedegaard BB, Knudsen LB, Kirk O. Structure-activity studies of glucagon-like peptide-1. J. Biol. Chem. 1994;269:6275–6278. [PubMed] [Google Scholar]
- Aiyar N, Daines RA, Disa J, et al. Pharmacology of sb-273779, a nonpeptide calcitonin gene-related peptide 1 receptor antagonist. J. Pharmacol. Exp. Ther. 2001;296:768–775. [PubMed] [Google Scholar]
- Aiyar N, Rand K, Elshourbagy NA, et al. A cDNA encoding the calcitonin gene-related peptide type 1 receptor. J. Biol. Chem. 1996;271(11):325–11,329. doi: 10.1074/jbc.271.19.11325. [DOI] [PubMed] [Google Scholar]
- Aldecoa A, Gujer R, Fischer JA, Born W. Mammalian calcitonin receptor-like receptor/receptor activity modifying protein complexes define calcitonin gene-related peptide and adrenomedullin receptors in drosophila schneider 2 cells. FEBS Lett. 2000;471:156–160. doi: 10.1016/s0014-5793(00)01387-9. [DOI] [PubMed] [Google Scholar]
- Al-Sabah S, Donnelly D. Amodel for receptor-peptide binding at the glucagon-like peptide-1 (GLP-1) receptor through the analysis of truncated ligands and receptors. Br. J. Pharmacol. 2003;140:339–346. doi: 10.1038/sj.bjp.0705453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298:240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Baetens D, Rufener C, Srikant BC, Dobbs R, Unger R, Orci L. Identification of glucagon-producing cells (A cells) in dog gastric mucosa. J. Cell Biol. 1976;69:455–464. doi: 10.1083/jcb.69.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Goulet M, Rusche JR, Niehoff ML, Boismenu R. Differential transport of a secretin analog across the blood-brain and blood-cerebrospinal fluid barriers of the mouse. J. Pharmacol. Exp. Ther. 2002;302:1062–1069. doi: 10.1124/jpet.102.036129. [DOI] [PubMed] [Google Scholar]
- Barragan JM, Eng J, Rodriguez R, Blazquez E. Neural contribution to the effect of glucagon-like peptide-1-(7-36) amide on arterial blood pressure in rats. Am. J. Physiol. 1999;277:E784–791. doi: 10.1152/ajpendo.1999.277.5.E784. [DOI] [PubMed] [Google Scholar]
- Barzilai A, Melamed E. Molecular mechanisms of selective dopaminergic neuronal death in Parkinson's disease. Trends Mol. Med. 2003;9:126–132. doi: 10.1016/s1471-4914(03)00020-0. [DOI] [PubMed] [Google Scholar]
- Bassan M, Zamostiano R, Davidson A, et al. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J. Neurochem. 1999;72:1283–1293. doi: 10.1046/j.1471-4159.1999.0721283.x. [DOI] [PubMed] [Google Scholar]
- Baum J, Simons BE, Jr., Unger RH, Madison LL. Localization of glucagon in the alpha cells in the pancreatic islet by immunofluorescent techniques. Diabetes. 1962;11:371–374. [PubMed] [Google Scholar]
- Bazarsuren A, Grauschopf U, Wozny M, et al. In vitro folding, functional characterization, and disulfide pattern of the extracellular domain of human GLP-1 receptor. Biophys. Chem. 2002;96:305–318. doi: 10.1016/s0301-4622(02)00023-6. [DOI] [PubMed] [Google Scholar]
- Beaumont K, Pittner RA, Moore CX, et al. Regulation of muscle glycogen metabolism by CGRP and amylin: CGRP receptors not involved. Br. J. Pharmacol. 1995;115:713–715. doi: 10.1111/j.1476-5381.1995.tb14991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan DP, Grigoriadis DE, Lovenberg T, et al. Neurobiology of corticotropin releasing factor (CRF) receptors and CRF-binding protein: implications for the treatment of CNS disorders. Mol. Psychiatry. 1996;1:265–277. [PubMed] [Google Scholar]
- Berelowitz M, Szabo M, Frohman LA, Firestone S, Chu L, Hintz RL. Somatomedin-C mediates growth hormone negative feedback by effects on both the hypothalamus and the pituitary. Science. 1981;212:1279–1281. doi: 10.1126/science.6262917. [DOI] [PubMed] [Google Scholar]
- Bergwitz C, Gardella TJ, Flannery MR, et al. Full activation of chimeric receptors by hybrids between parathyroid hormone and calcitonin. Evidence for a common pattern of ligand-receptor interaction. J. Biol. Chem. 1996;271(26):469–26,472. doi: 10.1074/jbc.271.43.26469. [DOI] [PubMed] [Google Scholar]
- Besson J, Sarrieau A, Vial M, Marie JC, Rosselin G, Rostene W. Characterization and autoradiographic distribution of vasoactive intestinal peptide binding sites in the rat central nervous system. Brain Res. 1986;398:329–336. doi: 10.1016/0006-8993(86)91493-9. [DOI] [PubMed] [Google Scholar]
- Bissette G, Reynolds GP, Kilts CD, Widerlov E, Nemeroff CB. Corticotropin-releasing factor-like immunoreactivity in senile dementia of the Alzheimer type. Reduced cortical and striatal concentrations. JAMA. 1985;254:3067–3069. [PubMed] [Google Scholar]
- Blondel O, Collin C, McCarran WJ, et al. A glia-derived signal regulating neuronal differentiation. J. Neurosci. 2000;20:8012–8020. doi: 10.1523/JNEUROSCI.20-21-08012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman DE, Eiden LE. Vasoactive intestinal peptide and electrical activity influence neuronal survival. Proc. Natl. Acad. Sci. USA. 1986;83:1159–1162. doi: 10.1073/pnas.83.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines ML, Ling Z, Broadus AE. Parathyroid hormone-related protein protects against kainic acid excitotoxicity in rat cerebellar granule cells by regulating L-type channel calcium flux. Neurosci. Lett. 1999;274:13–16. doi: 10.1016/s0304-3940(99)00664-3. [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- Buggy JJ, Livingston JN, Rabin DU, Yoo-Warren H. Glucagon-glucagon-like peptide I receptor chimeras reveal domains that determine specificity of glucagon binding. J. Biol. Chem. 1995;270:7474–7478. doi: 10.1074/jbc.270.13.7474. [DOI] [PubMed] [Google Scholar]
- Buhlmann N, Leuthauser K, Muff R, Fischer JA, Born W. A receptor activity modifying protein (ramp)2-dependent adrenomedullin receptor is a calcitonin gene-related peptide receptor when coexpressed with human ramp1. Endocrinology. 1999;140:2883–2890. doi: 10.1210/endo.140.6.6783. [DOI] [PubMed] [Google Scholar]
- Burton PB, Moniz C, Knight DE. Parathyroid hormone related peptide can function as an autocrine growth factor in human renal cell carcinoma. Biochem. Biophys. Res. Commun. 1990;167:1134–1138. doi: 10.1016/0006-291x(90)90641-y. [DOI] [PubMed] [Google Scholar]
- Canonico PL, Cronin MJ, Thorner MO, Macleod RM. Human pancreatic GRF stimulates phosphatidylinositol labeling in cultured anterior pituitary cells. Am. J. Physiol. 1983;245:E587–E590. doi: 10.1152/ajpendo.1983.245.6.E587. [DOI] [PubMed] [Google Scholar]
- Cavallaro S, Copani A, D'agata V, et al. Pituitary adenylate cyclase activating polypeptide prevents apoptosis in cultured cerebellar granule neurons. Mol. Pharmacol. 1996;50:60–66. [PubMed] [Google Scholar]
- Chen R, Lewis KA, Perrin MH, Vale WW. Expression cloning of a human corticotropin-releasing-factor receptor. Proc. Natl. Acad. Sci. USA. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WJ, Armour S, Way J, et al. Expression cloning and receptor pharmacology of human calcitonin receptors from mcf-7 cells and their relationship to amylin receptors. Mol. Pharmacol. 1997;52:1164–1175. doi: 10.1124/mol.52.6.1164. [DOI] [PubMed] [Google Scholar]
- Cheng B, Mattson MP. IGF-I and IGF-II protect cultured hippocampal and septal neurons against calcium-mediated hypoglycemic damage. J. Neurosci. 1992;12:1558–1566. doi: 10.1523/JNEUROSCI.12-04-01558.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T, Hashimoto Y, Tajima H, et al. Neuroprotective effect of activity-dependent neurotrophic factor against toxicity from familial amyotrophic lateral sclerosis-linked mutant SOD1 in vitro and in vivo. J. Neurosci. Res. 2004;78:542–552. doi: 10.1002/jnr.20305. [DOI] [PubMed] [Google Scholar]
- Chow BK. Functional antagonism of the human secretin receptor by a recombinant protein encoding the N-terminal ectodomain of the receptor. Recept. Signal Transduct. 1997;7:143–150. [PubMed] [Google Scholar]
- Christopoulos A, Christopoulos G, Morfis M, et al. Novel receptor partners and function of receptor activity-modifying proteins. J. Biol. Chem. 2003;278:3293–3297. doi: 10.1074/jbc.C200629200. [DOI] [PubMed] [Google Scholar]
- Christopoulos G, Perry KJ, Morfis M, et al. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol. Pharmacol. 1999;56:235–242. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- Clemens TL, Cormier S, Eichinger A, et al. Parathyroid hormone-related protein and its receptors: nuclear functions and roles in the renal and cardiovascular systems, the placental trophoblasts and the pancreatic islets. Br. J. Pharmacol. 2001;134:1113–1136. doi: 10.1038/sj.bjp.0704378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coniglio SJ, Lewis JD, Lang C, et al. A randomized, double-blind, placebo-controlled trial of single-dose intravenous secretin as treatment for children with autism. J. Pediatr. 2001;138:649–655. doi: 10.1067/mpd.2001.112474. [DOI] [PubMed] [Google Scholar]
- Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc. Natl. Acad. Sci. USA. 1987;84:8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste SC, Kesterson RA, Heldwein KA, et al. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat. Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Mevenkamp G, Wille S, Hauger RL. N-terminal splice variants of the type I PACAP receptor: isolation, characterization and ligand binding/selectivity determinants. J. Neuroendocrinol. 1999;11:941–949. doi: 10.1046/j.1365-2826.1999.00411.x. [DOI] [PubMed] [Google Scholar]
- DeLecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Pozo D, Martinez C, et al. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit endotoxin-induced TNF-alpha production by macrophages: in vitro and in vivo studies. J. Immunol. 1999;162:2358–2367. [PubMed] [Google Scholar]
- Di Angelantonio S, Costa V, Carloni P, Messori L, Nistri A. A novel class of peptides with facilitating action on neuronal nicotinic receptors of rat chromaffin cells in vitro: Functional and molecular dynamics studies. Mol. Pharmacol. 2002;61:43–54. doi: 10.1124/mol.61.1.43. [DOI] [PubMed] [Google Scholar]
- Dias MS. Traumatic brain and spinal cord injury. Pediatr. Clin. North Am. 2004;51:271–303. doi: 10.1016/S0031-3955(03)00211-6. [DOI] [PubMed] [Google Scholar]
- Dogan A, Suzuki Y, Koketsu N, et al. Intravenous infusion of adrenomedullin and increase in regional cerebral blood flow and prevention of ischemic brain injury after middle cerebral artery occlusion in rats. J. Cereb. Blood Flow Metab. 1997;17:19–25. doi: 10.1097/00004647-199701000-00004. [DOI] [PubMed] [Google Scholar]
- Drucker D, Bataille D, Göke B, Mayo K, Miller L, Thorens B. The IUPHAR Compendium of Receptor Characterization and Classification. 2nd edition IUPHAR Media; London, UK: 2000. Glucagon receptor family. [Google Scholar]
- Drucker DJ. Glucagon-like peptides. Diabetes. 1998;47:159–169. doi: 10.2337/diab.47.2.159. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol. Endocrinol. 2003;17:161–171. doi: 10.1210/me.2002-0306. [DOI] [PubMed] [Google Scholar]
- Drouot X, Moutereau S, Nguyen JP, et al. Low levels of ventricular CSF orexin/hypocretin in advanced PD. Neurology. 2003;61:540–543. doi: 10.1212/01.wnl.0000078194.53210.48. [DOI] [PubMed] [Google Scholar]
- Dunbar ME, Dann PR, Robinson GW, Hennighausen L, Zhang JP, Wysolmerski JJ. Parathyroid hormone-related protein signaling is necessary for sexual dimorphism during embryonic mammary development. Development. 1999;126:3485–3493. doi: 10.1242/dev.126.16.3485. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res. Brain Res. Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- Dunn-Geier J, Ho HH, Auersperg E, et al. Effect of secretin on children with autism: a randomized controlled trial. Dev. Med. Child Neurol. 2000;42:796–802. doi: 10.1017/s0012162200001481. [DOI] [PubMed] [Google Scholar]
- Dunphy JL, Taylor RG, Fuller PJ. Tissue distribution of rat glucagon receptor and GLP-1 receptor gene expression. Mol. Cell. Endocrinol. 1998;141:179–186. doi: 10.1016/s0303-7207(98)00096-3. [DOI] [PubMed] [Google Scholar]
- Eichinger A, Fiaschi-Taesch N, Massfelder T, Fritsch S, Barthelmebs M, Helwig JJ. Transcript expression of the tuberoinfundibular peptide (TIP)39/PTH2 receptor system and non-PTH1 receptor-mediated tonic effects of TIP39 and other PTH2 receptor ligands in renal vessels. Endocrinology. 2002;143:3036–3043. doi: 10.1210/endo.143.8.8960. [DOI] [PubMed] [Google Scholar]
- Elliott-Hunt CR, Kazlauskaite J, Wilde GJ, Grammatopoulos DK, Hillhouse EW. Potential signalling pathways underlying corticotrophin-releasing hormone-mediated neuroprotection from excitotoxicity in rat hippocampus. J. Neurochem. 2002;80:416–425. doi: 10.1046/j.0022-3042.2001.00712.x. [DOI] [PubMed] [Google Scholar]
- Escobar DC, Vicentini LM, Ghigo E, et al. Growth hormone-releasing factor does not stimulate phosphoinositides breakdown in primary cultures of rat and human pituitary cells. Acta. Endocrinol. (Copenh) 1986;112:345–350. doi: 10.1530/acta.0.1120345. [DOI] [PubMed] [Google Scholar]
- Facci L, Stevens DA, Pangallo M, Franceschini D, Skaper SD, Strijbos PJ. Corticotropin-releasing factor (CRF) and related peptides confer neuroprotection via type 1 CRF receptors. Neuropharmacology. 2003;45:623–636. doi: 10.1016/s0028-3908(03)00211-9. [DOI] [PubMed] [Google Scholar]
- Foord SM, Wise A, Brown J, Main MJ, Fraser NJ. The N-terminus of RAMPs is a critical determinant of the glycosylation state and ligand binding of calcitonin receptor-like receptor. Biochem. Soc. Trans. 1999;27:535–539. doi: 10.1042/bst0270535. [DOI] [PubMed] [Google Scholar]
- Fraser NJ, Wise A, Brown J, Mclatchie LM, Main MJ, Foord SM. The amino terminus of receptor activity modifying proteins is a critical determinant of glycosylation state and ligand binding of calcitonin receptor-like receptor. Mol. Pharmacol. 1999;55:1054–1059. doi: 10.1124/mol.55.6.1054. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr., Jensen RT, Charlton CG, Miller RL, O'donohue TL, Moody TW. Secretin: specific binding to rat brain membranes. J. Neurosci. 1983;3:1620–1625. doi: 10.1523/JNEUROSCI.03-08-01620.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French MB, Lussier BT, Moor BC, Kraicer J. Effect of growth hormone-releasing factor on phosphoinositide hydrolysis in somatotrophs. Mol. Cell. Endocrinol. 1990;72:221–226. doi: 10.1016/0303-7207(90)90146-y. [DOI] [PubMed] [Google Scholar]
- Frimurer TM, Bywater RP. Structure of the integral membrane domain of the GLP1 receptor. Proteins. 1999;35:375–386. [PubMed] [Google Scholar]
- Frohman MA, Downs TR, Chomczynski P, Frohman LA. Cloning and characterization of mouse growth hormone-releasing hormone (GRH) complementary DNA: increased GRH messenger RNA levels in the growth hormone-deficient lit/lit mouse. Mol. Endocrinol. 1989;3:1529–1536. doi: 10.1210/mend-3-10-1529. [DOI] [PubMed] [Google Scholar]
- Gardella TJ, Juppner H. Molecular properties of the PTH/PTHrP receptor. Trends Endocrinol. Metab. 2001;12:210–217. doi: 10.1016/s1043-2760(01)00409-x. [DOI] [PubMed] [Google Scholar]
- Gasparini L, Xu H. Potential roles of insulin and IGF-1 in Alzheimer's disease. Trends Neurosci. 2003;26:404–406. doi: 10.1016/S0166-2236(03)00163-2. [DOI] [PubMed] [Google Scholar]
- Gaudin P, Rouyer-Fessard C, Couvineau A, Maoret JJ, Laburthe M. Constitutive activation of the human VIP1 receptor. Ann. N. Y. Acad. Sci. 1998;865:382–385. doi: 10.1111/j.1749-6632.1998.tb11200.x. [DOI] [PubMed] [Google Scholar]
- Gaylinn BD, Harrison JK, Zysk JR, Lyons CE, Lynch KR, Thorner MO. Molecular cloning and expression of a human anterior pituitary receptor for growth hormone-releasing hormone. Mol. Endocrinol. 1993;7:77–84. doi: 10.1210/mend.7.1.7680413. [DOI] [PubMed] [Google Scholar]
- Gebre-Medhin S, Mulder H, Pekny M, et al. Increased insulin secretion and glucose tolerance in mice lacking islet amyloid polypeptide (amylin) Biochem. Biophys. Res. Commun. 1998;250:271–277. doi: 10.1006/bbrc.1998.9308. [DOI] [PubMed] [Google Scholar]
- Gebre-Medhin S, Mulder H, Zhang Y, Sundler F, Betsholtz C. Reduced nociceptive behavior in islet amyloid polypeptide (amylin) knockout mice. Brain Res. Mol. Brain Res. 1998;63:180–183. doi: 10.1016/s0169-328x(98)00269-1. [DOI] [PubMed] [Google Scholar]
- Gelling RW, Wheeler MB, Xue J, et al. Localization of the domains involved in ligand binding and activation of the glucose-dependent insulinotropic polypeptide receptor. Endocrinology. 1997;138:2640–2643. doi: 10.1210/endo.138.6.9104. [DOI] [PubMed] [Google Scholar]
- Gilman CP, Perry T, Furukawa K, Greig NH, Egan JM, Mattson MP. Glucagon-like peptide 1 modulates calcium responses to glutamate and membrane depolarization in hippocampal neurons. J. Neurochem. 2003;87:1137–1144. doi: 10.1046/j.1471-4159.2003.02073.x. [DOI] [PubMed] [Google Scholar]
- Glazner GW, Boland A, Dresse AE, Brenneman DE, Gozes I, Mattson MP. Activity-dependent neurotrophic factor peptide (ADNF9) protects neurons against oxidative stress-induced death. J. Neurochem. 1999;73:2341–2347. doi: 10.1046/j.1471-4159.1999.0732341.x. [DOI] [PubMed] [Google Scholar]
- Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Identification of specific binding sites for glucagon-like peptide-1 on the posterior lobe of the rat pituitary. Neuroendocrinology. 1995;62:130–134. doi: 10.1159/000126997. [DOI] [PubMed] [Google Scholar]
- Gonzalez BJ, Basille M, Mei YA, et al. Ontogeny of PACAP and PACAP receptors in the rat brain: role of PACAP in the cerebellum during development. Ann. N. Y. Acad. Sci. 1996;805:302–313. doi: 10.1111/j.1749-6632.1996.tb17492.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez BJ, Basille M, Vaudry D, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide promotes cell survival and neurite outgrowth in rat cerebellar neuroblasts. Neuroscience. 1997;78:419–430. doi: 10.1016/s0306-4522(96)00617-3. [DOI] [PubMed] [Google Scholar]
- Goodyer CG, De Stephano L, Lai WH, Guyda HJ, Posner BI. Characterization of insulin-like growth factor receptors in rat anterior pituitary, hypothalamus, and brain. Endocrinology. 1984;114:1187–1195. doi: 10.1210/endo-114-4-1187. [DOI] [PubMed] [Google Scholar]
- Goulet M, Shiromani PJ, Ware CM, Strong RA, Boismenu R, Rusche JR. A secretin i.v. infusion activates gene expression in the central amygdala of rats. Neuroscience. 2003;118:881–888. doi: 10.1016/s0306-4522(02)00782-0. [DOI] [PubMed] [Google Scholar]
- Gozes I, Brenneman DE. A new concept in the pharmacology of neuroprotection. J. Mol. Neurosci. 2000;14:61–68. doi: 10.1385/JMN:14:1-2:061. [DOI] [PubMed] [Google Scholar]
- Gozes I, Divinsky I, Pilzer I, Fridkin M, Brenneman DE, Spier AD. From vasoactive intestinal peptide (VIP) through activity-dependent neuroprotective protein (ADNP) to NAP: a view of neuroprotection and cell division. J. Mol. Neurosci. 2003;20:315–322. doi: 10.1385/JMN:20:3:315. [DOI] [PubMed] [Google Scholar]
- Gozes I, Brenneman DE. VIP: molecular biology and neurobiological function. Mol. Neurobiol. 1989;3:201–236. doi: 10.1007/BF02740606. [DOI] [PubMed] [Google Scholar]
- Grauschopf U, Lilie H, Honold K, et al. The N-terminal fragment of human parathyroid hormone receptor 1 constitutes a hormone binding domain and reveals a distinct disulfide pattern. Biochemistry. 2000;39:8878–8887. doi: 10.1021/bi0001426. [DOI] [PubMed] [Google Scholar]
- Grinevich V, Fournier A, Pelletier G. Effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on corticotropin-releasing hormone (CRH) gene expression in the rat hypothalamic paraventricular nucleus. Brain Res. 1997;773:190–196. doi: 10.1016/s0006-8993(97)01011-1. [DOI] [PubMed] [Google Scholar]
- Guan J, Miller OT, Waugh KM, McCarthy DC, Gluckman PD. Insulin-like growth factor-1 improves somatosensory function and reduces the extent of cortical infarction and ongoing neuronal loss after hypoxia-ischemia in rats. Neuroscience. 2001;105:299–306. doi: 10.1016/s0306-4522(01)00145-2. [DOI] [PubMed] [Google Scholar]
- Guo J, Iida-Klein A, Huang X, Abou-Samra AB, Segre GV, Bringhurst FR. Parathyroid hormone (PTH)/PTH-related peptide receptor density modulates activation of phospholipase C and phosphate transport by PTH in LLC-PK1 cells. Endocrinology. 1995;136:3884–3891. doi: 10.1210/endo.136.9.7649096. [DOI] [PubMed] [Google Scholar]
- Guo Q, Sebastian L, Sopher BL, et al. Neurotrophic factors [activity-dependent neurotrophic factor (ADNF) and basic fibroblast growth factor (bFGF)] interrupt excitotoxic neurodegenerative cascades promoted by a PS1 mutation. Proc. Natl. Acad. Sci. USA. 1999;96:4125–4130. doi: 10.1073/pnas.96.7.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzwiller JP, Drewe J, Goke B, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am. J. Physiol. 1999;276:1541–1544. doi: 10.1152/ajpregu.1999.276.5.R1541. [DOI] [PubMed] [Google Scholar]
- Hallbrink M, Holmqvist T, Olsson M, Ostenson CG, Efendic S, Langel U. Different domains in the third intracellular loop of the GLP-1 receptor are responsible for Galpha(s) and Galpha(i)/Galpha(o) activation. Biochem. Biophys. Acta. 2001;1546:79–86. doi: 10.1016/s0167-4838(00)00270-3. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Jamen F, Nielsen HS, Journot L, Brabet P, Fahrenkrug J. Dissociation between light-induced phase shift of the circadian rhythm and clock gene expression in mice lacking the pituitary adenylate cyclase activating polypeptide type 1 receptor. J. Neurosci. 2001;21:4883–4890. doi: 10.1523/JNEUROSCI.21-13-04883.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen LH, Gromada J, Bouchelouche P, et al. Glucagon-mediated Ca2+ signaling in BHK cells expressing cloned human glucagon receptors. Am. J. Physiol. 1998;274:1552–1562. doi: 10.1152/ajpcell.1998.274.6.C1552. [DOI] [PubMed] [Google Scholar]
- Harmar AJ. Family-B G protein-coupled receptors. Genome Biol. 2001;2:3013.1. doi: 10.1186/gb-2001-2-12-reviews3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey S, Hayer S. Parathyroid hormone binding sites in the brain. Peptides. 1993;14:1187–1191. doi: 10.1016/0196-9781(93)90174-f. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Rockenstein E, Crews L, Masliah E. Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer's and Parkinson's diseases. Neuromolecular Med. 2003;4:21–36. doi: 10.1385/NMM:4:1-2:21. [DOI] [PubMed] [Google Scholar]
- Heraud C, Hilairet S, Muller JM, Leterrier JF, Chadeneau C. Neuritogenesis induced by vasoactive intestinal peptide, pituitary adenylate cyclase-activating polypeptide, and peptide histidine methionine in SH-SY5y cells is associated with regulated expression of cytoskeleton mRNAs and proteins. J. Neurosci. Res. 2004;75:320–329. doi: 10.1002/jnr.10866. [DOI] [PubMed] [Google Scholar]
- Heuser IJ, Baronti F, Marin CA, et al. Growth hormone secretion in Alzheimer's disease: 24-hour profile of basal levels and response to stimulation and suppression studies. Neurobiol. Ageing. 1992;13:255–260. doi: 10.1016/0197-4580(92)90037-x. [DOI] [PubMed] [Google Scholar]
- Hilairet S, Belanger C, Bertrand J, Laperriere A, Foord SM, Bouvier M. Agonist-promoted internalization of a ternary complex between calcitonin receptor-like receptor, receptor activity-modifying protein 1 (RAMP1). and beta-arrestin. J. Biol. Chem. 2001;276(42):182–42,190. doi: 10.1074/jbc.M107323200. [DOI] [PubMed] [Google Scholar]
- Hirasawa T, Nakamura T, Mizushima A, et al. Adverse effects of an active fragment of parathyroid hormone on rat hippocampal organotypic cultures. Br. J. Pharmacol. 2000;129:21–28. doi: 10.1038/sj.bjp.0702949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth SA, Adelhorst K, Pedersen BB, Kirk O, Schwartz TW. Glucagon and glucagon-like peptide 1: selective receptor recognition via distinct peptide epitopes. J. Biol. Chem. 1994;269(30):121–30,124. [PubMed] [Google Scholar]
- Hjorth SA, Orskov C, Schwartz TW. Constitutive activity of glucagon receptor mutants. Mol. Endocrinol. 1998;12:78–86. doi: 10.1210/mend.12.1.0045. [DOI] [PubMed] [Google Scholar]
- Holl RW, Thorner MO, Leong DA. Intracellular calcium concentration and growth hormone secretion in individual somatotropes: effects of growth hormone-releasing factor and somatostatin. Endocrinology. 1988;122:2927–2932. doi: 10.1210/endo-122-6-2927. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Barden N. Antidepressants and hypothalamic-pituitary-adrenocortical regulation. Endocr. Rev. 1996;17:187–205. doi: 10.1210/edrv-17-2-187. [DOI] [PubMed] [Google Scholar]
- Holt EH, Broadus AE, Brines ML. Parathyroid hormone-related peptide is produced by cultured cerebellar granule cells in response to L-type voltage-sensitive Ca2+ channel flux via a Ca2+/calmodulin-dependent kinase pathway. J. Biol. Chem. 1996;271(28):105–28,111. doi: 10.1074/jbc.271.45.28105. [DOI] [PubMed] [Google Scholar]
- Holtmann MH, Ganguli S, Hadac EM, Dolu V, Miller LJ. Multiple extracellular loop domains contribute critical determinants for agonist binding and activation of the secretin receptor. J. Biol. Chem. 1996;271(14):944–14,949. doi: 10.1074/jbc.271.25.14944. [DOI] [PubMed] [Google Scholar]
- Holz GG, Leech CA, Heller RS, Castonguay M, Habener JF. cAMP-dependent mobilization of intracellular Ca2+ stores by activation of ryanodine receptors in pancreatic beta-cells. A Ca2+ signaling system stimulated by the insulinotropic hormone glucagon-like peptide-1-(7-37) J. Biol. Chem. 1999;274(14):147–14,156. doi: 10.1074/jbc.274.20.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoosein NM, Gurd RS. Human glucagon-like peptides 1 and 2 activate rat brain adenylate cyclase. FEBS Lett. 1984;178:83–86. doi: 10.1016/0014-5793(84)81245-4. [DOI] [PubMed] [Google Scholar]
- Hoppener JW, Ahren B, Lips CJ. Islet amyloid and type 2 diabetes mellitus. N. Engl. J. Med. 2000;343:411–419. doi: 10.1056/NEJM200008103430607. [DOI] [PubMed] [Google Scholar]
- Horvath K, Stefanatos G, Sokolski KN, Wachtel R, Nabors L, Tildon JT. Improved social and language skills after secretin administration in patients with autistic spectrum disorders. J. Assoc. Acad. Minor. Phys. 1998;9:9–15. [PubMed] [Google Scholar]
- Husmann K, Sexton PM, Fischer JA, Born W. Mouse receptor-activity-modifying proteins 1, -2 and -3: Amino acid sequence, expression and function. Mol. Cell. Endocrinol. 2000;162:35–43. doi: 10.1016/s0303-7207(00)00212-4. [DOI] [PubMed] [Google Scholar]
- Irwin DM. cDNA cloning of proglucagon from the stomach and pancreas of the dog. DNA Seq. 2001;12:253–260. doi: 10.3109/10425170109024999. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Nakamura S, Kaziro Y, Takahashi T, Takahashi K, Nagata S. Molecular cloning and expression of a cDNA encoding the secretin receptor. EMBO J. 1991;10:1635–1641. doi: 10.1002/j.1460-2075.1991.tb07686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Furuya T, Ozaki K, Ohta M, Kawasaki T. The secretin precursor gene. Structure of the coding region and expression in the brain. J. Biol. Chem. 1991;266(12):595–12,598. [PubMed] [Google Scholar]
- Itoh N, Obata K, Yanaihara N, Okamoto H. Human preprovasoactive intestinal polypeptide contains a novel PHI-27-like peptide, PHM-27. Nature. 1983;304:547–549. doi: 10.1038/304547a0. [DOI] [PubMed] [Google Scholar]
- Jahnke GD, Brunssen S, Maier WE, Harry GJ. Neurotoxicant-induced elevation of adrenomedullin expression in hippocampus and glia cultures. J. Neurosci. Res. 2001;66:464–474. doi: 10.1002/jnr.1237. [DOI] [PubMed] [Google Scholar]
- Jamen F, Persson K, Bertrand G, et al. PAC1 receptor-deficient mice display impaired insulinotropic response to glucose and reduced glucose tolerance. J. Clin. Invest. 2000;105:1307–1315. doi: 10.1172/JCI9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RT, Tatemoto K, Mutt V, Lemp GF, Gardner JD. Actions of a newly isolated intestinal peptide PHI on pancreatic acini. Am. J. Physiol. 1981;241:G498–502. doi: 10.1152/ajpgi.1981.241.6.G498. [DOI] [PubMed] [Google Scholar]
- Jhamandas JH, Mactavish D. Antagonist of the amylin receptor blocks beta-amyloid toxicity in rat cholinergic basal forebrain neurons. J. Neurosci. 2004;24:5579–5584. doi: 10.1523/JNEUROSCI.1051-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund PK. Distribution of glucagonlike peptide I (GLP-I). glucagon, and glicentin in the rat brain: an immunocytochemical study. J. Comp. Neurol. 1988;271:519–532. doi: 10.1002/cne.902710405. [DOI] [PubMed] [Google Scholar]
- Jouishomme H, Whitfield JF, Gagnon L, et al. Further definition of the protein kinase C activation domain of the parathyroid hormone. J. Bone. Miner. Res. 1994;9:943–949. doi: 10.1002/jbmr.5650090620. [DOI] [PubMed] [Google Scholar]
- Jongsma H, Pettersson LM, Zhang Y, et al. Markedly reduced chronic nociceptive response in mice lacking the PAC1 receptor. Neuroreport. 2001;12:2215. doi: 10.1097/00001756-200107200-00034. [DOI] [PubMed] [Google Scholar]
- Kang G, Chepurny OG, Holz GG. cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic beta-cells. J. Physiol. 2001;536:375–385. doi: 10.1111/j.1469-7793.2001.0375c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karapalis AC, Luz A, Glowacki J, et al. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 1994;8:277–289. doi: 10.1101/gad.8.3.277. [DOI] [PubMed] [Google Scholar]
- Kashima Y, Miki T, Shibasaki T, et al. Critical role of cAMP-GEFII—Rim2 complex in incretinpotentiated insulin secretion. J. Biol. Chem. 2001;276(46):046–46,053. doi: 10.1074/jbc.M108378200. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J. Mol. Neurosci. 2002;18:7–14. doi: 10.1385/JMN:18:1-2:07. [DOI] [PubMed] [Google Scholar]
- Kawahara M, Kuroda Y, Arispe N, Rojas E. Alzheimer's beta-amyloid, human islet amylin, and prion protein fragment evoke intracellular free calcium elevations by a common mechanism in a hypothalamic GnRH neuronal cell line. J. Biol. Chem. 2000;275(14):077–14,083. doi: 10.1074/jbc.275.19.14077. [DOI] [PubMed] [Google Scholar]
- Kenny AJ. Extractable glucagon of the human pancreas. J. Clin. Endocrinol. Metab. 1955;15:1089–1105. doi: 10.1210/jcem-15-9-1089. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Radulovic J, Radulovic M, et al. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat. Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Kangawa K, Kawamoto M, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem. Biophys. Res. Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- Knudsen SM, Tams JW, Fahrenkrug J. Functional roles of conserved transmembrane pro-lines in the human VPAC(1) receptor. FEBS Lett. 2001;503:126–130. doi: 10.1016/s0014-5793(01)02716-8. [DOI] [PubMed] [Google Scholar]
- Kong LY, Maderdrut JL, Jeohn GH, Hong JS. Reduction of lipopolysaccharide-induced neurotoxicity in mixed cortical neuron/glia cultures by femtomolar concentrations of pituitary adenylate cyclase-activating polypeptide. Neuroscience. 1999;91:493–500. doi: 10.1016/s0306-4522(98)00606-x. [DOI] [PubMed] [Google Scholar]
- Konturek SJ, Pepera J, Zabielski K, et al. Brain-gut axis in pancreatic secretion and appetite control. J. Physiol. Pharmacol. 2003;54:293–317. [PubMed] [Google Scholar]
- Kourie JI, Henry CL. Ion channel formation and membrane-linked pathologies of misfolded hydrophobic proteins: The role of dangerous unchaperoned molecules. Clin. Exp. Pharmacol. Physiol. 2002;29:741–753. doi: 10.1046/j.1440-1681.2002.03737.x. [DOI] [PubMed] [Google Scholar]
- Kourie JI, Culverson AL, Farrelly PV, Henry CL, Laohachai KN. Heterogeneous amyloid-formed ion channels as a common cytotoxic mechanism: Implications for therapeutic strategies against amyloidosis. Cell Biochem. Biophys. 2002;36:191–207. doi: 10.1385/CBB:36:2-3:191. [DOI] [PubMed] [Google Scholar]
- Koves K, Arimura A, Somogyvari-Vigh A, Vigh S, Miller J. Immunohistochemical demonstration of a novel hypothalamic peptide, pituitary adenylate cyclase-activating polypeptide, in the ovine hypothalamus. Endocrinology. 1990;127:264–271. doi: 10.1210/endo-127-1-264. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Yanaihara H, Arimura A. Distribution of urocortin-like immunoreactivity in the central nervous system of the rat. J. Comp. Neurol. 1998;391:1–10. doi: 10.1002/(sici)1096-9861(19980202)391:1<1::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Kuwahara S, Kesuma Sari D, Tsukamoto Y, Tanaka S, Sasaki F. Age-related changes in growth hormone (GH)-releasing hormone and somatostatin neurons in the hypothalamus and in GH cells in the anterior pituitary of female mice. Brain Res. 2004;1025:113–122. doi: 10.1016/j.brainres.2004.08.012. [DOI] [PubMed] [Google Scholar]
- La Buda CJ, Usdin TB. Tuberoinfundibular peptide of 39 residues decreases pain-related affective behavior. Neuroreport. 2004;15:1779–1782. doi: 10.1097/01.wnr.0000134849.63755.15. [DOI] [PubMed] [Google Scholar]
- Laburthe M, Couvineau A, Gaudin P, Maoret JJ, Rouyer-Fessard C, Nicole P. Receptors for VIP, PACAP, secretin, GRF, glucagon, GLP-1, and other members of their new family of G protein-linked receptors: structure-function relationship with special reference to the human VIP-1 receptor. Ann. N. Y. Acad. Sci. 1996;805:94–109. doi: 10.1111/j.1749-6632.1996.tb17476.x. [DOI] [PubMed] [Google Scholar]
- Lambeir AM, Proost P, Scharpe S, De Meester I. Akinetic study of glucagon-like peptide-1 and glucagon-like peptide-2 truncation by dipeptidyl peptidase IV, in vitro. Biochem. Pharmacol. 2002;64:1753–1756. doi: 10.1016/s0006-2952(02)01415-6. [DOI] [PubMed] [Google Scholar]
- Lanfranco F, Gianotti L, Giordano R, Pellegrino M, Maccario M, Arvat E. Ageing, growth hormone and physical performance. J. Endocrinol. Invest. 2003;26:861–872. doi: 10.1007/BF03345237. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997;138:4445–4455. doi: 10.1210/endo.138.10.5270. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ. Historical review: a brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol. Sci. 2004;25:413–422. doi: 10.1016/j.tips.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Ihl R, Frolich L, et al. Endocrine responses to growth hormone releasing hormone and corticotropin releasing hormone in early-onset Alzheimer's disease. Psychiatry Res. 1990;33:107–112. doi: 10.1016/0165-1781(90)90063-b. [DOI] [PubMed] [Google Scholar]
- Leuthauser K, Gujer R, Aldecoa A, et al. Receptor-activity-modifying protein 1 forms heterodimers with two G protein-coupled receptors to define ligand recognition. Biochem. J. 2000;351:347–351. [PMC free article] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc. Natl. Acad. Sci. USA. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezoualc'h F, Engert S, Berning B, Behl C. Corticotropin-releasing hormone-mediated neuroprotection against oxidative stress is associated with the increased release of non-amyloidogenic amyloid beta precursor protein and with the suppression of nuclear factor-kappaB. Mol. Endocrinol. 2000;14:147–159. doi: 10.1210/mend.14.1.0403. [DOI] [PubMed] [Google Scholar]
- Li S, Grinevich V, Fournier A, Pelletier G. Effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on gonadotropin-releasing hormone and somatostatin gene expression in the rat brain. Brain Res. Mol. Brain Res. 1996;41:157–162. doi: 10.1016/0169-328x(96)00086-1. [DOI] [PubMed] [Google Scholar]
- Li Y, Hansotia T, Yusta B, Ris F, Halban PA, Drucker DJ. Glucagon-like peptide-1 receptor signaling modulates beta cell apoptosis. J. Biol. Chem. 2003;278:471–478. doi: 10.1074/jbc.M209423200. [DOI] [PubMed] [Google Scholar]
- Liaw CW, Lovenberg TW, Barry G, Oltersdorf T, Grigoriadis DE, De Souza EB. Cloning and characterization of the human corticotropin-releasing factor-2 receptor complementary deoxyribonucleic acid. Endocrinology. 1996;137:72–77. doi: 10.1210/endo.137.1.8536644. [DOI] [PubMed] [Google Scholar]
- Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- Light PE, Manning Fox JE, Riedel MJ, Wheeler MB. Glucagon-like peptide-1 inhibits pancreatic ATP-sensitive potassium channels via a protein kinase A- and ADP-dependent mechanism. Mol. Endocrinol. 2002;16:2135–2144. doi: 10.1210/me.2002-0084. [DOI] [PubMed] [Google Scholar]
- Lightdale JR, Hayer C, Duer A, et al. Effects of intravenous secretin on language and behavior of children with autism and gastrointestinal symptoms: a single-blinded, open-label pilot study. Pediatrics. 2001;108:E90. doi: 10.1542/peds.108.5.e90. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- Lin C, Lin SC, Chang CP, Rosenfeld MG. Pit-1-dependent expression of the receptor for growth hormone releasing factor mediates pituitary cell growth. Nature. 1992;360:765–768. doi: 10.1038/360765a0. [DOI] [PubMed] [Google Scholar]
- Liu GJ, Madsen BW. PACAP38 modulates activity of NMDAreceptors in cultured chick cortical neurons. J. Neurophysiol. 1997;78:2231–2234. doi: 10.1152/jn.1997.78.4.2231. [DOI] [PubMed] [Google Scholar]
- Lopez De Maturana R, Willshaw A, Kuntzsch A, Rudolph R, Donnelly D. The isolated N-terminal domain of the glucagon-like peptide-1 (GLP-1) receptor binds exendin peptides with much higher affinity than GLP-1. J. Biol. Chem. 2003;278(10):195–10,200. doi: 10.1074/jbc.M212147200. [DOI] [PubMed] [Google Scholar]
- Lovshin JA, Huang Q, Seaberg R, Brubaker PL, Drucker DJ. Extrahypothalamic expression of the glucagon-like peptide-2 receptor is coupled to reduction of glutamate-induced cell death in cultured hippocampal cells. Endocrinology. 2004;145:3495–3506. doi: 10.1210/en.2004-0100. [DOI] [PubMed] [Google Scholar]
- Lund PK, Goodman RH, Dee PC, Habener JF. Pancreatic preproglucagon cDNA contains two glucagon-related coding sequences arranged in tandem. Proc. Natl. Acad. Sci. USA. 1982;79:345–349. doi: 10.1073/pnas.79.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz-Bucher B, Monnier D, Koch B. Evidence for the presence of receptors for pituitary adenylate cyclase-activating polypeptide in the neurohypophysis that are positively coupled to cyclic AMP formation and neurohypophyseal hormone secretion. Neuroendocrinology. 1996;64:153–161. doi: 10.1159/000127113. [DOI] [PubMed] [Google Scholar]
- Lynch CD, Lyons D, Khan A, Bennett SA, Sonntag WE. Insulin-like growth factor-1 selectively increases glucose utilization in brains of aged animals. Endocrinology. 2001;142:506–509. doi: 10.1210/endo.142.1.8053. [DOI] [PubMed] [Google Scholar]
- Main MJ, Brown J, Brown S, Fraser NJ, Foord SM. The cgrp receptor can couple via pertussis toxin sensitive and insensitive G proteins. FEBS Lett. 1998;441:6–10. doi: 10.1016/s0014-5793(98)01507-5. [DOI] [PubMed] [Google Scholar]
- Malhotra RK, Wakade TD, Wakade AR. Vasoactive intestinal polypeptide and muscarine mobilize intracellular Ca2+ through breakdown of phosphoinositides to induce catecholamine secretion. Role of IP3 in exocytosis. J. Biol. Chem. 1988;263:2123–2126. [PubMed] [Google Scholar]
- Mangin M, Ikeda K, Dreyer BE, Broadus AE. Identification of an up-stream promoter of the human parathyroid hormone-related peptide gene. Mol. Endocrinol. 1990;4:851–858. doi: 10.1210/mend-4-6-851. [DOI] [PubMed] [Google Scholar]
- Martin JL, Dietl MM, Hof PR, Palacios JM, Magistretti PJ. Autoradiographic mapping of [mono[125I]iodo-Tyr10, MetO17]vasoactive intestinal peptide binding sites in the rat brain. Neuroscience. 1987;23:539–565. doi: 10.1016/0306-4522(87)90075-3. [DOI] [PubMed] [Google Scholar]
- Martin JL, Gasser D, Magistretti PJ. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide potentiate c-fos expression induced by glutamate in cultured cortical neurons. J. Neurochem. 1995;65:1–9. doi: 10.1046/j.1471-4159.1995.65010001.x. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Ohtaki T, Masuda Y, Tsuda M, Fujino M. Binding sites for pituitary adenylate cyclase activating polypeptide (PACAP). comparison with vasoactive intestinal polypeptide (VIP) binding site localization in rat brain sections. Brain Res. 1992;575:113–123. doi: 10.1016/0006-8993(92)90430-h. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Goodman Y. Different amyloidogenic peptides share a similar mechanism of neurotoxicity involving reactive oxygen species and calcium. Brain Res. 1995;676:219–224. doi: 10.1016/0006-8993(95)00148-j. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolecular Med. 2003;3:65–94. doi: 10.1385/NMM:3:2:65. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res. Rev. 2004;3:445–464. doi: 10.1016/j.arr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- May PC, Boggs LN, Fuson KS. Neurotoxicity of human amylin in rat primary hippocampal cultures: Similarity to alzheimer 's disease amyloid-beta neurotoxicity. J. Neurochem. 1993;61:2330–2333. doi: 10.1111/j.1471-4159.1993.tb07480.x. [DOI] [PubMed] [Google Scholar]
- Mayo KE. Molecular cloning and expression of a pituitary-specific receptor for growth hormone-releasing hormone. Mol. Endocrinol. 1992;6:1734–1744. doi: 10.1210/mend.6.10.1333056. [DOI] [PubMed] [Google Scholar]
- Mayo KE, Cerelli GM, Lebo RV, Bruce BD, Rosenfeld MG, Evans RM. Gene encoding human growth hormone-releasing factor precursor: structure, sequence, and chromosomal assignment. Proc. Natl. Acad. Sci. USA. 1985;82:63–67. doi: 10.1073/pnas.82.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo KE, Vale W, Rivier J, Rosenfeld MG, Evans RM. Expression-cloning and sequence of a cDNA encoding human growth hormone-releasing factor. Nature. 1983;306:86–88. doi: 10.1038/306086a0. [DOI] [PubMed] [Google Scholar]
- McKnight AJ, Gordon S. EGF-TM7: a novel subfamily of seven-transmembrane-region leukocyte cell-surface molecules. Immunol. Today. 1996;17:283–287. doi: 10.1016/0167-5699(96)80546-9. [DOI] [PubMed] [Google Scholar]
- Mclatchie LM, Fraser NJ, Main MJ, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Mei YA. High-voltage-activated calcium current and its modulation by dopamine D4 and pituitary adenylate cyclase activating polypeptide receptors in cerebellar granule cells. Zhongguo Yao Li Xue Bao. 1999;20:3–9. [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J. Comp. Neurol. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Thomas CR, Arimura A. Immunocytochemical localization of growth hormone releasing factor (GHRF)-containing structures in the rat brain using anti-rat GHRF serum. Peptides. 1984;5:1071–1075. doi: 10.1016/0196-9781(84)90173-6. [DOI] [PubMed] [Google Scholar]
- Mergenthaler P, Dirnagl U, Meisel A. Pathophysiology of stroke: lessons from animal models. Metab. Brain Dis. 2004;19:151–167. doi: 10.1023/b:mebr.0000043966.46964.e6. [DOI] [PubMed] [Google Scholar]
- Miyata A, Arimura A, Dahl RR, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- Miyata A, Jiang L, Dahl RD, et al. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38) Biochem. Biophys. Res. Commun. 1990;170:643–648. doi: 10.1016/0006-291x(90)92140-u. [DOI] [PubMed] [Google Scholar]
- Morio H, Tatsuno I, Hirai A, Tamura Y, Saito Y. Pituitary adenylate cyclase-activating polypeptide protects rat-cultured cortical neurons from glutamate-induced cytotoxicity. Brain Res. 1996;741:82–88. doi: 10.1016/s0006-8993(96)00920-1. [DOI] [PubMed] [Google Scholar]
- Muff R, Buhlmann N, Fischer JA, Born W. An amylin receptor is revealed following co-transfection of a calcitonin receptor with receptor activity modifying proteins-1 or -3. Endocrinology. 1999;140:2924–2927. doi: 10.1210/endo.140.6.6930. [DOI] [PubMed] [Google Scholar]
- Munroe DG, Gupta AK, Kooshesh F, et al. Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc. Natl. Acad. Sci. USA. 1999;96:1569–1573. doi: 10.1073/pnas.96.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase T, Kondo K, Otake K, Oiso Y. Pituitary adenylate cyclase-activating polypeptide stimulates arginine vasopressin release in conscious rats. Neuroendocrinology. 1993;57:1092–1096. doi: 10.1159/000126475. [DOI] [PubMed] [Google Scholar]
- Mutt V. Chemistry, isolation and purification of gastrointestinal hormones. Biochem. Soc. Trans. 1980;8:11–14. doi: 10.1042/bst0080011. [DOI] [PubMed] [Google Scholar]
- Mutt V, Carlquist M, Tatemoto K. Secretin-like bioactivity in extracts of porcine brain. Life Sci. 1979;25:1703–1707. doi: 10.1016/0024-3205(79)90472-7. [DOI] [PubMed] [Google Scholar]
- Nabhan C, Xiong Y, Xie LY, Abou-Samra AB. The alternatively spliced type II corticotropin-releasing factor receptor, stably expressed in LLCPK-1 cells, is not well coupled to the G protein(s) Biochem. Biophys. Res. Commun. 1995;212:1015–1021. doi: 10.1006/bbrc.1995.2071. [DOI] [PubMed] [Google Scholar]
- Nagai K. Role of VIP-neurons in the hypothalamic suprachiasmatic nucleus in the control of blood glucose. Nippon Yakurigaku Zasshi. 2004;123:253–260. doi: 10.1254/fpj.123.253. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Krishnan KR, Belkin BM, et al. Growth hormone response to growth hormone releasing factor in Alzheimer's disease. Neuroendocrinology. 1989;50:663–666. doi: 10.1159/000125296. [DOI] [PubMed] [Google Scholar]
- Nielsen SM, Nielsen LZ, Hjorth SA, Perrin MH, Vale WW. Constitutive activation of tethered-peptide/corticotropin-releasing factor receptor chimeras. Proc. Natl. Acad. Sci. USA. 2000;97(10):277–10,281. doi: 10.1073/pnas.97.18.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzveig DR, Mathew S, Gershengorn MC. Alternative splicing of a 48-nucleotide exon generates two isoforms of the human calcitonin receptor. Endocrinology. 1995;136:2047–2051. doi: 10.1210/endo.136.5.7720653. [DOI] [PubMed] [Google Scholar]
- Nutt RF, Caulfield MP, Levy JJ, Gibbons SW, Rosenblatt M, McKee RL. Removal of partial agonism from parathyroid hormone (PTH)-related protein-(7-34)NH2 by substitution of PTH amino acids at positions 10 and 11. Endocrinology. 1990;127:491–493. doi: 10.1210/endo-127-1-491. [DOI] [PubMed] [Google Scholar]
- O'Donohue TL, Charlton CG, Miller RL, Boden G, Jacobowitz DM. Identification, characterization, and distribution of secretin immunoreactivity in rat and pig brain. Proc. Natl. Acad. Sci. USA. 1981;78:5221–5224. doi: 10.1073/pnas.78.8.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offen D, Sherki Y, Melamed E, Fridkin M, Brenneman DE, Gozes I. Vasoactive intestinal peptide (VIP) prevents neurotoxicity in neuronal cultures: relevance to neuroprotection in Parkinson's disease. Brain Res. 2000;854:257–262. doi: 10.1016/s0006-8993(99)02375-6. [DOI] [PubMed] [Google Scholar]
- Ohlsson L, Lindstrom P. The correlation between calcium outflow and growth hormone release in perifused rat somatotrophs. Endocrinology. 1990;126:488–497. doi: 10.1210/endo-126-1-488. [DOI] [PubMed] [Google Scholar]
- Oliver KR, Kane SA, Salvatore CA, et al. Cloning, characterization and central nervous system distribution of receptor activity modifying proteins in the rat. Eur. J. Neurosci. 2001;14:618–628. doi: 10.1046/j.0953-816x.2001.01688.x. [DOI] [PubMed] [Google Scholar]
- Onoue S, Endo K, Ohshima K, Yajima T, Kashimoto K. The neuropeptide PACAP attenuates beta-amyloid (1-42)-induced toxicity in PC12 cells. Peptides. 2002;23:1471–1478. doi: 10.1016/s0196-9781(02)00085-2. [DOI] [PubMed] [Google Scholar]
- Orskov C, Wettergren A, Holst JJ. Biological effects and metabolic rates of glucagonlike peptide-1 7-36 amide and glucagonlike peptide-1 7-37 in healthy subjects are indistinguishable. Diabetes. 1993;42:658–661. doi: 10.2337/diab.42.5.658. [DOI] [PubMed] [Google Scholar]
- Otto C, Kovalchuk Y, Wolfer DP, et al. Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice. J. Neurosci. 2001;21:5520–5527. doi: 10.1523/JNEUROSCI.21-15-05520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol. Rev. 1991;43:425–473. [PubMed] [Google Scholar]
- Owley T, Mcmahon W, Cook EH, et al. Multisite, double-blind, placebo-controlled trial of porcine secretin in autism. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:1293–1299. doi: 10.1097/00004583-200111000-00009. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- Pedersen WA, McCullers D, Culmsee C, Haughey NJ, Herman JP, Mattson MP. Corticotropin-releasing hormone protects neurons against insults relevant to the pathogenesis of Alzheimer's disease. Neurobiol. Dis. 2001;8:492–503. doi: 10.1006/nbdi.2001.0395. [DOI] [PubMed] [Google Scholar]
- Pedersen WA, Wan R, Zhang P, Mattson MP. Urocortin, but not urocortin II, protects cultured hippocampal neurons from oxidative and excitotoxic cell death via corticotropin-releasing hormone receptor type I. J. Neurosci. 2002;22:404–412. doi: 10.1523/JNEUROSCI.22-02-00404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann. N. Y. Acad. Sci. 1999;885:312–328. doi: 10.1111/j.1749-6632.1999.tb08687.x. [DOI] [PubMed] [Google Scholar]
- Perrin MH, Fischer WH, Kunitake KS, et al. Expression, purification, and characterization of a soluble form of the first extracellular domain of the human type 1 corticotropin releasing factor receptor. J. Biol. Chem. 2001;276(31):528–31,534. doi: 10.1074/jbc.M101838200. [DOI] [PubMed] [Google Scholar]
- Perry T, Haughey NJ, Mattson MP, Egan JM, Greig NH. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J. Pharmacol. Exp. Ther. 2002a;302:881–888. doi: 10.1124/jpet.102.037481. [DOI] [PubMed] [Google Scholar]
- Perry T, Lahiri DK, Chen D, et al. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J. Pharmacol. Exp. Ther. 2002b;300:958–966. doi: 10.1124/jpet.300.3.958. [DOI] [PubMed] [Google Scholar]
- Perry T, Lahiri DK, Sambamurti K, et al. Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J. Neurosci. Res. 2003;72:603–612. doi: 10.1002/jnr.10611. [DOI] [PubMed] [Google Scholar]
- Philbrick WM, Wysolmerski JJ, Galbraith S, et al. Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol. Rev. 1996;76:127–173. doi: 10.1152/physrev.1996.76.1.127. [DOI] [PubMed] [Google Scholar]
- Pinhasov A, Mandel S, Torchinsky A, et al. Activity-dependent neuroprotective protein: a novel gene essential for brain formation. Brain Res. Dev. Brain Res. 2003;144:83–90. doi: 10.1016/s0165-3806(03)00162-7. [DOI] [PubMed] [Google Scholar]
- Pisegna JR, Moody TW, Wank SA. Differential signaling and immediate-early gene activation by four splice variants of the human pituitary adenylate cyclase-activating polypeptide receptor (hPACAP-R) Ann. N. Y. Acad. Sci. 1996;805:54–64. doi: 10.1111/j.1749-6632.1996.tb17473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittner RA, Albrandt K, Beaumont K, et al. Molecular physiology of amylin. J. Cell. Biochem. 1994;55:19–28. doi: 10.1002/jcb.240550004. [DOI] [PubMed] [Google Scholar]
- Pomara N, Singh RR, Deptula D, et al. CSF corticotropin-releasing factor (CRF) in Alzheimer's disease: its relationship to severity of dementia and monoamine metabolites. Biol. Psychiatry. 1989;26:500–504. doi: 10.1016/0006-3223(89)90071-1. [DOI] [PubMed] [Google Scholar]
- Pombo CM, Zalvide J, Gaylinn BD, Dieguez C. Growth hormone-releasing hormone stimulates mitogen-activated protein kinase. Endocrinology. 2000;141:2113–2119. doi: 10.1210/endo.141.6.7513. [DOI] [PubMed] [Google Scholar]
- Purdue BW, Tilakaratne N, Sexton PM. Molecular pharmacology of the calcitonin receptor. Receptors Channels. 2002;8:243–255. [PubMed] [Google Scholar]
- Qi LJ, Leung AT, Xiong Y, Marx KA, Abou-Samra AB. Extracellular cysteines of the corticotropin-releasing factor receptor are critical for ligand interaction. Biochemistry. 1997;36(12):442–12,448. doi: 10.1021/bi970997r. [DOI] [PubMed] [Google Scholar]
- Rao AV, Balachandran B. Role of oxidative stress and antioxidants in neurodegenerative diseases. Nutr. Neurosci. 2002;5:291–309. doi: 10.1080/1028415021000033767. [DOI] [PubMed] [Google Scholar]
- Reichlin S. Neuroendocrine significance of vasoactive intestinal polypeptide. Ann. N. Y. Acad. Sci. 1988;527:431–449. doi: 10.1111/j.1749-6632.1988.tb26998.x. [DOI] [PubMed] [Google Scholar]
- Rodbell M, Birnbaumer L, Pohl SL. Characteristics of glucagon action on the hepatic adenylate cyclase system. Biochem. J. 1971;125:58–59. doi: 10.1042/bj1250058p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe SY, McGowan EM, Rothwell NJ. Evidence for the involvement of corticotrophin-releasing hormone in the pathogenesis of traumatic brain injury. Eur. J. Neurosci. 1998;10:553–559. doi: 10.1046/j.1460-9568.1998.00064.x. [DOI] [PubMed] [Google Scholar]
- Rosenfeld RG, Ceda G, Wilson DM, Dollar LA, Hoffman AR. Characterization of high affinity receptors for insulin-like growth factors I and II on rat anterior pituitary cells. Endocrinology. 1984;114:1571–1575. doi: 10.1210/endo-114-5-1571. [DOI] [PubMed] [Google Scholar]
- Said SI. VIP as a modulator of lung inflammation and airway constriction. Am. Rev. Respir. Dis. 1991;143:22–24. doi: 10.1164/ajrccm/143.3_Pt_2.S22. [DOI] [PubMed] [Google Scholar]
- Said SI, Mutt V. Potent peripheral and splanchnic vasodilator peptide from normal gut. Nature. 1970;225:863–864. doi: 10.1038/225863a0. [DOI] [PubMed] [Google Scholar]
- Salomon R, Couvineau A, Rouyer-Fessard C, et al. Characterization of a common VIP-PACAP receptor in human small intestinal epithelium. Am. J. Physiol. 1993;264:294–300. doi: 10.1152/ajpendo.1993.264.2.E294. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Rivier J, Vale WW. The distribution of growth-hormone-releasing factor (GRF) immunoreactivity in the central nervous system of the rat: an immunohistochemical study using antisera directed against rat hypothalamic GRF. J. Comp. Neurol. 1985;237:100–115. doi: 10.1002/cne.902370108. [DOI] [PubMed] [Google Scholar]
- Schipani E, Langman C, Hunzelman J, et al. Anovel parathyroid hormone (PTH)/PTH-related peptide receptor mutation in Jansen's metaphyseal chondrodysplasia. J. Clin. Endocrinol. Metab. 1999;84:3052–3057. doi: 10.1210/jcem.84.9.6000. [DOI] [PubMed] [Google Scholar]
- Schirra J, Kuwert P, Wank U, et al. Differential effects of subcutaneous GLP-1 on gastric emptying, antroduodenal motility, and pancreatic function in men. Proc. Assoc. Am. Physicians. 1997;109:84–97. [PubMed] [Google Scholar]
- Segre GV. Receptors for secretion, calcitonin, parathyroid hormone (PTH)/PTH-related peptide, vasoactive intestinal peptide, glucagon-like peptide 1, growth hormone-releasing hormone, and glucagons belong to a newly discovered G-protein-linked receptor family. Trends Endocrinol. Metab. 1993;4:309–314. doi: 10.1016/1043-2760(93)90071-l. [DOI] [PubMed] [Google Scholar]
- Seki Y, Suzuki Y, Baskaya MK, et al. Central cardiovascular effects induced by intracisternal PACAP in dogs. Am. J. Physiol. 1995;269:135–139. doi: 10.1152/ajpheart.1995.269.1.H135. [DOI] [PubMed] [Google Scholar]
- Serrano J, Alonso D, Encinas JM, et al. Adrenomedullin expression is up-regulated by ischemia-reperfusion in the cerebral cortex of the adult rat. Neuroscience. 2002;109:717–731. doi: 10.1016/s0306-4522(01)00532-2. [DOI] [PubMed] [Google Scholar]
- Serrano J, Alonso D, Fernandez AP, et al. Adrenomedullin in the central nervous system. Microsc. Res. Tech. 57:76–90. doi: 10.1002/jemt.10053. [DOI] [PubMed] [Google Scholar]
- Servoss SJ, Lee SJ, Gibney G, Gozes I, Brenneman DE, Hill JM. IGF-I as a mediator of VIP/activity-dependent neurotrophic factor-stimulated embryonic growth. Endocrinology. 2001;142:3348–3353. doi: 10.1210/endo.142.8.8335. [DOI] [PubMed] [Google Scholar]
- Sexton PM, Albiston A, Morfis M, Tilakaratne N. Receptor activity modifying proteins. Cell. Signal. 2001;13:73–83. doi: 10.1016/s0898-6568(00)00143-1. [DOI] [PubMed] [Google Scholar]
- Sheitman BB, Knable MB, Jarskog LF, et al. Secretin for refractory schizophrenia. Schizophr. Res. 2004;66:177–181. doi: 10.1016/S0920-9964(03)00068-9. [DOI] [PubMed] [Google Scholar]
- Shen S, Spratt C, Sheward WJ, et al. Overexpression of the human VPAC2 receptor in the suprachiasmatic nucleus alters the circadian phenotype of mice. Proc. Natl. Acad. Sci. USA. 2000;97(11):575–11,580. doi: 10.1073/pnas.97.21.11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki T, Yamauchi N, Hotta M, et al. In vitro release of growth hormone-releasing factor from rat hypothalamus: effect of insulin-like growth factor-1. Regul. Pept. 1986;15:47–53. doi: 10.1016/0167-0115(86)90074-1. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Carter PH, Gardella TJ. Autoactivation of type-1 parathyroid hormone receptors containing a tethered ligand. J. Biol. Chem. 2000;275(19):456–19,460. doi: 10.1074/jbc.M001596200. [DOI] [PubMed] [Google Scholar]
- Shoge K, Mishima HK, Saitoh T, et al. Attenuation by PACAP of glutamate-induced neurotoxicity in cultured retinal neurons. Brain Res. 1999;839:66–73. doi: 10.1016/s0006-8993(99)01690-x. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Glucagon-like peptide-1 receptor (GLP1-R) mRNA in the rat hypothalamus. Endocrinology. 1996;137:5159–5162. doi: 10.1210/endo.137.11.8895391. [DOI] [PubMed] [Google Scholar]
- Siegel JM. Narcolepsy: a key role for hypocretins (orexins) Cell. 1999;98:409–412. doi: 10.1016/s0092-8674(00)81969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieradzan KA, Mann DM. The selective vulnerability of nerve cells in Huntington's disease. Neuropathol. Appl. Neurobiol. 2001;27:1–21. doi: 10.1046/j.0305-1846.2001.00299.x. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, et al. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Somogyvari-Vigh A, Reglodi D. Pituitary adenylate cyclase activating polypeptide: a potential neuroprotective peptide. Curr. Pharm. Des. 2004;10:2861–2869. doi: 10.2174/1381612043383548. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J. Anat. 2000;197:575–585. doi: 10.1046/j.1469-7580.2000.19740575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg O, Magistretti PJ. Vasoactive intestinal peptide and noradrenaline exert long-term control on glycogen levels in astrocytes: blockade by protein synthesis inhibition. J. Neurosci. 1992;12:4923–4931. doi: 10.1523/JNEUROSCI.12-12-04923.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergh PH, Hoppener JW, Zandberg J, Lips CJ, Jansz HS. A second human calcitonin/CGRP gene. FEBS Lett. 1985;183:403–407. doi: 10.1016/0014-5793(85)80820-6. [DOI] [PubMed] [Google Scholar]
- Sugihara H, Emoto N, Tamura H, et al. Effect of insulin-like growth factor-I on growth hormone-releasing factor receptor expression in primary rat anterior pituitary cell culture. Neurosci. Lett. 1999;276:87–90. doi: 10.1016/s0304-3940(99)00801-0. [DOI] [PubMed] [Google Scholar]
- Svoboda M, Tastenoy M, Vertongen P, Robberecht P. Relative quantitative analysis of glucagon receptor mRNA in rat tissues. Mol. Cell. Endocrinol. 1994;105:131–137. doi: 10.1016/0303-7207(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Simmons DM. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: a hybridization histochemical study in the rat. J. Comp. Neurol. 1989;285:413–435. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- Takei N, Skoglosa Y, Lindholm D. Neurotrophic and neuroprotective effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on mesencephalic dopaminergic neurons. J. Neurosci. Res. 1998;54:698–706. doi: 10.1002/(SICI)1097-4547(19981201)54:5<698::AID-JNR15>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Tamas A, Reglodi D, Szanto Z, Borsiczky B, Nemeth J, Lengvari I. Comparative neuroprotective effects of preischemic PACAP and VIP administration in permanent occlusion of the middle cerebral artery in rats. Neuro. Endocrinol. Lett. 2002;23:249–254. [PubMed] [Google Scholar]
- Tay J, Goulet M, Rusche J, Boismenu R. Age-related and regional differences in secretin and secretin receptor mRNAlevels in the rat brain. Neurosci. Lett. 2004;366:176–181. doi: 10.1016/j.neulet.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Thornton PL, Ingram RL, Sonntag WE. Chronic [D-Ala2]-growth hormone-releasing hormone administration attenuates age-related deficits in spatial memory. J. Gerontol. A. Biol. Sci. Med. Sci. 2000;55:106–112. doi: 10.1093/gerona/55.2.b106. [DOI] [PubMed] [Google Scholar]
- Tilakaratne N, Christopoulos G, Zumpe ET, Foord SM, Sexton PM. Amylin receptor phenotypes derived from human calcitonin receptor/ramp coexpression exhibit pharmacological differences dependent on receptor isoform and host cell environment. J. Pharmacol. Exp. Ther. 2000;294:61–72. [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat. Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Trimble ER, Bruzzone R, Biden TJ, Meehan CJ, Andreu D, Merrifield RB. Secretin stimulates cyclic AMP and inositol trisphosphate production in rat pancreatic acinar tissue by two fully independent mechanisms. Proc. Natl. Acad. Sci. USA. 1987;84:3146–3150. doi: 10.1073/pnas.84.10.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CC, Lin L. A point mutation in the glucose-dependent insulinotropic peptide receptor confers constitutive activity. Biochem. Biophys. Res. Commun. 1997;232:96–100. doi: 10.1006/bbrc.1997.6231. [DOI] [PubMed] [Google Scholar]
- Turton MD, O'shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- Uchida D, Arimura A, Somogyvari-Vigh A, Shioda S, Banks WA. Prevention of ischemia-induced death of hippocampal neurons by pituitary adenylate cyclase activating polypeptide. Brain Res. 1996;736:280–286. doi: 10.1016/0006-8993(96)00716-0. [DOI] [PubMed] [Google Scholar]
- Ulrich CD, 2nd, Wood P, Hadac EM, Kopras E, Whitcomb DC, Miller LJ. Cellular distribution of secretin receptor expression in rat pancreas. Am. J. Physiol. 1998;275:1437–1444. doi: 10.1152/ajpgi.1998.275.6.G1437. [DOI] [PubMed] [Google Scholar]
- Unson CG, Macdonald D, Merrifield RB. The role of histidine-1 in glucagon action. Arch. Biochem. Biophys. 1993;300:747–750. doi: 10.1006/abbi.1993.1103. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Gruber C, Bonner TI. Identification and functional expression of a receptor selectively recognizing parathyroid hormone, the PTH2 receptor. J. Biol. Chem. 1995;270(15):455–15,458. doi: 10.1074/jbc.270.26.15455. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Hoare SR, Wang T, Mezey E, Kowalak JA. TIP39: a new neuropeptide and PTH2-receptor agonist from hypothalamus. Nat. Neurosci. 1999;2:941–943. doi: 10.1038/14724. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Basille M, Anouar Y, Fournier A, Vaudry H, Gonzalez BJ. The neurotrophic activity of PACAP on rat cerebellar granule cells is associated with activation of the protein kinase A pathway and c-fos gene expression. Ann. N. Y. Acad. Sci. 1998a;865:92–99. doi: 10.1111/j.1749-6632.1998.tb11167.x. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Anouar Y, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide stimulates both c-fos gene expression and cell survival in rat cerebellar granule neurons through activation of the protein kinase A pathway. Neuroscience. 1998b;84:801–812. doi: 10.1016/s0306-4522(97)00545-9. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Pamantung TF, Fournier A, Vaudry H. PACAP acts as a neurotrophic factor during histogenesis of the rat cerebellar cortex. Ann. N. Y. Acad. Sci. 2000;921:293–299. doi: 10.1111/j.1749-6632.2000.tb06980.x. [DOI] [PubMed] [Google Scholar]
- Vilardaga JP, Frank M, Krasel C, Dees C, Nissenson RA, Lohse MJ. Differential conformational requirements for activation of G proteins and the regulatory proteins arrestin and G protein-coupled receptor kinase in the G protein-coupled receptor for parathyroid hormone (PTH)/PTH-related protein. J. Biol. Chem. 2001;276(33):435–33,443. doi: 10.1074/jbc.M011495200. [DOI] [PubMed] [Google Scholar]
- Villalba M, Bockaert J, Journot L. Pituitary adenylate cyclase-activating polypeptide (PACAP-38) protects cerebellar granule neurons from apoptosis by activating the mitogen-activated protein kinase (MAP kinase) pathway. J. Neurosci. 1997;17:83–90. doi: 10.1523/JNEUROSCI.17-01-00083.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi R, Caffarra P, Scaglioni A, et al. Defective 5-HT 1-receptor-mediated neurotransmission in the control of growth hormone secretion in Parkinson's disease. Neuropsychobiology. 1997;35:79–83. doi: 10.1159/000119395. [DOI] [PubMed] [Google Scholar]
- Wakelam MJ, Murphy GJ, Hruby VJ, Houslay MD. Activation of two signal-transduction systems in hepatocytes by glucagon. Nature. 1986;323:68–71. doi: 10.1038/323068a0. [DOI] [PubMed] [Google Scholar]
- Wang X, Yue TL, Barone FC, et al. Discovery of adrenomedullin in rat ischemic cortex and evidence for its role in exacerbating focal brain ischemic damage. Proc. Natl. Acad. Sci. USA. 1995;92(11):480–11,484. doi: 10.1073/pnas.92.25.11480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver DR, Deeds JD, Lee K, Segre GV. Localization of parathyroid hormone-related peptide (PTHrP) and PTH/PTHrP receptor mRNAs in rat brain. Brain Res. Mol. Brain Res. 1995;28:296–310. doi: 10.1016/0169-328x(94)00222-z. [DOI] [PubMed] [Google Scholar]
- Weir EC, Brines ML, Ikeda K, Burtis WJ, Broadus AE, Robbins RJ. Parathyroid hormone-related peptide gene is expressed in the mammalian central nervous system. Proc. Natl. Acad. Sci. USA. 1990;87:108–112. doi: 10.1073/pnas.87.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JH, Hartley DM, Koh J, Choi DW. The calcium channel blocker nifedipine attenuates slow excitatory amino acid neurotoxicity. Science. 1990;247:1474–1477. doi: 10.1126/science.247.4949.1474. [DOI] [PubMed] [Google Scholar]
- Welch MG, Keune JD, Welch-Horan TB, Anwar N, Anwar M, Ruggiero DA. Secretin activates visceral brain regions in the rat including areas abnormal in autism. Cell. Mol. Neurobiol. 2003;23:817–837. doi: 10.1023/A:1025013322194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysolmerski JJ, Philbrick WM, Dunbar ME, Lanske B, Kronenberg H, Broadus AE. Rescue of the parathyroid hormone-related protein knockout mouse demonstrates that parathyroid hormone-related protein is essential for mammary gland development. Development. 1998;125:1285–1294. doi: 10.1242/dev.125.7.1285. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Giguere J, Parisien M, et al. Biological activities of glucagon-like peptide-1 analogues in vitro and in vivo. Biochemistry. 2001;40:2860–2869. doi: 10.1021/bi0014498. [DOI] [PubMed] [Google Scholar]
- Yakar S, Pennisi P, Zhao H, Zhang Y, Leroith D. Circulating IGF-1 and its role in cancer: lessons from the IGF-1 gene deletion (LID) mouse. Novartis Found. Symp. 2004;262:3–9. discussion 9–18, 265–268. [PubMed] [Google Scholar]
- Yamamoto H, Lee CE, Marcus JN, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J. Clin. Invest. 2002;110:43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S, Melmed S. Insulin-like growth factor I action on rat anterior pituitary cells: suppression of growth hormone secretion and messenger ribonucleic acid levels. Endocrinology. 1986;118:176–182. doi: 10.1210/endo-118-1-176. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Minamitani N, Maeda K. Peptide histidine methionine in cerebrospinal fluid of patients with senile dementia of the Alzheimer type. Jpn. J. Psychiatry Neurol. 1993;47:85–90. doi: 10.1111/j.1440-1819.1993.tb02034.x. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Maeda K, Kakigi T, Minamitani N, Kawaguchi T, Tanaka C. Low cerebrospinal fluid concentrations of peptide histidine valine and somatostatin-28 in Alzheimer's disease: altered processing of prepro-vasoactive intestinal peptide and prepro-somatostatin. Neuropeptides. 1995;29:325–330. doi: 10.1016/0143-4179(95)90003-9. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Williams SJ, Bishop AE, Polak JM, Bloom SR. Peptide histidine-methionine immunoreactivity in plasma and tissue from patients with vasoactive intestinal peptide-secreting tumors and watery diarrhea syndrome. J. Clin. Endocrinol. Metab. 1987;64:131–139. doi: 10.1210/jcem-64-1-131. [DOI] [PubMed] [Google Scholar]
- Yung WH, Leung PS, Ng SS, Zhang J, Chan SC, Chow BK. Secretin facilitates GABA transmission in the cerebellum. J. Neurosci. 2001;21:7063–7068. doi: 10.1523/JNEUROSCI.21-18-07063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusta B, Somwar R, Wang F, et al. Identification of glucagon-like peptide-2 (GLP-2)-activated signaling pathways in baby hamster kidney fibrob-lasts expressing the rat GLP-2 receptor. J. Biol. Chem. 1999;274(30):459–30,467. doi: 10.1074/jbc.274.43.30459. [DOI] [PubMed] [Google Scholar]
- Yusta B, Boushey RP, Drucker DJ. The glucagon-like peptide-2 receptor mediates direct inhibition of cellular apoptosis via a cAMP-dependent protein kinase-independent pathway. J. Biol. Chem. 2000;275(35):345–35,352. doi: 10.1074/jbc.M005510200. [DOI] [PubMed] [Google Scholar]
- Yusta B, Estall J, Drucker DJ. Glucagon-like peptide-2 receptor activation engages bad and glycogen synthase kinase-3 in a protein kinase A-dependent manner and prevents apoptosis following inhibition of phosphatidylinositol 3-kinase. J. Biol. Chem. 2002;277(24):896–24,906. doi: 10.1074/jbc.M201358200. [DOI] [PubMed] [Google Scholar]
- Zamostiano R, Pinhasov A, Bassan M, et al. A femtomolar-acting neuroprotective peptide induces increased levels of heat shock protein 60 in rat cortical neurons: a potential neuroprotective mechanism. Neurosci. Lett. 1999;264:9–12. doi: 10.1016/s0304-3940(99)00168-8. [DOI] [PubMed] [Google Scholar]
- Zeitler P, Siriwardana G. Stimulation of mitogen-activated protein kinase pathway in rat somatotrophs by growth hormone-releasing hormone. Endocrine. 2000;12:257–264. doi: 10.1385/ENDO:12:3:257. [DOI] [PubMed] [Google Scholar]
- Zerr P, Feltz A. Forskolin blocks the transient K current of rat cerebellar granule neurons. Neurosci. Lett. 1994;181:153–157. doi: 10.1016/0304-3940(94)90582-7. [DOI] [PubMed] [Google Scholar]
- Zumpe ET, Tilakaratne N, Fraser NJ, Christopoulos G, Foord SM, Sexton PM. Multiple ramp domains are required for generation of amylin receptor phenotype from the calcitonin receptor gene product. Biochem. Biophys. Res. Commun. 2000;267:368–372. doi: 10.1006/bbrc.1999.1943. [DOI] [PubMed] [Google Scholar]


